Abstract
The aging process is often paralleled by decreases in muscle and increases in fat mass. At the extreme these two processes lead to a condition known as “sarcopenic obesity” (Roubenoff R. Ann NY Acad Sci 904: 553–557, 2000). Research suggests that inflammatory cytokines produced by adipose tissue, especially visceral fat, accelerate muscle catabolism and thus contribute to the vicious cycle that initiates and sustains sarcopenic obesity. We tested the hypothesis that obesity and poor muscle strength, hallmarks of sarcopenic obesity, are associated with high circulating levels of proinflammatory cytokines in a random sample of the residents of two municipalities in the Chianti geographic area (Tuscany, Italy). The study sample consisted of 378 men and 493 women 65 yr and older with complete data on anthropometrics, handgrip strength, and inflammatory markers. Participants were cross-classified according to sex-specific tertiles of waist circumference and grip strength and according to a cut point for obesity of body mass index ≥30 kg/m2. After adjusting for age, sex, education, smoking history, physical activity, and history of comorbid diseases, components of sarcopenic obesity were associated with elevated levels of IL-6, C-reactive protein, IL-1 receptor antagonist, and soluble IL-6 receptor (P < 0.05). Our findings suggest that global obesity and, to a greater extent, central obesity directly affect inflammation, which in turn negatively affects muscle strength, contributing to the development and progression of sarcopenic obesity. These results suggest that proinflammatory cytokines may be critical in both the development and progression of sarcopenic obesity.
Keywords: sarcopenia, central obesity, proinflammatory cytokines
Aging is accompanied by changes in body composition characterized by a relative decline of muscle mass (1) and an increase in fat mass (23). In certain individuals, these changes are extreme and produce a combination of substantial overweight and muscle weakness, a condition recently termed “sarcopenic obesity” (33). Previous research on sarcopenia has focused largely on factors affecting loss of muscle mass, without adequately considering gains in fat mass. However, studies addressing the relationship between changes in body composition and physical function in older individuals have found that both reduced lean body mass and increased fat mass contribute independently to mobility impairment (40). These findings are consistent with the notion that one of the main causes of mobility disability in older persons is a decline in strength to a level that becomes inadequate to support the efficient locomotion of a disproportionately high body mass.
The combination of low muscle strength and mass and obesity may be particularly deleterious because of its possible association with a proinflammatory state. Adipocytes actively secrete leptin and proinflammatory cytokines (25, 30), both of which stimulate muscle catabolism (6, 21, 34) and thus activate a vicious cycle leading to accelerated sarcopenia, additional weight gain largely in the form of fat, and, ultimately, physical disability.
Consistent with this hypothesis, elevated levels of proinflammatory cytokines are associated with global obesity (3, 7, 22) and central obesity (26, 42) and have been shown to predict accelerated decline of muscle strength and increased risk of disability and mortality in older individuals (8, 15, 19, 28). However, to our knowledge, no studies have directly compared circulating levels of inflammatory markers in individuals with varying degrees and combinations of sarcopenia and obesity. In this study, using data from a representative population, we tested the hypothesis that older persons with high body mass index (BMI) and/or waist circumference and low muscle strength are characterized by a proinflammatory state revealed by high circulating levels of proinflammatory cytokines.
METHODS
The present study used data from the “Invecchiare in Chianti” (Aging in the Chianti Area, InCHIANTI) study, a prospective population-based study of older people designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (INRCA), Florence, Italy. The InCHIANTI Study is a representative epidemiological study performed in Greve in Chianti and Bagno a Ripoli, two small towns in the Tuscany region of Italy. In 1998, 1,270 persons 65 years or older were randomly selected from the population. Of these, 378 men and 493 women had complete data for the present analyses. The study design was approved by the Italian National Institute of Research and Care on Aging Review Board and has been reported elsewhere (13).
Muscle strength
Grip strength was measured with a handheld dynamometer (hydraulic hand BASELINE; Smith and Nephew). Participants were asked to perform the task twice with each hand, and the maximum strength attained during the four trials was used for the present analyses. Although lower extremity muscle strength was measured in the InCHIANTI study, it was not used in this study because it was not available for the weakest and frailest participants. Additionally, in a previous study we demonstrated that handgrip and lower extremity muscle strength are similarly associated with lower extremity function (24).
Anthropometric measures
Height and weight were measured at the study clinic, and BMI was calculated as weight (in kg) divided by the square of height (in m). Waist circumference was measured to the nearest 0.5 cm by using a nonelastic plastic tape, with the participant standing upright, at the midpoint between the lower rib margin and the iliac crest (normally umbilical level).
Physical activity measures
On the basis of responses to a standardized questionnaire administered during the home interview, participants were assigned to one of three categories of physical activity during the previous 12 mo. Data on current physical activity level were collected by asking participants to specify the type of each physical activity, the number of months performed, the number of times per month, and the number of minutes per exercise bout. Using these responses and the physical activity conversion tables, participants were classified according to recommendations by the U.S. Department of Health and Human Services, as reported previously (39): 1) sedentary, including participants who were completely inactive and those who performed light-intensity physical activity [i.e., walking, dancing; ≤4 metabolic equivalents (METs)] less than 1 h/wk; 2) moderately active, including participants who performed light-intensity physical activity 2–4 h/wk; 3) highly active, including participants who performed at least light physical activity 5 h/wk or more and those who performed moderate physical activity (i.e., gymnastics, swimming; > 4 METs) 1–2 h/wk or more.
Inflammatory markers
Blood samples were obtained from participants after a 12-h fast and a 15-min rest period. Aliquots of serum and plasma were immediately obtained and stored in a deep freezer at −80°C and were subsequently used for assessment of cytokines. Assays were done in duplicate for all cytokine measures and were repeated if the second measure differed by >10% from the first. The average of the two measures was used in the analyses. A detailed description of the sampling procedure and data collection method has been previously published (13).
Serum IL-6, soluble IL-6 receptors (sIL-6r, 80 kDa), IL-1 receptor antagonist (IL-1ra), and tumor necrosis factor (TNF)-α were measured in duplicate by high-sensitivity enzyme-linked immunosorbent assays (ELISA) (BIOSOURCE, Camarillo, CA). The lowest detectable concentrations were 0.10 pg/ml for IL-6, 8.00 pg/ml for sIL-6r, 4.00 pg/ml for IL-1ra, and 0.09 pg/ml for TNF-α. The interassay coefficient of variation was 4.5% for IL-1ra and 7% for IL-6, sIL-6r, and TNF-α. Serum IL-18 levels were measured in duplicate using highly sensitive quantitative sandwich assays (Quantikine HS, R&D Systems, Minneapolis, MN). The lower limit of detection for this test was 0.70 pg/ml, and the interassay coefficient of variation was 7%.
High-sensitivity C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay. The minimum detectable concentration was 0.03 mg/l, and the interassay coefficient of variation was 5%.
Statistical analyses
Descriptive values are given as means (SD). For analytical purposes, central obesity was defined as being in the upper sex-specific tertile of waist circumference, global obesity as having a BMI > 30 kg/m2, and poor muscle strength as being in the lowest sex-specific tertile of grip strength. Participants were then cross-classified according to these three dichotomous variables into eight groups as illustrated in Fig. 1. Division into tertiles of muscle strength allows for the exploration of sarcopenic obesity across the entire spectrum of strength and not only limited to the small number of individuals that, in the InCHIANTI population, had a standard definition of sarcopenia (2 SD below the population mean for strength).
Fig. 1.
Cross-classification of study participants according to global obesity, central obesity, and muscle strength. Schematic representation is shown of 4 cross-classified groups for men (M) and women (W) based on tertiles of waist circumference (WC) and body mass index (BMI) ≥ 30 kg/m2. Within each of these 4 body composition groups, M and W in the lowest tertile of grip strength were considered sarcopenic. Cutpoints for BMI, WC, and strength are also shown.
Since plasma levels of inflammatory markers were not normally distributed, analyses were performed using log-transformed values, which were then back-transformed for data presentation. Linear regression models were used to estimate mean values of TNF-α, IL-6, s-IL-6r, IL-1ra, IL-18, and CRP across the eight groups defined by cross-classification of participants according to BMI, waist circumference, and muscle strength as described above. These values were adjusted for group differences in age, sex, education, smoking history, physical activity, and history of comorbid diseases, including diabetes mellitus, hypertension, myocardial infarction, stroke, and heart failure. In addition, adjusted logistic regression models were fitted to the data to test the hypothesis that sarcopenia and obesity are associated with increased likelihood of having IL-6 > 2.5 pg/ml or CRP > 3.0 mg/l on the likelihood of having Il-6 > 2.5 pg/ml or CRP > 3.0 mg/l. Finally, structural equation modeling (20) was used to further examine interrelationships among age, sex, physical activity, BMI, waist circumference, strength, and proinflammatory markers, according to a predefined interpretative model, which is shown in Fig. 3. All analyses were performed using the SAS statistical software version 8.2 (SAS Institute, Cary, NC), except the structural equation modeling that was implemented via the program R under the terms of the Free Software Foundation’s GNU General Public License.
Fig. 3.
Proposed final structural equation model for the relationships between age, obesity, central obesity, inflammation, and poor muscle strength. Inflammation is represented by a latent variable (ellipse), which expresses the common relationships between the 6 inflammatory markers. Each variable in the model has a series of arrows that reflect the direction of the a priori proposed relationship between the variables. Path coefficients are standardized and represent the change that occurs in the variable at the head of an arrow for each SD change in the variable at the tail of the arrow. The inflammation latent variable is similar to a factor that is derived during confirmatory factor analysis. It represents the shared variance that exists between the 6 inflammatory markers that are included in the model. All the markers contribute to the variable proportionately to their path coefficient.
RESULTS
Participant characteristics are shown in Table 1. The mean age of the sample population was 74.0 yr (SD 7.1), and 56.6% of the participants were women.
Table 1.
Characteristics of men and women included in the study population
| Central Obesity |
Global Obesity |
Low Strength |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P Value | Yes | No | P Value | Yes | No | P Value | |
| Age, yr | |||||||||
| Men | 72.7(6.1) | 73.9(7.2) | 0.13 | 72.9(6.1) | 73.6(7.0) | 0.41 | 77.2(7.3) | 71.5(5.7) | <0.001 |
| Women | 73.6(6.6) | 74.7(7.7) | 0.13 | 73.6(6.7) | 74.7(7.6) | 0.11 | 77.6(7.7) | 72.8(6.6) | <0.001 |
| Site, Greve, % | 48.1 | 55.6 | 0.037 | 51.8 | 53.6 | 0.65 | 31.6 | 64.0 | <0.001 |
| Smokers, % | |||||||||
| Current | 13.1 | 14.8 | 9.2 | 15.9 | 14.1 | 14.3 | |||
| Former | 29.7 | 25.7 | 24.3 | 27.9 | 28.5 | 26.2 | |||
| Never | 57.2 | 59.5 | 0.74 | 66.5 | 56.2 | 0.004 | 57.4 | 59.5 | 0.64 |
| BMI, kg/m2 | |||||||||
| Men | 29.6(2.7) | 25.5(2.5) | <0.001 | 31.7(1.5) | 25.8(2.4) | <0.001 | 26.8(3.0) | 26.9(3.3) | 0.65 |
| Women | 32.0(4.1) | 25.9(3.3) | <0.001 | 33.3(3.0) | 25.5(2.8) | <0.001 | 28.0(4.9) | 27.8(4.4) | 0.64 |
| Waist circumference, cm | |||||||||
| Men | 104.2(4.9) | 90.0(6.0) | <0.001 | 105.4(6.2) | 92.4(7.4) | <0.001 | 94.6(9.0) | 95.0(8.7) | 0.65 |
| Women | 102.6(5.8) | 84.9(7.7) | <0.001 | 100.8(7.8) | 86.1(8.8) | <0.001 | 91.6(10.8) | 89.9(10.9) | 0.11 |
| Grip Strength, kg | |||||||||
| Men | 38.2(11.2) | 37.9(10.5) | 0.76 | 38.5(10.5) | 37.9(10.8) | 0.66 | 25.9(5.4) | 44.3(6.6) | <0.001 |
| Women | 21.4(7.6) | 22.2(7.4) | 0.27 | 21.7(8.0) | 22.1(7.2) | 0.58 | 13.9(3.7) | 26.0(5.3) | <0.001 |
| Physical activity level, % | |||||||||
| Sedentary | 18.4 | 17.5 | 20.6 | 16.9 | 27.5 | 12.9 | |||
| Moderate | 77.0 | 75.9 | 76.6 | 76.1 | 68.0 | 80.3 | |||
| High | 4.6 | 6.6 | 0.044 | 2.8 | 7.0 | 0.001 | 4.5 | 6.7 | <0.001 |
| CHD, % | 6.00 | 4.93 | 0.52 | 6.42 | 4.90 | 0.39 | 7.22 | 4.31 | 0.066 |
| CHF, % | 19.79 | 25.68 | 0.04 | 22.47 | 24.2 | 0.48 | 27.83 | 21.72 | 0.066 |
| Stroke, % | 8.83 | 5.44 | 0.062 | 7.79 | 6.13 | 0.40 | 9.28 | 5.17 | 0.024 |
| Diabetes, % | 19.44 | 8.84 | <0.001 | 17.44 | 10.56 | 0.01 | 14.08 | 11.38 | 0.27 |
| Education, yr | 5.5(3.2) | 5.5(3.3) | 0.99 | 5.1(3.2) | 5.6(3.3) | 0.08 | 5.7(3.3) | 4.9(3.1) | 0.001 |
Values are means (SD). BMI, body mass index; CHD, coronary heart disease; CHF, congestive heart failure; Greve, Greve in Chianti, Tuscany, Italy.
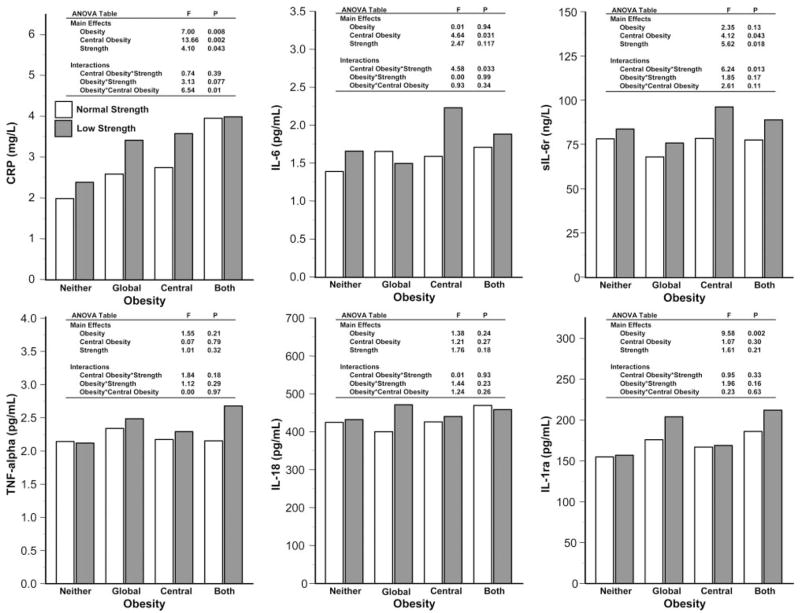
Figure 2 shows the mean values of the six inflammatory markers for the eight groups identified by a combination of measures of body composition and strength, adjusted for age, sex, education, smoking history, comorbidities, and physical activity. In models predicting the different inflammatory markers, we found no significant sex × age, sex × BMI, sex × waist circumference, or sex × strength interactions. Therefore, all subsequent analyses were performed in men and women combined.
Fig. 2.
Adjusted group mean values for inflammatory markers. Back-transformed mean inflammatory marker level values are shown for C-reactive protein (CRP), IL-6, IL-1 receptor antagonist (IL-1ra), soluble IL-6 receptor (sIL-6r), IL-18, and TNF-α across the 8 cross-classified groups (adjusted for group differences in age, sex, education, smoking history, physical activity, and history of comorbid diseases including diabetes mellitus, hypertension, myocardial infarction, stroke, and heart failure).
In general, BMI and waist circumference were positively and muscle strength negatively associated with proinflammatory cytokines, but there was specificity of effect across different inflammatory markers (Fig. 2). Global obesity, central obesity, and low muscle strength had an additive effect on CRP levels, with a significant synergistic effect for global obesity with central obesity. Regardless of global obesity status, participants with both central obesity and poor muscle strength had higher IL-6 levels. Similar to IL-6, levels of sIL-6r were highest in individuals with both central obesity and low strength. Only global obesity had an effect on levels of IL-1ra, with no effect of strength.
TNF-α levels were essentially similar across the eight groups, with no substantial differences according to global obesity, central obesity, or muscle strength (Fig. 2). For IL-18, participants with obesity and central obesity tended to have higher serum levels, but differences were not statistically significant (Fig. 2).
Figure 3 depicts the proposed structural equation model of the overall relationships between age, BMI, waist circumference, grip strength, and inflammation. Briefly, the model includes standardized coefficients for each relationship in the analysis, and each coefficient represents the change that occurs in the variable at the arrowhead for each SD change in the variable at the tail of the arrow. The arrows represent the proposed direction of the path relationships, consistent with our a priori hypotheses. In the proposed model, inflammation is a latent variable composed of individual components, i.e., individual inflammatory markers that have common characteristics. The advantage of using this approach is that the models allow for addressing the common features between individual inflammatory markers, which can be lost by examination of each marker alone. To evaluate the proposed model, we compared the covariance matrix that resulted from the model to the covariance matrix of the actual data. Because the χ2 test was nonsignificant (χ2 = 64.8; df = 27, P = 0.41), indicating that the proposed model adequately explained the underlying covariance matrix of the data, this model was accepted.
As shown in Fig. 3, age appears to have a positive association with inflammation. Additionally, our specified structural equation model is consistent with the presence of a direct effect of waist circumference on inflammation. Our model also suggests the presence of a direct inverse effect of BMI on inflammation but also a separate direct effect on individual inflammatory markers such as IL-1ra, CRP, and IL-18. Together, the effects of waist circumference and BMI on inflammation and individual inflammatory markers suggest a more complex relationship between central and global obesity and inflammation, not addressed in the model. Finally, according to our model, inflammation has a direct inverse effect on grip strength.
DISCUSSION
Confirming the finding reported by Cesari et al. (9) in participants in the TRAIN Study, we found that obesity is associated with high circulating levels of inflammatory markers. Specifically, sarcopenic obesity is associated with elevated levels of CRP, IL-6, and sIL-6r, and obesity is associated with elevated levels of IL-1ra. Our study points out that the distribution of the fat mass is also important and that central obesity is more proinflammatory than generic obesity. This is consistent with previous work demonstrating that visceral fat produces more proinflammatory adipokines than subcutaneous fat (16). In addition, we found a significant effect of muscle strength in the models predicting CRP and sIL-6r and significant interactions between central obesity and strength for models predicting IL-6 and sIL-6r. Thus our findings provide evidence that central obesity may negatively affect muscle strength through the upregulation of proinflammatory cytokine production and stimulation of the IL-6 pathway, which is consistent with previous work that has demonstrated that higher levels of IL-6, along with CRP (37), are associated with lower muscle strength or mass and mobility disability in older individuals (14, 37, 41).
An alternative hypothesis is based on recent experimental data suggesting that IL-6 may have both inflammatory and anti-inflammatory properties. Thus increased IL-6 levels may be compensatory to some other inflammatory or noninflammatory biological processes that are primarily responsible for the reduction of muscle strength. Ultimately, only intervention studies testing the effect of compounds that interfere with the IL-6 signaling pathway in the prevention and/or reversal of sarcopenia can address this issue. Previous reports have found obese individuals have elevated levels of inflammatory markers. This appears to follow directly from the secretion of proinflammatory cytokines (25) and indirectly from the secretion by adipose tissue of leptin, which may directly stimulate the production of CRP independent of cytokines produced by adipose tissue in older adults (5). Individuals with central obesity appear to be particularly prone to inflammation. In a recent report, inflammatory markers were more strongly associated with central obesity, as indicated by a large waist circumference, than with global obesity (26).
A possible mechanism by which inflammation may contribute to sarcopenia is through the direct catabolic effect of cytokines on muscle mass (34). Specifically, there is substantial evidence in both animal and human studies that high levels of IL-6 inhibit the anabolic effects of IGF-I on muscle (10, 35, 36), and that high levels of IL-6 and low levels of IGF-I contribute synergistically to impaired mobility (8), presumably through the acceleration of sarcopenia. IL-6 and other cytokines may also induce insulin resistance (32), which has been suggested as a significant contributing factor to sarcopenia (18, 29). Another possible mechanism, apoptosis, has been linked to sarcopenia in animal models (11), and it has been suggested that TNF-α and other cytokines could stimulate apoptosis in muscle cells (32). The lack of a significant finding for TNF-α is potentially due to a measurement problem: circulating levels of TNF-α are very low and the half-life of TNF-α is very short. The lack of significant finding for IL-18 is not clear, nor is the lack of effect of strength on IL-1ra. At the molecular level, IL-1ra is a natural antagonist of the proinflammatory cytokine IL-1, and in animal models of chronic inflammation, the administration of IL-1ra prevents tissue damage (2). Despite this, as a circulating biomarker, IL-1ra is considered an acute-phase protein and a more reliable measure of proinflammatory state than IL-1 (4). Similar triggers induce the production of IL-1 and IL-1ra, but IL-1 is produced locally, and only small quantities are released in the serum, whereas IL-1ra is produced by the liver in large quantities and fully released in the circulation (17). Thus the fact that in our data IL-1ra appear to be inflammatory markers is not completely surprising.
It is difficult to comment on whether the difference in inflammatory markers related to obesity and sarcopenia are clinically relevant. However, using logistic regression analysis we found that both central obesity and sarcopenia were associated with a significantly higher likelihood of having levels of IL-6 above those associated with clinical risk (>2.5 pg/ml) and that both central and global obesity were associated with the likelihood of having >3.0 mg/l for CRP (with a nonsignificant trend for the effect of muscle strength) (14, 31). To our knowledge, there are no established diagnostic values for the other four inflammatory markers.
The primary limitation of this study is the cross-sectional design. Even though our analyses, especially the structural equation model, suggest that high proinflammatory cytokines lead to low muscle strength, a mechanism of reverse causality cannot be excluded, and sarcopenia could be viewed as a risk factor for the development of a proinflammatory state. Furthermore, our proposed structural equation model adequately explained the underlying covariance matrix of the data, and the proposed relationships it contains are supported by evidence from previous research. However, it is not the only plausible model. Thus our hypothesis requires further testing in the context of appropriately designed longitudinal studies. Specifically, it would be useful to examine whether gains in waist circumference and/or BMI precede increases in proinflammatory cytokine levels and loss of muscle mass and strength.
Another potential limitation concerns the use of waist circumference as a measure of central obesity. This probably has a small effect, however, since waist circumference has been found to be robustly correlated with measures of visceral adiposity derived from computerized tomography (38). Use of handgrip strength to assess sarcopenia constitutes another minor limitation. Nevertheless, age-related sarcopenia is a generalized phenomenon, and handgrip strength has demonstrated value as a measure of overall strength and predictor of functional limitation in older persons (27).
In sum, study findings indicate that proinflammatory cytokines play a key role in the development of sarcopenic obesity and potentially contribute to a process by which sarcopenic obesity leads to diminished mobility. The age-related proinflammatory state of aging and obesity may prove to be a critical target of intervention. If inflammation can be blocked, either pharmacologically, or through the apparent anti-inflammatory (12, 30) and established body composition effects of exercise, the progression of sarcopenia could be attenuated as well.
Acknowledgments
GRANTS
The InCHIANTI study was supported as a targeted project (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the National Institute on Aging (contracts 263 MD 9164 13 and 263 MD 821336).
References
- 1.Aniansson A, Sperling L, Rundgren A, Lehnberg E. Muscle function in 75-year-old men and women. A longitudinal study. Scand J Rehabil Med Suppl. 1983;9:92–102. [PubMed] [Google Scholar]
- 2.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 3.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 4.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–2084. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 5.Ble A, Windham BG, Bandinelli S, Taub DD, Volpato S, Bartali B, Tracy RP, Guralnik JM, Ferrucci L. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) Am J Cardiol. 2005;96:991–995. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 6.Bullo-Bonet M, Garcia-Lorda P, Lopez-Soriano FJ, Argiles JM, Salas-Salvado J. Tumour necrosis factor, a key role in obesity? FEBS Lett. 1999;451:215–219. doi: 10.1016/s0014-5793(99)00540-2. [DOI] [PubMed] [Google Scholar]
- 7.Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 8.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 9.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 10.De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 12.Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60:760–767. doi: 10.1093/gerona/60.6.760. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 16.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 17.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab. 2005;31(Spec 2):5S20–25S26. doi: 10.1016/s1262-3636(05)73648-x. [DOI] [PubMed] [Google Scholar]
- 19.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 20.Hayduk L. Structural Equation Modeling with LISREL: Essentials and Advances. Baltimore, MD: The Johns Hopkins Univ. Press; 1987. [Google Scholar]
- 21.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 24.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 26.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Bassuk SS, Toth PP. C-reactive protein and risk of cardiovascular disease: evidence and clinical application. Curr Atheroscler Rep. 2003;5:341–349. doi: 10.1007/s11883-003-0004-3. [DOI] [PubMed] [Google Scholar]
- 32.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6:295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 33.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann NY Acad Sci. 2000;904:553–557. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 34.Roubenoff R, Freeman LM, Smith DE, Abad LW, Dinarello CA, Kehayias JJ. Adjuvant arthritis as a model of inflammatory cachexia. Arthritis Rheum. 1997;40:534–539. doi: 10.1002/art.1780400320. [DOI] [PubMed] [Google Scholar]
- 35.Serri O, St-Jacques P, Sartippour M, Renier G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: effect of substitutive GH therapy. J Clin Endocrinol Metab. 1999;84:58–63. doi: 10.1210/jcem.84.1.5374. [DOI] [PubMed] [Google Scholar]
- 36.Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111–122. doi: 10.7326/0003-4819-133-2-200007180-00010. [DOI] [PubMed] [Google Scholar]
- 37.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72:490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 39.Valenti G, Denti L, Maggio M, Ceda G, Volpato S, Bandinelli S, Ceresini G, Cappola A, Guralnik JM, Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 42.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S25–S28. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]