Abstract
Objective
We hypothesized that low serum selenium was associated with anemia in humans.
Subjects
A total of 2092 adults aged 65 and older, in the third National Nutrition Examination Survey, Phase 2 (1991–1994) (NHANES III).
Methods
Examination of the relationship between serum selenium and hematological indices in NHANES III.
Results
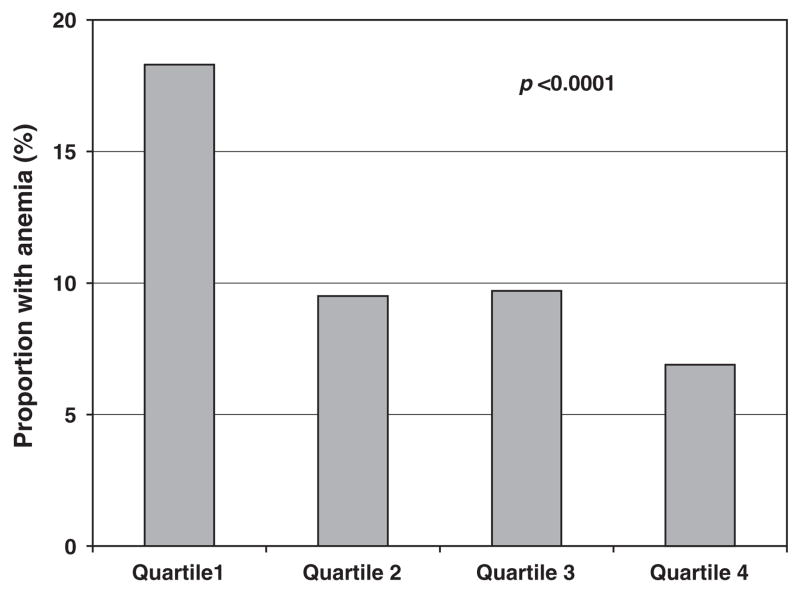
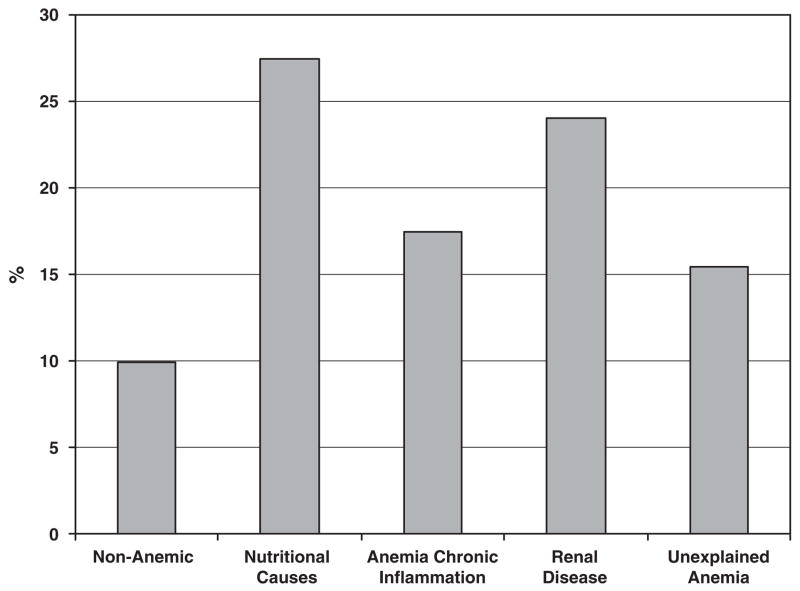
Anemia, defined by World Health Organization criteria, was present in 12.9%. Mean serum selenium among non-anemic and anemic adults was 1.60 and 1.51 μmol l−1 (P=0.0003). The prevalence of anemia among adults in the lowest to highest quartiles of serum selenium was 18.3, 9.5, 9.7 and 6.9%, respectively (P=0.0005). The proportion of adults in the lowest quartile of selenium among those who were non-anemic or who had anemia due to nutritional causes, chronic inflammation, renal disease or unexplained anemia was 9.9, 27.5, 17.5, 24.0 and 15.4%, respectively. An increase in loge selenium was associated with a reduced risk of anemia (odds ratio per one standard deviation increase 0.75, 95% confidence interval 0.58–0.97, P=0.03), adjusting for age, race, education, body mass index and chronic diseases.
Conclusion
Low serum selenium is independently associated with anemia among older men and women in the United States.
Keywords: aging, anemia, inflammation, hemoglobin, selenium
Introduction
Anemia is common in older adults, and the prevalence of anemia increases with advancing age (Beghé et al., 2004; Woodman et al., 2005). Among older adults, anemia has been associated with a wide spectrum of adverse outcomes (Lipschitz, 2003), including reduced quality of life (Thomas, 1998; Valderrabano, 2000), decreased muscle strength (Cesari et al., 2004), increased disability (Penninx et al., 2004), higher risk of Alzheimer’s disease (Beard et al., 1997) and increased all-cause mortality among nursing home residents (Kikuchi et al., 2001), and among moderately to severely disabled women living in the community (Chaves et al., 2004). Anemia has also been linked with congestive heart failure (Silverberg et al., 2000) and impaired cognitive function (Nissenson, 1992). The reduction of oxygen-carrying capacity of the blood that occurs with anemia may account for fatigue, cardiovascular complications and impaired physical performance (Lipschitz, 2003). Anemia among older adults is caused by renal failure, chronic inflammation and nutritional deficiencies, and about one-third of the anemia is unexplained (Guralnik et al., 2004).
Selenium is an essential trace element and a normal constituent of the diet. Selenium is a component of selenoproteins, including selenoenzymes such as glutathione peroxidase, selenoprotein-P and thioredoxin reductase (Zimmermann and Köhrle, 2002; Moghadaszadeh and Beggs, 2006). Serum selenium concentrations seem to decrease with age (Savarino et al., 2001; Bates et al., 2002) and are lower in persons with chronic diseases (Bates et al., 2002). Low serum selenium concentrations have been associated with increased mortality from cancer (Kok et al., 1987; Kornitzer et al., 2004) and all-cause mortality among older women living in the community (Ray et al., 2006). Low activity of glutathione peroxidase, a major selenoenzyme, was associated with increased risk of cardiovascular events among adults with suspected coronary artery disease (Blankenberg et al., 2003).
Animal and human studies suggest that there is a link between selenium deficiency and anemia. Among animals, selenium deficiency is associated with anemia in hens (Latshaw et al., 1977) and cattle (Morris et al., 1984), but not rats (Hu et al., 1984). In humans, selenium deficiency may contribute to anemia among dialysis patients (Hampel et al., 1985), adults with pulmonary tuberculosis (van Lettow et al., 2005), adults in Vietnam (Van Nhien et al., 2006) and disabled older women living in the community (Semba et al., 2006). However, no information is currently available on serum selenium concentrations and the risk of anemia in the older population. We hypothesized that low serum selenium levels are associated with low hemoglobin among older adults. To examine this hypothesis, we characterized serum selenium and hemoglobin among older adults in the third National Health and Nutrition Examination Survey (1988–1994) (NHANES III).
Methods
The study population consisted of men and women who participated in phase 2 of NHANES III (1991–1994). Subjects were sampled using a stratified, multistage probability design that was representative of a national probability sample of the civilian noninstitutionalized population of the United States (National Center for Health Statistics, 1994). A full assessment in NHANES III included a home interview and examination, including phlebotomy in a mobile examination center. Some people were unable to come to the mobile examination center and underwent a modified home examination that included phlebotomy.
Laboratory methods for NHANES III have been described in detail elsewhere (Gunter et al., 1996) and are briefly summarized. Hemoglobin was measured using a Coulter-S-Plus Jr electronic counter (Coulter Electronics, Hialeah, FL, USA). Serum iron was measured colorimetrically (Alpkem RFA Analyzer, Clackamas, OR, USA). Serum ferritin was measured using the Quantimmune Ferritin IRMA kit (Bio-Rad Laboratories, Hercules, CA, USA). Folate and vitamin B12 were measured using the Quantaphase Folate radioassay kit (Bio-Rad). Serum creatinine was measured by the Jaffe reaction using a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA), and creatinine clearance was computed using the Cockroft–Gault equation: creatinine clearance (ml min−1)= ((140−age)×kg body weight)/(mg dl−1 plasma creatinine×72). A 15% reduction was given for women (Cockcroft and Gault, 1976). Serum selenium was measured using a graphite furnace atomic absorption spectrometer (PerkinElmer, Shelton, CT, USA) with Zeeman background correction (Niskar et al., 2003).
Anemia was defined as hemoglobin <12 g dl−1 for women and <13 g dl−1 for men (World Health Organization, 1968). Different pathophysiological types of anemia were distinguished according to the criteria already used by Guralnik et al. (2004) in the same study population. Anemia was divided into types of anemia to gain insight into serum selenium concentrations within each type of anemia that was previously described (Guralnik et al., 2004). Among anemic adults, iron-deficiency anemia was defined as having two or three of the following criteria: transferrin saturation rate less than 15%, serum ferritin concentration<12 ng ml−1 and erythrocyte protoporphyrin concentration greater than 1.24 μM (Looker et al., 1997), folate-deficiency anemia was defined as red blood cell folate <232.49 nmol l−1 or serum folate <5.89 nmol l−1, and anemia due to vitamin B12 deficiency was defined as serum B12 <200 pg ml−1. Red blood cell folate was used for those who were seen in the mobile examination center, and serum folate was used as the indicator of folate status for those in the modified home examination with phlebotomy, since red blood cell folate was not measured in the home visits. Nutritional anemia was defined as anemia due to either iron deficiency, folate deficiency or deficiency in vitamin B12. Among anemic adults, the anemia due to chronic inflammation was defined as serum iron <60 μgdl−1, creatinine clearance ≥30 ml min−1 and no evidence of iron deficiency. Anemia due to renal disease was defined in the presence of creatinine clearance <30 ml min−1. Unexplained anemia was defined as anemia that was not due to iron, folate or vitamin B12 deficiencies or due to the anemia of chronic inflammation or renal disease. The quartiles of selenium were based upon all participants aged 65 or greater and were defined with cutoffs of ≤1.33, >1.33; ≤1.49, >1.49 and ≤1.65, >1.65 μmol l−1.
The National Center for Health Statistics was the source for the original data files used in this analysis (US Department of Health and Human Services, 1996). Prevalence rates and distributions were estimated for the US population using probability weights specific to NHANES III. Descriptive statistics were used to characterize the study population, provide information on the distribution of biochemical measurements of micronutrients and calculate the prevalence of deficiencies. χ2- and trend tests were used to examine the associations between anemia and dichotomized covariates. Univariate and multivariate logistic regression was used to examine the relationship between selenium and anemia, with sequential models adjusting for demographic, chronic disease, nutritional and inflammation variables that were significant (P<0.05) in univariate analyses. Selenium was transformed by loge to achieve a normal distribution. To assess the relative strength of associations between selenium and anemia, odds ratios and 95% confidence intervals (CIs) associated with one standard deviation increase in loge selenium were reported. Simple and multiple linear regression was used to examine the relationship of selenium and other risk factors with hemoglobin. Variables that were associated with hemoglobin at a level of P<0.05 were included as covariates in final multivariate linear regression models. Standardized β’s were also reported for each predictor to show the strength of the associations.
Results
There were 2092 adults (934 men and 1158 women) in phase 2 of NHANES III who had measurements of hemoglobin, serum selenium and other blood tests to characterize the causes of anemia. Overall, 270 (12.9%) of the participants were anemic. The main characteristics of study participants are shown in Table 1 according to anemia status. Compared to participants who were not anemic, those anemic were older (P=0.007), more likely to have education <12 years (P=0.01), to be black (P<0.001) and more often affected by osteoarthritis (P=0.01), diabetes (P=0.003) and renal disease (P<0.0001). Serum selenium levels were lower in anemic compared with non-anemic adults (P=0.0003). There were borderline differences in body mass index (BMI) between anemic and non-anemic adults (P=0.09), and no significant differences in the prevalence of smoking, hypertension, congestive heart failure, stroke, angina, cancer, myocardial infarction and cardiovascular disease. The prevalence of anemia was highest in the lowest quartile and lowest in the highest quartile of serum selenium (Figure 1).
Table 1.
Characteristics of adults ≥65 years with and without anemia in NHANES III, Phase 2, 1991–1994
| Characteristicsa | Anemic (N=270) | Non-anemic (N=1822) | P-value |
|---|---|---|---|
| Age (years) | 75.2 (0.6) | 73.6 (0.2) | 0.007 |
| Sex, women (%) | 48.1 | 58.2 | 0.026 |
| Race, Black (%) | 19.1 | 6.8 | <0.0001 |
| Body mass index, kgm−2 (%) | |||
| <18.5 | 4.2 | 2.8 | 0.09 |
| 18.5–24.9 | 31.7 | 34.4 | |
| 25.0–29.9 | 46.7 | 37.6 | |
| ≥30 | 17.4 | 25.2 | |
| Education <12 years (%) | 53.1 | 41.8 | 0.01 |
| Current smokers (%) | 14.3 | 23.2 | 0.13 |
| Serum selenium (μmol l−1)b | 1.51 (1.47, 1.56) | 1.60 (1.59, 1.61) | 0.0003 |
| Hypertension (%) | 52.3 | 46.5 | 0.21 |
| Osteoarthritis (%) | 28.3 | 16.4 | 0.01 |
| Diabetes (%) | 20.3 | 11.3 | 0.003 |
| Congestive heart failure (%) | 10.8 | 7.5 | 0.16 |
| Stroke (%) | 9.9 | 8.4 | 0.5 |
| Angina (%) | 34.9 | 31.9 | 0.5 |
| Cancer (%) | 24.3 | 21.7 | 0.5 |
| Myocardial infarction (%) | 13.4 | 12.9 | 0.87 |
| Cardiovascular disease (%) | 42.9 | 39.9 | 0.51 |
| Renal disease (%) | 21.2 | 5.6 | 0.0001 |
Population-weighted means and percentages.
Geometric mean (95% CI).
Figure 1.
Proportion of adults with anemia by quartile of serum selenium in NHANES III, Phase 2, 1991–1994.
Multivariate logistic regression models were used to examine the relationship between different risk factors and anemia (Table 2). Selenium was strongly and independently associated with anemia in models adjusted for demographic factors (model 2) and demographic factors and chronic diseases (model 3). Multiple linear regression models were also used to examine the relationship between serum selenium and hemoglobin (Table 3). Selenium was significantly associated with hemoglobin in a model that was adjusted for demographic factors (model 2). In model 2, for example, each one-unit increase in loge selenium was associated with an increase of 7.39 g l−1 hemoglobin, and male was associated with a 10.09 g l−1 increase in hemoglobin. A level of education <12 years was associated with a decrease of 0.98 g l−1 of hemoglobin. Selenium was related to hemoglobin in a final model (model 3) (P=0.06) that was adjusted for demographic factors, BMI and chronic diseases.
Table 2.
Multivariate logistic regression models of risk factors for anemia among adults ≥65 years in NHANES III, Phase 2, 1991–1994
| Variable | ORa | 95% CI | P-value |
|---|---|---|---|
| Model 1 | |||
| Log selenium | 0.68 | 0.56–0.84 | <0.0001 |
| Model 2 | |||
| Log selenium | 0.74 | 0.60–0.91 | 0.005 |
| Age (years) | 1.04 | 1.01–1.07 | 0.005 |
| Male | 1.60 | 1.10–2.35 | 0.015 |
| Black | 2.97 | 2.04–4.33 | <0.0001 |
| Education <12 years | 1.21 | 0.81–1.80 | 0.36 |
| Model 3 | |||
| Log selenium | 0.75 | 0.58–0.97 | 0.03 |
| Age (years) | 0.99 | 0.96–1.04 | 0.87 |
| Male | 1.27 | 0.79–2.05 | 0.32 |
| Black | 3.23 | 1.95–5.35 | <0.0001 |
| Education <12 years | 1.14 | 0.69–1.86 | 0.61 |
| Body mass index (kgm−2)b | |||
| <18.5 | 0.80 | 0.23–2.78 | 0.73 |
| 25.0–29.9 | 1.54 | 0.88–2.71 | 0.13 |
| ≥30 | 1.08 | 0.52–2.25 | 0.83 |
| Diabetes mellitus | 1.39 | 0.30–0.74 | 0.30 |
| Cardiovascular disease | 0.86 | 0.53–1.40 | 0.55 |
| Renal disease | 5.48 | 2.63–11.41 | <0.0001 |
| Osteoarthritis | 2.37 | 1.28–4.38 | 0.006 |
Abbreviations: CI, confidence intervals; OR, odds ratio.
OR per 1 s.d. increase in loge selenium.
BMI of 18.5–24.9 kgm−2 is the reference category.
Table 3.
Multiple linear regression models of selenium and other risk factors with hemoglobin (g dl−1) as the outcome, among adults ≥65 years in NHANES III, Phase 2, 1991–1994
| Variable | Coefficient | s.e. | Standardized β | P-value |
|---|---|---|---|---|
| Model 1 | ||||
| Log selenium | 9.72 | 2.88 | 0.103 | 0.001 |
| Model 2 | ||||
| Log selenium | 7.39 | 2.70 | 0.078 | 0.006 |
| Age (years) | −0.16 | 0.05 | −0.079 | 0.002 |
| Male | 10.09 | 0.76 | 0.371 | <0.0001 |
| Black | −6.83 | 0.86 | −0.136 | <0.0001 |
| Education <12 years | −0.99 | 0.73 | −0.036 | 0.18 |
| Model 3 | ||||
| Log selenium | 5.52 | 2.94 | 0.061 | 0.06 |
| Age (years) | −0.03 | 0.06 | −0.015 | 0.63 |
| Male | 10.65 | 0.82 | 0.410 | <0.0001 |
| Black | −6.49 | 1.02 | −0.124 | <0.0001 |
| Education <12 years | −0.81 | 0.81 | −0.031 | 0.31 |
| Body mass index (kgm−2)a | ||||
| <18.5 | 0.02 | 1.96 | 0.001 | 0.99 |
| 25.0–29.9 | 0.09 | 0.94 | 0.004 | 0.92 |
| ≥30 | 1.81 | 1.07 | 0.585 | 0.09 |
| Diabetes mellitus | −0.58 | 1.24 | −0.014 | 0.63 |
| Cardiovascular disease | 0.31 | 0.80 | 0.011 | 0.69 |
| Renal disease | −4.55 | 1.68 | −0.089 | 0.007 |
| Osteoarthritis | −2.94 | 1.15 | −0.086 | 0.01 |
BMI of 18.5–24.9 kgm−2 is the reference category.
Among the 270 anemic participants, the proportion of anemia due to nutritional causes, chronic inflammation, renal disease and unexplained anemia was 35.9, 20.4, 16.3 and 27.4%, respectively. The characteristics of participants who were non-anemic and who had various types of anemia are shown in Table 4. The participants with anemia due to renal disease tended to be the oldest (P=0.0003) and had the highest proportion with BMI <18.5 kgm−2 (P=0.007). Mean selenium concentrations were lowest among those with anemia due to nutritional causes and anemia of chronic inflammation and highest among those who were non-anemic (P=0.009). The prevalence of hypertension was highest among participants who had anemia of chronic inflammation (P=0.02), and the prevalence of diabetes was highest among those with anemia of chronic inflammation or unexplained anemia (P=0.02). The proportion of adults who were in the lowest quartile of serum selenium in the categories of non-anemic and anemia due to nutritional causes, chronic inflammation and/or renal disease, and unexplained anemia are shown in Figure 2. The highest proportion of participants with low selenium was in those with anemia due to nutritional causes and anemia due to renal disease.
Table 4.
Serum selenium levels in the types of anemia among adults ≥65 Years in NHANES III, Phase 2, 1991–1994a
| Characteristicsb,c | Non-anemic | Anemia due to nutritional causes | Anemia of chronic inflammation | Anemia due to renal disease | Unexplained anemia | P-value |
|---|---|---|---|---|---|---|
| N | 1822 | 97 | 55 | 44 | 74 | |
| Age (years) | 73.9 (0.2) | 75.7 (1.1) | 72.3 (0.8) | 81.0 (1.9) | 74.3 (0.9) | 0.0003 |
| Black (%) | 6.8 | 18.5 | 22.2 | 23.4 | 16.2 | <0.0001 |
| Women (%) | 58.2 | 45.2 | 56.7 | 61.0 | 40.9 | 0.08 |
| Body mass index (kgm−2, %) | ||||||
| <18.5 | 2.8 | 8.1 | 1.7 | 10.3 | 0 | 0.007 |
| 18.5–24.9 | 34.4 | 29.6 | 22.4 | 55.1 | 30.7 | |
| 25–29.9 | 37.6 | 49.3 | 46.9 | 31.9 | 49.5 | |
| ≥30 | 25.2 | 13.0 | 30.5 | 2.7 | 19.9 | |
| Education <12 years (%) | 41.8 | 59.0 | 57.6 | 52.2 | 44.6 | |
| Selenium (μmol l−1) | 1.60 (1.59, 1.61) | 1.50 (1.42, 1.57) | 1.50 (1.40, 1.61) | 1.54 (1.40, 1.68) | 1.54 (1.47, 1.60) | 0.009 |
| Hemoglobin (g l−1) | 141.7 (0.4) | 114.8 (1.6) | 117.0 (1.5) | 111.0 (1.8) | 117.8 (1.2) | <0.0001 |
| Current smokers (%) | 23.2 | 28.0 | 6.8 | 15.3 | 6.5 | 0.08 |
| Chronic diseases (%) | ||||||
| Hypertension | 46.5 | 53.0 | 69.4 | 65.5 | 36.2 | 0.02 |
| Osteoarthritis | 16.4 | 27.7 | 31.1 | 12.7 | 32.3 | 0.06 |
| Diabetes | 11.3 | 16.9 | 23.7 | 15.0 | 23.9 | 0.02 |
| Congestive heart | ||||||
| Failure | 7.5 | 8.2 | 20.3 | 11.8 | 7.4 | 0.11 |
| Stroke | 8.4 | 8.2 | 9.0 | 11.2 | 11.7 | 0.82 |
| Angina | 31.9 | 43.7 | 31.3 | 38.2 | 26.4 | 0.42 |
| Myocardial infarction | 12.9 | 15.8 | 16.3 | 5.6 | 12.1 | 0.78 |
| Cardiovascular disease | 39.9 | 48.5 | 38.9 | 53.0 | 35.4 | 0.53 |
| Cancer | 21.7 | 21.3 | 28.6 | 13.2 | 29.1 | 0.52 |
Weighted analysis for all means and percentages using NHANES III weights.
Mean (s.e.) for age and hemoglobin.
Geometric mean (CI) for selenium.
Figure 2.
Proportion of adults ≥65 years in the lowest selenium quartile by anemia status in NHANES III, Phase 2, 1991–1994.
Discussion
This study shows that low serum selenium is an independent risk factor for anemia among older men and women living in the community in the United States. Low selenium has not usually been considered as a possible cause of anemia in older adults (Balducci, 2003; Guralnik et al., 2004) and has only been previously implicated in anemia among patients on hemodialysis (Hampel et al., 1985) or with pulmonary tuberculosis (van Lettow et al., 2005). However, a correlation between low plasma selenium concentrations and low hemoglobin had already been noted in the British National Diet and Nutrition Survey among 1134 men and women aged 65 years and older (Bates et al., 2002), and more recently low serum selenium was found to be associated with anemia among older disabled women living in the community (Semba et al., 2006).
A potential biological mechanism by which low selenium could contribute to anemia is through maintenance of an optimal concentration of glutathione peroxidase, a key antioxidant selenoenzyme, in erythrocytes (Chow and Chen, 1980). Glutathione peroxidase protects hemoglobin against oxidation in erythrocytes (Nagababu et al., 2003). Although there is little direct evidence that serum selenium is related to erythrocyte and hemoglobin stability in humans, there is a possible relevant example of erythrocyte damage due to increased oxidative stress in animals (McPhail and Sibbald, 1992) with protection by selenium. Livestock that forage on Brassica (mustard, rape) are susceptible to brassica anemia in which oxidative stress causes excessive erythrocyte damage (Prache, 1994), and selenium supplementation has been shown to protect animals against erythrocyte damage (Rotruck et al., 1972; Gutzwiller, 1991, 1998). If low serum selenium levels are associated with reduced lifespan of erythrocytes in humans, then it would be anticipated that selenium deficiency would be associated with an increased reticulocyte count and high-erythropoietin levels. Studies in selenium-deficient pigs suggest that selenium deficiency limits erythropoiesis but does not affect red cell half-life (Fontaine et al., 1977). Further studies are needed to examine this issue.
Another mechanism by which selenium could potentially contribute to anemia is through modulation of inflammation. Low serum selenium concentrations were found in adults with anemia of chronic inflammation. Low serum selenium concentrations among disabled older women living in the community were predictive of subsequent rises in interleukin (IL)-6 (Walston et al., 2006). Thus, selenium could potentially play a role in the anemia of chronic inflammation through its relationship with the upregulation of IL-6 through the redox-sensitive transcription factor nuclear factor-κB. In turn, IL-6 has been implicated in the upregulation of hepcidin, the iron regulatory hormone that blocks iron absorption in the gut and iron release from macrophages and the liver (Roy and Andrews, 2005). The role of hepcidin in the anemia of chronic inflammation is not well understood, and little is known is about the relationship between selenium levels, hepcidin and anemia.
Another possible mechanism by which selenium could be involved in the pathogenesis of anemia is through heme oxygenase-1. Low selenium status can upregulate the activity of hepatic heme oxygenase-1, which catalyzes the initial step of heme catabolism and reduces heme to biliverdin, carbon monoxide and free divalent iron (Mostert et al., 2003; Tron et al., 2005). Whether upregulation of heme oxygenase-1 plays a role in the relationship of low serum selenium to anemia is not known.
Given that the study design of NHANES III is cross-sectional, the direction of the association between low serum selenium levels and anemia cannot be absolutely determined. It is possible that anemia causes low serum selenium concentrations through some uncharacterized mechanism, but there is less evidence to support such a biological explanation. The data from animal studies suggest that selenium deficiency causes anemia (Rotruck et al., 1972; Latshaw et al., 1977; Morris et al., 1984; Gutzwiller, 1991, 1998), but whether the same biological mechanism is found in humans would need to be determined by well-designed controlled clinical trials.
In this study, low levels of serum selenium occurred across all types of anemia. Anemia in older adults is multifactorial, and according to one hypothesized mechanism, a reduced half-life of erythrocytes related to low selenium could predispose in a general way to anemia. The association between low serum selenium and anemia may reflect the complex multisystem decline that occurs with aging and may possibly be caused by diverse biological mechanisms rather than the specific mechanisms described above.
The dietary intake of selenium varies widely worldwide, as selenium concentrations in plant-based foods reflect the concentrations of selenium in the soil where the plants were grown. The selenium concentrations in animal sources of food, in turn, depend upon the selenium content of the plants used for forage, or whether animal feed was fortified with selenium. Clinical selenium deficiency is rare and is seen in Keshan disease, an endemic cardiomyopathy found in low-selenium areas of China where affected persons may have serum selenium <0.25 μmol l−1 (20 μg l−1) (Food and Nutrition Board, 2000). The current dietary requirements for selenium by the Food and Nutrition Board of the Institute of Medicine are based in part upon maintenance of optimal levels of selenoproteins in blood, especially plasma glutathione peroxidase (Food and Nutrition Board, 2000).
The selenium requirement for prevention of chronic disease has not yet been definitively determined (Thomson, 2004). In addition, the selenium requirements for older adults are largely extrapolations from the middle-aged population (Thomson, 2004). The activity of glutathione peroxidase appears to be optimal when serum selenium concentrations are at least 1.27 μmol l−1 (100 μg l−1) or greater (Food and Nutrition Board, 2000). The baseline serum selenium concentrations at which there appeared to be a protective effect of selenium supplementation against cancer in two US trials were 1.34–1.54 μmol l−1 (Thomson, 2004). It is notable that in the present study, the highest prevalence of anemia was found among older subjects from NHANES III who were in the lowest quartile of serum selenium, which was <1.33 μmol l−1 and would be considered in the suboptimal range. It has been previously reported that 13.8% of NHANES III participants were taking supplements that contained selenium, but that serum selenium values were not significantly associated with taking a supplement that contained selenium (Niskar et al., 2003). Except for overt clinical selenium deficiency, the health consequences of an inadequate selenium intake are incompletely understood, and further work is needed to define optimal selenium status in the elderly population.
In conclusion, low serum selenium was independently associated with anemia among older men and women living in the community. This study raises a potentially important public health question: has selenium deficiency been overlooked as a cause of anemia among older adults? This study may represent a first important step toward determining whether selenium deficiency is a potential cause of anemia among older adults. To our knowledge, it is not known whether improving dietary selenium intake will increase hemoglobin concentrations among older adults with low serum selenium concentrations. Further work is needed to corroborate these findings in other populations, provide evidence for longitudinal causal associations, identify underlying biological mechanisms and determine whether improving selenium status has an impact upon anemia in older adults.
This study was sponsored by National Institute on Aging, National Institutes of Health (R01 AG027012, R01 AG029148 and Intramural Research Branch).
References
- Balducci L. Epidemiology of anemia in the elderly: information on diagnostic evaluation. J Am Geriatr Soc. 2003;51:S2–S9. doi: 10.1046/j.1532-5415.51.3s.4.x. [DOI] [PubMed] [Google Scholar]
- Bates CJ, Thane CW, Prentice A, Delves HT. Selenium status and its correlates in a British National Diet and Nutrition Survey: people aged 65 years and over. J Trace Elem Med Biol. 2002;16:1–8. doi: 10.1016/s0946-672x(02)80002-5. [DOI] [PubMed] [Google Scholar]
- Beard CM, Kokmen E, O’Brien PC, Anía BJ, Melton LJ., III Risk of Alzheimer’s disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann Epidemiol. 1997;7:219–224. doi: 10.1016/s1047-2797(97)00015-x. [DOI] [PubMed] [Google Scholar]
- Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116:3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Lauretani F, Russo CR, Carter C, Bandinelli S, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:238–241. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- Chaves PHM, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- Chow CK, Chen CJ. Dietary selenium and age-related susceptibility of rat erythrocytes to oxidative damage. J Nutr. 1980;110:2460–2466. doi: 10.1093/jn/110.12.2460. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Fontaine M, Valli VE, Young LG. Studies on vitamin E and selenium deficiency in young pigs. III. Effects on kinetics of erythrocyte production and destruction. Can J Comp Med. 1977;41:57–63. [PMC free article] [PubMed] [Google Scholar]
- Food and Nutrition Board and Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Centers for Disease Control and Prevention; Hyattsville, MD: 1996. [Google Scholar]
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- Gutzwiller A. Einfluss der Selenversorgungslage der Ziege die Resistenz der Erythrozyten gegen oxidative Schädigung. Schweiz Arch Tierheilk. 1991;133:157–161. [PubMed] [Google Scholar]
- Gutzwiller A. Erythrocyte resistance to oxidative damage and leucocyte capacity to reduce nitroblue tetrazolium in selenium-deficient cattle. Zentralbl Veterinarmed A. 1998;45:271–278. doi: 10.1111/j.1439-0442.1998.tb00827.x. [DOI] [PubMed] [Google Scholar]
- Hampel G, Schaller KH, Rosenmüller M, Oefele C. Seleniumdeficiency as contributing factor to anemia and thrombocytopenia in dialysis patients. Life Support Syst. 1985;3(Suppl 1):36–40. [PubMed] [Google Scholar]
- Hu ML, Chung C, Spallholz JE. Hematologic data of seleniumdeficient and selenium-supplemented rats. J Inorg Biochem. 1984;22:165–173. doi: 10.1016/0162-0134(84)80025-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Inagaki T, Shinagawa N. Five-year survival of older people with anemia: variation with hemoglobin concentration. J Am Geriatr Soc. 2001;49:1226–1228. doi: 10.1046/j.1532-5415.2001.49241.x. [DOI] [PubMed] [Google Scholar]
- Kok FJ, de Bruijn AM, Vermeeren R, Hofman A, van Laar A, de Bruin M, et al. Serum selenium, vitamin antioxidants, and cardiovascular mortality: a 9-year follow-up study in the Netherlands. Am J Clin Nut. 1987;45:462–468. doi: 10.1093/ajcn/45.2.462. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Valente F, de Bacquer D, Neve J, de Backer G. Serum selenium and cancer mortality: a nested case–control study within an age- and sex-stratified sample of the Belgian adult population. Eur J Clin Nutr. 2004;58:98–104. doi: 10.1038/sj.ejcn.1601754. [DOI] [PubMed] [Google Scholar]
- Latshaw JD, Ort JF, Diesem CD. The selenium requirements of the hen and effects of a deficiency. Poultry Sci. 1977;56:1876–1881. doi: 10.3382/ps.0561876. [DOI] [PubMed] [Google Scholar]
- Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51:S10–S13. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- McPhail DB, Sibbald AM. The role of free radicals in brassica-induced anaemia of sheep: an ESR spin trapping study. Free Radic Res Commun. 1992;16:277–284. doi: 10.3109/10715769209049180. [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology. 2006;21:307–315. doi: 10.1152/physiol.00021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JG, Cripe WS, Chapman HL, Jr, Walker DF, Armstrong JB, Alexander JD, Jr, et al. Selenium deficiency in cattle associated with Heinz bodies and anemia. Science. 1984;223:491–493. doi: 10.1126/science.6691160. [DOI] [PubMed] [Google Scholar]
- Mostert V, Hill KE, Burk RF. Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1. FEBS Lett. 2003;541:85–88. doi: 10.1016/s0014-5793(03)00309-0. [DOI] [PubMed] [Google Scholar]
- Nagababu E, Chrest FJ, Rifkind JM. Hydrogen-peroxide-induced heme degradation in red blood cells: the protective roles of catalase and glutathione peroxidase. Biochim Biophys Acta. 2003;1620:211–217. doi: 10.1016/s0304-4165(02)00537-8. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. National Center for Health Statistics; Hyattsville, MD: 1994. [PubMed] [Google Scholar]
- Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, Gunter EW, et al. Serum selenium levels in the US population. Third National Health and Nutrition Examination Survey, 1998–1994. Biol Trace Elem Res. 2003;91:1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- Nissenson AR. Epoietin and cognitive function. Am J Kidney Dis. 1992;20(Suppl 1):21–24. [PubMed] [Google Scholar]
- Penninx BWJH, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- Prache S. Haemolytic anaemia in ruminants fed forage Brassicas: a review. Vet Res. 1994;25:497–520. [PubMed] [Google Scholar]
- Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the Women’s Health and Aging Studies. J Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Hoekstra WG. Prevention of oxidative damage to rat erythrocytes by dietary selenium. J Nutr. 1972;102:689–696. doi: 10.1093/jn/102.5.689. [DOI] [PubMed] [Google Scholar]
- Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Savarino L, Granchi D, Ciapetti G, Cenni E, Ravaglia G, Forti P, et al. Serum concentrations of zinc and selenium in elderly people: results in healthy nonagenarians/centenarians. Exp Gerontol. 2001;36:327–339. doi: 10.1016/s0531-5565(00)00218-7. [DOI] [PubMed] [Google Scholar]
- Semba RD, Ferrucci L, Cappola AR, Ricks MO, Ray AL, Xue QL, et al. Low serum selenium is associated with anemia among older women living in the community. Biol Trace Elem Res. 2006;112:97–107. doi: 10.1385/BTER:112:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- Thomas ML. Impact of anemia and fatigue on quality of life in cancer patients: a brief review. Med Oncol. 1998;15(Suppl 1):S3–S7. [PubMed] [Google Scholar]
- Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- Tron K, Novosyadlyy R, Dudas J, Samoylenko A, Kietzmann T, Ramadori G. Upregulation of heme oxygenase-1 gene by turpentine oil-induced localized inflammation: involvement of interleukin-6. Lab Investig. 2005;85:376–387. doi: 10.1038/labinvest.3700228. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (DHHS) Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III Laboratory Data File (CD-ROM). Public Use Data File Documentation Number 76200. Centers for Disease Control and Prevention; Hyattsville, MD: 1996. National Center for Health Statistics. [Google Scholar]
- Valderrabano F. Quality of life benefits of early anaemia treatment. Nephrol Dial Transplant. 2000;15(Suppl 3):23–28. doi: 10.1093/oxfordjournals.ndt.a027972. [DOI] [PubMed] [Google Scholar]
- van Lettow M, West CE, van der Meer JWM, Wieringa FT, Semba RD. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr. 2005;59:526–532. doi: 10.1038/sj.ejcn.1602116. [DOI] [PubMed] [Google Scholar]
- Van Nhien N, Khan KC, Yabutani T, Ninh NX, Kassu A, Huong BT, et al. Serum levels of trace elements and iron deficiency anemia in adult Vietnamese. Biol Trace Elem Res. 2006;111:1–9. doi: 10.1385/BTER:111:1:1. [DOI] [PubMed] [Google Scholar]
- Walston J, Xue Q, Semba RD, Ferrucci L, Cappola A, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Nutritional Anemia: Report of a WHO Scientific Group. World Health Organization; Geneva, Switzerland: 1968. [Google Scholar]
- Zimmermann MB, Köhrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12:867–878. doi: 10.1089/105072502761016494. [DOI] [PubMed] [Google Scholar]