Abstract
OBJECTIVES
To examine cross-sectional associations between vitamin D status and musculoskeletal pain and whether they differ by sex.
DESIGN
Population-based study of persons living in the Chianti geographic area (Tuscany, Italy).
SETTING
Community.
PARTICIPANTS
Nine hundred fifty-eight persons (aged ≥65) selected from city registries of Greve and Bagno a Ripoli.
MEASUREMENTS
Pain was categorized as mild or no pain in the lower extremities and back; moderate to severe back pain, no lower extremity pain; moderate to severe lower extremity pain, no back pain; and moderate to severe lower extremity and back pain (dual region). Vitamin D was measured according to radioimmunoassay, and deficiency was defined as 25-hydroxyvitamin D (25(OH)D) less than 25 nmol/L.
RESULTS
The mean age ± standard deviation was 75.1 ± 7.3 for women and 73.9 ± 6.8 for men. Fifty-eight percent of women had at least moderate pain in some location, compared with 27% of men. After adjusting for potential confounders, vitamin D deficiency was not associated with lower extremity pain or dual-region pain, although it was associated with a significantly higher prevalence of at least moderate back pain without lower extremity pain in women (odds ratio = 1.96, 95% confidence interval = 1.01–3.59) but not in men.
CONCLUSION
Lower concentrations of 25(OH)D are associated with significant back pain in older women but not men. Because vitamin D deficiency and chronic pain are fairly prevalent in older adults, these findings suggest it may be worthwhile to query older adults about their pain and screen older women with significant back pain for vitamin D deficiency.
Keywords: vitamin D, pain, aging
Musculoskeletal pain is a well-documented cause of functional decline and progressive disability in older adults.1,2 With the expectation that the older population will increase 75% over the next 25 years,3 there will inevitably be an increase in prevalence of chronic pain and its associated societal costs. Thus, clinicians and researchers need to gain a better understanding of underlying pain mechanisms that are potentially mutable to develop effective intervention strategies.
Vitamin D deficiency has been linked to multiple poor outcomes in older adults, such as greater fracture risk,4 impaired lower extremity function,5 osteomalacia, and pain.6–9 Vitamin D is known to be critically important for the maintenance of bone and muscle health through its role in calcium homeostasis.10 The role of vitamin D in development of pain syndromes is less well known. It has been proposed that hypovitaminosis D-induced pain is due to a lack of calcium phosphate available to mineralize the collagen matrix of bone, with the matrix expanding under the innervated periosteum, leading to diffuse pain.11
Although multiple studies have suggested a link between vitamin D status and pain, particularly back pain,6,7,9,12,13 there have been several studies that have found no relationship between vitamin D status and pain.14–16 The relationship between vitamin D status and pain needs to be further explored. No large-scale study in older adults has examined the association between 25-hydroxyvitamin D (25(OH)D) concentrations and pain in ambulatory older persons. The objective of this study was to examine cross-sectional associations between vitamin D status and musculoskeletal pain using data from the Invecchiare in Chianti (InCHIANTI) Study. The hypothesis was that lower levels of 25(OH)D would be associated with the presence of significant pain in older adults. Because chronic pain is more prevalent in older women,17 whether these relationships would differ according to sex was also examined.
METHODS
Study Population
The InCHIANTI Study is a prospective population-based study of the factors that contribute to mobility decline in older Italian adults. The study sample (1,155 participants aged 65–102) was randomly selected using a multistage stratified sampling method from two towns in the Chianti geographic area of Italy (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). The details of the data collection and sampling procedures have been described elsewhere.18 Baseline data was collected from September 1998 through March 2000. All participants gave written consent for study participation, and the ethical committee of the Italian National Research Council of Aging approved the study.
Of the 1,155 participants, 197 were excluded: 100 who did not have blood drawn, 50 who had blood drawn but did not have serum 25(OH)D values, and 47 who had 25(OH)D values but not provide complete pain information. Therefore, 958 participants (529 women and 429 men) were included in the final analysis.
Pain Measurement and Categories
At the baseline interview, participants were asked questions about back and lower extremity (hip, knee, and foot) pain. If participants reported back pain that had occurred quite often or almost every day during the previous year, they were asked to rate their pain using a numeric pain rating scale, which has been validated for use in older populations with varying cognitive statuses.19 This scale, which was shown on a card, included numbers from 0 to 10, with 0 indicating no pain and 10 indicating the worst pain imaginable. For the measurement of lower extremity pain, participants who reported having hip, knee, or foot pain for at least 1 month during the previous year were also asked to rate their pain in the same manner. Upper extremity pain in was not examined in the analyses, because these questions were not included in the database.
Pain was categorized into four categories based on location and severity. Location was divided as back pain (thoracic to lumbar) or lower extremity pain (hip, knee, and foot). If the participant reported pain anywhere in the spine from the cervical to the lumbar region, they were considered to have back pain. If the participant reported pain in the hip, knee, or foot, they were considered to have lower extremity pain. Based on previous work, moderate pain severity (>3 on a scale of 0–10), which is thought to be a clinically important level of pain, was used as the break point.18 Therefore, four pain categories were created: mild or no pain in the lower extremities or back; moderate to severe back pain, no lower extremity pain; moderate to severe lower extremity pain, no back pain; and lower extremity and back pain with moderate to severe intensity in at least one location.
Serum Vitamin D
Fasting blood samples were collected in the morning after a 12-hour fast, centrifuged, and stored at − 80°C. Serum levels of 25(OH)D were measured using radioimmunoassay (RIA kit, DiaSorin, Stillwater, MN). The intra- and interassay coefficients of variation for vitamin D were 8.1% and 10.2%, respectively.
Covariates
Covariates included specific variables thought to confound the association between serum 25(OH)D levels and pain, including age, body mass index (BMI), cognitive status, depressive symptoms, month of vitamin D assessment, calcium intake, and serum creatinine levels. BMI was calculated as weight in kg divided by height in m2. Cognitive status was measured according to Mini-Mental State Examination (MMSE) score. Depressive symptoms were examined using the Centers for Epidemiologic Studies Depression Scale (CES-D) score. Months of vitamin D assessment was included to account for seasonal variation in 25(OH)D levels (nmol/L), because greater sunlight exposure during spring and summer could increase measurement levels. Calcium intake (mg/d) was collected using the food-frequency questionnaire originally developed and validated for the assessment of dietary intake in Italian volunteers participating in the European Prospective Investigation into Cancer and Nutrition.20 Serum creatinine (mg/dL; a marker of kidney function) was measured using a standard creatinine Jaffe method (Roche Diagnostics, Mannheim, Germany). Parathyroid hormone (PTH) levels were measured using a two-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). The intra- and interassay coefficients of variation for vitamin D were less than 3.0% and 5.5%, respectively.
Statistical Analyses
Vitamin D deficiency was defined as 25(OH)D less than 25.0 nmol/L, consistent with definitions reported in the literature.21 Descriptive analysis included reporting means ± standard deviations for continuous variables and numbers and percentages for categorical variables according to gender and vitamin D category. Within-sex comparisons were made across vitamin D categories using t-tests for continuous variables and chi-square tests for categorical variables. The covariate-adjusted odds ratios were estimated using multinomial logistic regression in sex-stratified models.22 This approach is appropriate for semi-ordinal outcomes, because it does not assume a linear association between vitamin D deficiency status and pain category. Using the group with no or mild lower extremity and back pain as the reference outcome category, the association between vitamin D deficiency status and each pain category was separately determined. All analyses were conducted using SAS version 8.02 (SAS Institute, Inc., Cary, NC).
RESULTS
The 197 excluded participants were older and had more depressive symptoms, and poorer cognition. Also, men with low vitamin D levels had lower calcium and vitamin D intake than the remainder of the cohort. Excluded women had more moderate to severe lower extremity pain without back pain than those who were included, and excluded men had more sites with moderate to severe pain than men who were included.
The median 25(OH)D levels (interquartile range (IQR)) were 48.9 nmol/L (35.4, 73.6) for men and 33.9 nmol/L (23.5, 50.7) for women. The mean age was 75.1 ± 7.3 for women included in the study and 73.9 ± 6.8 for men. Selected baseline characteristics of the study sample are displayed in Table 1. Men and women with low vitamin D were older and had more depressive symptoms, poorer cognition, and higher PTH levels than those with high vitamin D. Also, men with low vitamin D had lower calcium intake than those with low vitamin D.
Table 1.
Selected Participant Characteristics According to Sex and Vitamin D Category
| 25(OH) Dnmol/L | ||||||
|---|---|---|---|---|---|---|
| Men (n = 429) | Women (n = 529) | |||||
| Characteristic | <25.0* (n = 55) | ≥25.0 (n = 374) | P-Value | <25.0* (n = 151) | ≥25.0 (n = 378) | P-Value |
| Age, mean ± SD | 78.8 ± 8.4 | 73.1 ± 6.2 | <.001 | 77.9 ± 8.0 | 74.0 ± 6.7 | <.001 |
| Body mass index, kg/m2, mean ± SD | 26.8 ± 3.0 | 27.1 ± 3.3 | .50 | 27.5 ± 5.0 | 27.9 ± 4.4 | .46 |
| Center for Epidemiologic Study Depression Scale score, mean ± SD | 10.0 ± 8.4 | 9.4 ± 6.8 | .61 | 16.6 ± 8.7 | 14.7 ± 9.2 | .04 |
| Mini-Mental State Examination score, mean ± SD | 22.8 ± 6.6 | 25.6 ± 3.8 | .003 | 23.2 ± 5.1 | 24.8 ± 3.8 | <.001 |
| Serum creatinine, mg/dL, mean ± SD | 1.07 ± 0.34 | 1.01 ± 0.19 | .25 | 0.86 ± 0.18 | 0.85 ± 0.18 | .47 |
| Calcium intake, mg/d, mean ± SD | 786 ± 276 | 869 ± 277 | .039 | 773 ± 287 | 803 ± 326 | .29 |
| Pain, n (%) | ||||||
| No or mild pain | 42 (76) | 269 (72) | reference | 65 (43) | 162 (44) | reference |
| Moderate-to-severe back pain, no lower extremity pain | 6 (11) | 40 (11) | .93 | 26 (17) | 35 (9) | .04 |
| Moderate-to-severe lower extremity pain, no back pain | 3 (5) | 14 (4) | .71 | 13(9) | 40 (10) | .55 |
| Back and lower extremity pain, moderate to severe in ≥1 site | 4 (8) | 51 (13) | .27 | 47 (31) | 141 (37) | .41 |
Vitamin D deficiency.
SD = standard deviation.
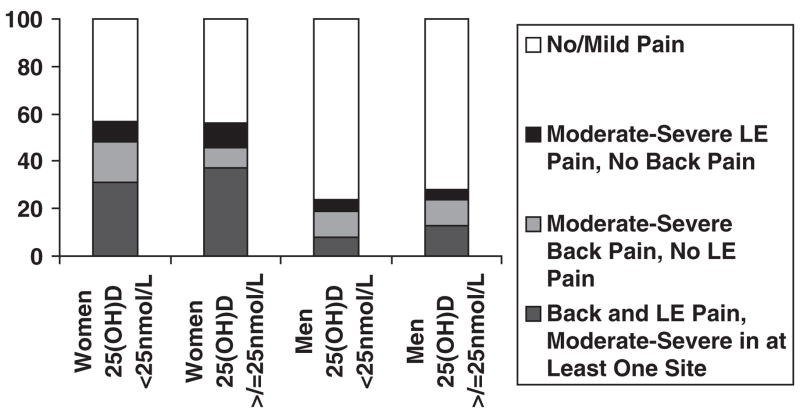
Reports of significant musculoskeletal pain were fairly common in the women in this cohort but not in the men (Figure 1); 57.1% of the women reported having at least moderate severity pain in one or more locations, compared with 27.5% of the men. Having dual regions of pain (lower extremity and back) was also more common in women than men (35.5% vs 12.8%). In terms of back pain alone, reports were similar between men (10.7%) and women (11.5%).
Figure 1.
Cumulative self-reported pain burden of the sample according to sex and vitamin D status.
LE = lower extremity.
With the exception of back pain alone in women, pain distributions of subjects classified as vitamin D deficient and those not deficient were similar (overall P-value .57 for men and .06 for women). The relationship between back pain alone and vitamin D status was largely responsible for the nearly significant overall P-value seen in women. As seen in Table 1 and Figure 1, in women who were vitamin D deficient, the percentage with significant back pain alone was nearly double that of the women who were not vitamin D deficient (P = .04).
As seen in Table 2, women who were classified as vitamin D deficient had 1.96 greater odds (95% confidence interval = 1.01–3.59) of at least moderate back pain without lower extremity pain versus no or mild pain only than those who were not classified as vitamin D deficient (P = .04), although vitamin D deficiency did not lead to significantly greater odds of moderate or severe pain in the lower extremities or of dual-region pain (lower extremity and back). The addition of PTH to the model attenuated the odds ratio for back pain only marginally. In men, the same relationship between vitamin D status and back pain did not exist; low vitamin D levels did not appear to be related to any pain pattern in men (Table 3).
Table 2.
Adjusted Odds Ratio (OR) for Specific Pain Categories According to Baseline Vitamin D Status in Women
| Model 1* | Model 2† | Model 3‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pain Category | No OR | Yes OR (95% CI) | P-Value | No OR | Yes OR (95% CI) | P-Value | No OR | Yes OR (95% CI) | P-Value |
| Moderate-to-severe back pain, no lower extremity pain, versus no or mild pain only | 1.00 | 1.83 (1.01–3.32) | .05 | 1.00 | 1.96 (1.01–3.59) | .04 | 1.00 | 1.88 (0.99–3.57) | .05 |
| Moderate-to-severe lower extremity pain, no back pain, versus no or mild pain only | 1.00 | 0.80 (0.39–1.61) | .52 | 1.00 | 0.58 (0.26–1.29) | .18 | 1.00 | 0.61 (0.27–1.40) | .25 |
| Back and lower extremity pain, moderate to severe in ≥1 sites, versus no or mild pain only | 1.00 | 0.83 (0.53–1.30) | .43 | 1.00 | 0.80 (0.49–1.30) | .36 | 1.00 | 0.89 (0.54–1.47) | .66 |
Adjusted for age.
Adjusted for age, body mass index, Center for Epidemiologic Study Depression Scale score, Mini-Mental State Examination score, calcium intake, time of vitamin D assessment, and creatinine levels (marker of kidney function).
Adjusted for covariates in Model 2 and parathyroid hormone.
CI = confidence interval.
Table 3.
Adjusted Odds Ratio (OR) for Specific Pain Categories According to Baseline Vitamin D Status in Men
| Model 1* | Model 2† | Model 3‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pain Category | No OR | Yes OR (95% CI) | P-Value | No OR | Yes OR (95% CI) | P-Value | No OR | Yes OR (95% CI) | P-Value |
| Moderate-to-severe back pain, no lower extremity pain, versus no or mild pain only | 1.00 | 0.95 (0.37–2.41) | .91 | 1.00 | 0.88 (0.32–2.47) | .81 | 1.00 | 0.86 (0.31–2.43) | .78 |
| Moderate-to-severe lower extremity pain, no back pain, versus no or mild pain only | 1.00 | 1.34 (0.36–4.93) | .66 | 1.00 | 0.49 (0.06–3.93) | .50 | 1.00 | 0.54 (0.07–4.31) | .56 |
| Back and lower extremity pain, moderate to severe in ≥1 sites, versus no or mild pain only | 1.00 | 0.51 (0.17–1.48) | .21 | 1.00 | 0.52 (0.17–1.57) | .25 | 1.00 | 0.61 (0.20–1.86) | .38 |
Adjusted for age.
Adjusted for age, body mass index, Center for Epidemiologic Study Depression Scale score, Mini-Mental State Examination score, calcium intake, time of vitamin D assessment, and creatinine levels (marker of kidney function).
Adjusted for covariates in Model 2 and parathyroid hormone.
CI = confidence interval.
DISCUSSION
The initial hypothesis was that lower levels of 25(OH)D would be associated with the presence of significant pain in older adults; the significant relationship between vitamin D status and moderate to severe back pain in older women but not men partially supported this hypothesis. According to the data, vitamin D deficiency was not associated with higher levels of lower extremity pain or dual-region pain; furthermore, non-deficient vitamin D status was not associated with lower levels of pain, although a unique relationship was found between vitamin D status and back pain, in that older women with vitamin D deficiency were more likely to have significant back pain. It is not completely clear why this specific relationship between back pain and vitamin D exists in this cohort. One plausible explanation is that vitamin D deficiency leads to osteomalacia, which commonly presents as chronic low back pain clinically and is more commonly seen in women.23 This may explain why the findings demonstrate this significant relationship between vitamin D status and back pain in older women. Similar to these findings, other studies have shown a relationship between back pain and vitamin D status, but none have shown a relationship between vitamin D and pain in other body regions.
In terms of sex differences, in general, older women appeared to bear a higher burden of vitamin D deficiency and pain, particularly having pain in more than one location. The findings regarding pain are consistent with other reports in the literature.2,17 Thus, it may be even more important to examine underlying causes of pain in older women, particularly the role of vitamin D status in back pain.
Public health awareness of the need for adequate vitamin D levels is steadily increasing in Europe and North America because of emerging evidence that vitamin D deficiency can lead to a host of poor health consequences, including greater fracture risk, functional decline, and pain.4–6,8,9 Due to multiple factors, vitamin D deficiency is common in older adults.24,25 With limited sources of vitamin D available in nature (primarily fatty fish), dietary intake is not a reliable source for vitamin D acquisition.26 Thus, the consumption of vitamin D-fortified foods and sunlight exposure and the resultant synthesis in the skin are the primary mechanisms by which vitamin D is acquired.27 In older adults, this is problematic because of the limited sunlight exposure, especially in the homebound, the reduced capacity of older skin to synthesize vitamin D, and the greater prevalence of nutritional deficiencies in general and intolerance to dairy products in particular in this age group.27 In addition to the aging subgroup, people with darker skin pigmentation are also at greater risk because of a need for longer exposure periods for maximal absorption of ultraviolet rays.28 Living at higher latitudes also adds to the deficiency problem because of the shorter periods of available ultraviolet light.28 Thus, special consideration should be given to screening people in these at-risk populations to prevent vitamin D deficiency and its negative sequelae.
Although the relationship between vitamin D status and pain has been examined in smaller studies, to the authors’ knowledge, this is the first large-scale population based study to examine this issue in older adults. A small case series described an unusual pain syndrome that failed to respond to traditional pain management strategies but was alleviated with the administration of a man-made form of vitamin D, ergocalciferol.7 Another study also demonstrated an association between vitamin D deficiency and persistent, nonspecific musculoskeletal pain in a nonelderly sample of 150 patients.9 They found that 93% of the sample would be considered vitamin D deficient and had never been tested for vitamin D status. The large prevalence of low vitamin D levels in this cohort may be a function of the study location (Minneapolis, MN), where people are unlikely to get enough sunlight exposure.9 An investigation of the contribution of vitamin D deficiency to chronic low back pain (CLBP) status in a cohort of 360 patients (90% women) found that 83% of all patients had serum 25(OH)D levels less than 22.5 nmol/L and that there was a significant reduction in symptoms after vitamin D supplementation in those who were deficient.6 In Saudi women, vitamin D deficiency is thought to be a serious problem due to limited sunlight exposure as a result of the intense heat and the avoidance of body exposure encouraged in Muslim communities.6 Although all of these studies suggest a reduction in symptoms related to vitamin D supplementation, none of these studies was conducted as a randomized or placebo-controlled trial. Randomized trials examining various forms of vitamin D supplementation need to be performed before advocating its use for back pain.
In contrast to the studies demonstrating a positive relationship between vitamin D status and pain, there are also several studies that have found no relationship. For example, one found that vitamin D levels were not correlated with back pain as measured using a visual analog scale in women of various ages with osteoporosis,14 although when comparing pain as measured using the Quality of Life Questionnaire of the European Foundation for Osteoporosis in those with those with deficient vitamin D status (<25 nmol/L) and those with sufficient status (25–50 nmol/L), it was found that the effect size was clinically relevant at 0.58 and was borderline significant (P = .05). In addition, there have been several studies that have evaluated pain as a secondary outcome when plain vitamin D was used as a supplement.15,16,29 These studies have found that plain vitamin D is not sufficient to ameliorate back pain,15,16,29 although use of a vitamin D-hormone analog, alfacalcidol, significantly reduced back pain severity in postmenopausal women with osteoporosis. All of these vitamin D studies (positive and negative), along with the findings of the current study, provide motivation for future work to understand the role of vitamin D in back pain and to evaluate the use of vitamin D analogs as potential treatments for pain.
Although the development of these vitamin D-related pain syndromes are not well understood, they are thought to be due to the development of osteomalacia, which is a condition characterized by softening of the bones, with resultant weakness, and bone fragility, caused by inadequate deposition of calcium or vitamin D.11 Osteomalacia often presents itself as nonspecific musculoskeletal pain, particularly in the lumbar spine region, and is more commonly seen in women.23 The mechanism by which vitamin D deficiency leads to pain has been proposed previously.8,11 It is proposed that vitamin D deficiency causes a reduction in calcium absorption and thereby greater production of parathyroid hormone (PTH) to maintain blood calcium levels. Then PTH increases urinary excretion of phosphorus, leading to hypophosphatemia. Without sufficient calcium phosphate product levels in the circulation, the collagen matrix on the endosteal and periosteal surfaces of bones cannot be properly mineralized. Thus, when the improperly supported collagen matrix hydrates and swells, it causes pressure on the sensory-innervated periosteum, resulting in pain.8,11 There is also evidence that vitamin D deficiency may lead to a faster rate of progression of osteoarthritis, the most common musculoskeletal disease in older adults.30 This association, which may be mediated, in part, through an effect of vitamin D on bone mineral density, could also result in greater prevalence of pain in and around the joints, including those of the axial skeleton.4,31
Although the prevalence of back pain is similar between men and women, the association between 25(OH)D levels and pain were not the same in both sexes. This finding suggests a potential sex difference in the mechanisms underlying back pain in older adults. In the same way that women are more prone to bone loss over time (osteoporotic changes), it is logical that women would be more vulnerable to vitamin D-related pain because osteomalacia is on the pathway to osteoporosis. In terms of typical structural adaptations seen in aging bone, men and women exhibit greater total cross-sectional area (CSA) and medullary area over time, but women have a much smaller increase in CSA and a larger increase in medullary area.32,33 As a result, women display a greater thinning of cortical area and thickness, resulting in a greater load per unit area (stress) being imposed on women’s bones than in men. This greater stress leads to a greater likelihood of structural damage to the bone.32,33 In addition, the greater cortical thinning seen in women may also make the bone more susceptible to the influences of low vitamin D status.32,33 Given the small proportion of men in the sample who were vitamin D deficient, it is also possible that a true association between vitamin D status and pain is being masked because of sample size. In any event, the sex difference seen in the relationship between vitamin D and pain needs to be explored further.
A primary advantage of this study is the use of a large population-based sample with plasma 25(OH)D levels, which is the best clinical indicator of vitamin D body stores. It is important to highlight possible intervention points on the pathway to disability by identifying factors, such as vitamin D status and pain, that may lead to functional decline. The cross-sectional nature of the data does not allow temporality of vitamin D and pain categories to be determined; therefore, the observed association may be due to a noncausal relationship (e.g., unmeasured causal agents that may affect both vitamin D levels and pain). Although it is biologically plausible that vitamin D deficiency caused significant back pain, it is also possible that the women with significant back pain were more likely to stay indoors and self-limit their activities, leading to less sunlight exposure and thus to vitamin D deficiency. It is also not known whether the results of this study are generalizable to groups other than older Italians. With regard to the sex difference, one must consider that men are more likely to underreport pain, which may have affected the overall findings of the study. Although the sample size was sufficiently large to demonstrate the association between vitamin D status and back pain in women, it may not be large enough to show the associations with other pain groupings, especially in men, who are more likely to underreport pain. Finally, because the vitamin D-deficient participants in this study had poorer cognition, there may be some concern that their ability to understand the use of the numeric pain rating scale (NPRS) was compromised. It is likely that this is not the case, given the established concurrent validity for use of the NPRS in older adults with and without cognitive impairment,19 as well as the evidence that 92% of nursing home residents with mild cognitive impairment (MMSE score 18–23) can complete the NPRS.34
In conclusion, low vitamin D status was associated with significant back pain in older women but not men. Given that low vitamin D status is fairly prevalent in older adults and that there are significant functional consequences to untreated chronic pain,2 these findings argue strongly for querying older adults about their pain and potentially screening older women with significant back pain for vitamin D deficiency. Prevention of vitamin D deficiency in older women may be beneficial in reducing back pain severity and its deleterious functional consequences, but randomized, controlled trials will be needed before practice guidelines can be changed. Recent work in the area of vitamin D status and pain suggest a need for continued research in this area, particularly in older women and ethnic populations with darker skin who require greater levels of sunlight exposure to synthesize vitamin D.
Acknowledgments
Conflict of Interest: The InCHIANTI Study was supported by the Italian Ministry of Health and in part by the National Institute on Aging (contracts 263 MD 916413 and 263 MD 821336). This research was partly supported by an unrestricted grant from Procter and Gamble, Italy. The work of Dr. Hicks, a Building Interdisciplinary Research Careers in Women’s Health scholar at the time a portion of this work was done, was supported by National Institutes of Health Contract K12 HD43489 (National Institute of Child Health and Human Development/Office of Research on Women’s Health/National Institute of Diabetes and Digestive and Kidney Diseases).
Sponsor’s Role: None.
Footnotes
Author Contributions: Gregory E. Hicks, Michelle Shardell, and Ram R. Miller: study concept and design, analysis and interpretation of data, preparation of manuscript. Jack Guralnik, Antonio Cherubini, and Fulvio Lauretani: analysis and interpretation of data, preparation of manuscript. Luigi Ferrucci and Stefania Bandinelli: study concept and design, acquisition of participants and data, analysis and interpretation of data, preparation of manuscript.
References
- 1.Leveille SG, Bean J, Ngo L, et al. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2006;128:69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135:1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 3.Government U. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1992–2050. Washington, DC: US Government Printing Office; 1992. [Google Scholar]
- 4.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 6.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28:177–179. doi: 10.1097/00007632-200301150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Gloth FM, III, Lindsay JM, Zelesnick LB, et al. Can vitamin D deficiency produce an unusual pain syndrome? Arch Intern Med. 1991;151:1662–1664. [PubMed] [Google Scholar]
- 8.Mascarenhas R, Mobarhan S. Hypovitaminosis D-induced pain. Nutr Rev. 2004;62:354–359. doi: 10.1111/j.1753-4887.2004.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 9.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 10.Gerdhem P, Ringsberg KA, Obrant KJ, et al. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency: What a pain it is. Mayo Clin Proc. 2003;78:1457–1459. doi: 10.4065/78.12.1457. [DOI] [PubMed] [Google Scholar]
- 12.Macfarlane GJ, Palmer B, Roy D, et al. An excess of widespread pain among South Asians: Are low levels of vitamin D implicated? Ann Rheum Dis. 2005;64:1217–1219. doi: 10.1136/ard.2004.032656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotfi A, Abdel-Nasser AM, Hamdy A, et al. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26:1895–1901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 14.Basaran S, Guzel R, Coskun-Benlidayi I, et al. Vitamin D status: Effects on quality of life in osteoporosis among Turkish women. Qual Life Res. 2007;16:1491–1499. doi: 10.1007/s11136-007-9257-6. [DOI] [PubMed] [Google Scholar]
- 15.Papadokostakis G, Katonis P, Damilakis J, et al. Does raloxifene treatment influence back pain and disability among postmenopausal women with osteoporosis? Eur Spine J. 2005;14:977–981. doi: 10.1007/s00586-005-0899-1. [DOI] [PubMed] [Google Scholar]
- 16.Helliwell PS, Ibrahim GH, Karim Z, et al. Unexplained musculoskeletal pain in people of South Asian ethnic group referred to a rheumatology clinic—relationship to biochemical osteomalacia, persistence over time and response to treatment with calcium and vitamin D. Clin Exp Rheumatol. 2006;24:424–427. [PubMed] [Google Scholar]
- 17.Leveille SG, Zhang Y, McMullen W, et al. Sex differences in musculoskeletal pain in older adults. Pain. 2005;116:332–338. doi: 10.1016/j.pain.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 19.Taylor LJ, Harris J, Epps CD, et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil Nurs. 2005;30:55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 20.Kroke A, Klipstein-Grobusch K, Voss S, et al. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr. 1999;70:439–447. doi: 10.1093/ajcn/70.4.439. [DOI] [PubMed] [Google Scholar]
- 21.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 22.Agresti A. Categorical Data Analysis. 2. New York: Wiley; 2002. [Google Scholar]
- 23.Francis RM, Selby PL. Osteomalacia. Baillieres Clin Endocrinol Metab. 1997;11:145–163. doi: 10.1016/s0950-351x(97)80569-1. [DOI] [PubMed] [Google Scholar]
- 24.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 25.Maggio D, Cherubini A, Lauretani F, et al. 25(OH)D serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J Gerontol A Biol Sci Med Sci. 2005;60A:1414–1419. doi: 10.1093/gerona/60.11.1414. [DOI] [PubMed] [Google Scholar]
- 26.Ovesen L, Andersen R, Jakobsen J. Geographical differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc. 2003;62:813–821. doi: 10.1079/PNS2003297. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 28.Calvo MS, Whiting SJ. Public health strategies to overcome barriers to optimal vitamin D status in populations with special needs. J Nutr. 2006;136:1135–1139. doi: 10.1093/jn/136.4.1135. [DOI] [PubMed] [Google Scholar]
- 29.Ringe JD, Faber H, Fahramand P, et al. Alfacalcidol versus plain vitamin D in the treatment of glucocorticoid/inflammation-induced osteoporosis. J Rheumatol Suppl. 2005;76:33–40. [PubMed] [Google Scholar]
- 30.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: The Framingham Study. J Rheumatol. 2000;27:1032–1037. [PubMed] [Google Scholar]
- 32.Russo CR, Lauretani F, Bandinelli S, et al. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 33.Russo CR, Lauretani F, Seeman E, et al. Structural adaptations to bone loss in aging men and women. Bone. 2006;38:112–118. doi: 10.1016/j.bone.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Closs SJ, Barr B, Briggs M, et al. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage. 2004;27:196–205. doi: 10.1016/j.jpainsymman.2003.12.010. [DOI] [PubMed] [Google Scholar]