Abstract
Steroidogenic factor-1 (SF-1) (Ad4BP, NR5A1) is a nuclear receptor that regulates many aspects of adrenal and reproductive development and function. Consequently, deletion of the gene (Nr5a1) encoding Sf-1 in XY mice results in impaired adrenal development, complete testicular dysgenesis with Müllerian structures, and female external genitalia. Initial efforts to identify NR5A1 changes in humans focused on 46,XY individuals with combined adrenogonadal failure and Müllerian structures. Although this combination of clinical features is rare, 2 such patients harboring NR5A1 mutations have been described within the past decade. More recently, however, it has emerged that heterozygous loss of function mutations in NR5A1 can be found relatively frequently in children and adults with 46,XY disorders of sex development (DSD) but with apparently normal adrenal function. The phenotypic spectrum associated with these changes ranges from complete testicular dysgenesis with Müllerian structures, through individuals with mild clitoromegaly or genital ambiguity, to severe penoscrotal hypospadias or even anorchia. Furthermore, a non-synonymous polymorphism in NR5A1 may be associated with micropenis or undescended testes within the population. Taken together, these reports suggest that variable loss of SF-1 function can be associated with a wide range of reproductive phenotypes in humans.
Keywords: Gonad; Gonadal dysgenesis; NR5A1; Nuclear receptor; Pituitary; Steroidogenesis; Steroidogenic Factor-1; 46,XY DSD
The Identification and Cloning of NR5A1
The concept of a common ‘steroidogenic factor’ that could activate multiple different steps in steroidogenesis was first proposed in the early 1990s following the identification of a number of similar regulatory elements in the proximal promoter regions of the cytochrome P450 steroid hydroxylase genes [Rice et al., 1991; Morohashi et al., 1992]. These elements contained variations on an AGGTCA DNA sequence motif, leading to the hypothesis that a common protein – termed ‘steroidogenic factor-1’ (SF-1) – could regulate their transcription.
The gene encoding Sf-1 in the mouse (now termed Nr5a1) was first cloned in 1992 from an adrenal cDNA library using a probe corresponding to the DNA-binding domain (DBD) of a related orphan receptor, retinoid X receptor [Lala et al., 1992]. The bovine homologue of this factor (termed adrenal 4-binding protein, Ad4BP) was identified shortly afterwards from an adrenal cDNA library using the partial sequence of a protein purified from bovine adrenal extracts [Honda et al., 1993]. Both these murine and bovine cDNAs were shown to encode proteins that could activate the promoters of steroid hydroxylase enzymes. Thus, it was concluded that a common steroidogenic factor had been identified.
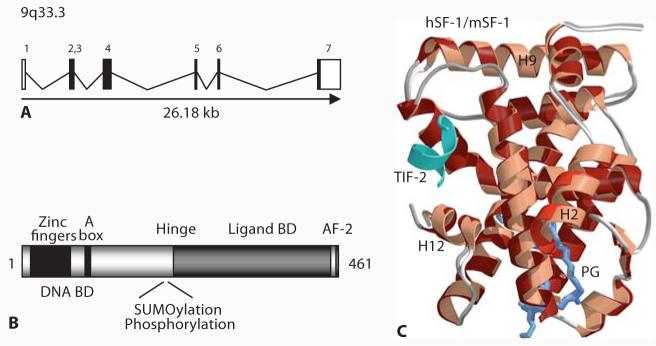
The mouse gene encoding steroidogenic factor-1 was initially termed FtzF1, as it resembles the Drosophila orphan nuclear receptor, fushi tarazu factor homolog 1 (FTZ-F1) [Ueda et al., 1990; Swift and Ashworth, 1995; Taketo et al., 1995]. This gene was mapped to chromosome 2. The corresponding human gene encoding SF-1 was initially called FTZF1 but is now more correctly referred to as NR5A1 (nuclear receptor subfamily 5 group A member 1). This gene was mapped to the long arm of chromosome 9 in humans (9q33) and consists of 7 exons spanning approximately 30 kb of genomic DNA (fig. 1A) [Taketo et al., 1995; Oba et al., 1996; Wong et al., 1996]. Exon 1 is untranslated.
Fig. 1.
A An overview of the genomic structure of NR5A1 (encoding SF-1). B Cartoon showing key structural domains of SF-1 (Ad4BP, NR5A1). C Crystal structure model of the ligand-binding domain of human SF-1 (dark red ribbons) superimposed with that of mouse Sf-1 (light red ribbons) bound to TIF-2. The small phospholipid ligand is shown in blue. Reproduced with permission from Krylova et al. [2005].
Structure of SF-1
Human SF-1 (NR5A1) is a 461 amino acid protein that shares structural homology with other members of the nuclear receptor superfamily (www.nursa.org). Critical functional domains of this protein include: an amino-terminal 2 zinc finger DNA-binding domain (DBD), an accessory DNA-binding region, a hinge region, and a ligand-binding domain (LBD) that forms an AF-2 structure (fig. 1B). Unlike some nuclear receptors (e.g., androgen receptor), SF-1 does not have a large AF1 domain.
The first zinc finger of the DBD of SF-1 contains a proximal (P) box that is involved in the specific recognition of DNA target sequences by nuclear receptors. This P box amino acid sequence is one of the main factors determining nuclear receptor DNA-binding specificity and interfaces with the major groove of DNA by recognizing variations on an AGGTCA motif [Evans, 1988]. The accessory DNA-binding region of SF-1 contains an A box (FTZ box) motif. This region is believed to stabilize DNA binding by interacting with the minor groove of DNA (e.g., an extended 3′ flanking sequence of T/CCA) [Ueda et al., 1992; Wilson et al., 1992; Ito et al., 2000; Little et al., 2006]. This accessory DNA-binding region is important as SF-1 is one of the few nuclear receptors that is thought to bind to target genes monomerically rather than as a homo- or heterodimer. However, other transcription factors (e.g., Pitx1, Egr-1, GATA-4, SOX proteins, Foxl2, Lhx3, Isl-1, Sp1, GR) may also play a role in stabilizing SF-1 binding within promoter complexes and conferring synergistic activity [Tremblay and Viger, 2003; Wang et al., 2007].
The hinge region of SF-1 lies between the amino-terminal DBD and carboxy-terminal LBD. This region is important for post-transcription/translation modification (e.g., phosphorylation, SUMOylation) [Hammer et al., 1999; Komatsu et al., 2004; Lee et al., 2005; Lewis et al., 2008]. These modifications up- or down-regulate SF-1 function directly or in response to intracellular signaling pathways. Indeed, SUMOylation may be critical for repressing SF-1 activity in the basal state, and may involve interaction with DP103 and an E3-SUMO ligase complex [Lee et al., 2005].
The LBD region of SF-1 has structural similarity to other nuclear receptors as it contains 12 helices (H1–H12) that can form an AF2 domain for co-factor recruitment. Several nuclear receptor co-activators have been identified that can interact with SF-1 to modulate its activity (e.g., SRC1, GRIP1, CBP/p300, RIP140, PNRC, TReP-132, EID-1) [Ito et al., 1998; Park et al., 2007]. The related nuclear receptor DAX1 (NR0B1) can also regulate SF-1 activity through direct and indirect mechanisms [Ito et al., 1997]. SF-1 was thought to be an ‘orphan’ nuclear receptor for many years as no high-affinity naturally occurring ligand was identified, and it was proposed that the LBD of SF-1 could adopt a stable conformation in the absence of such a molecule [Desclozeaux et al., 2002]. However, several groups have now been able to crystallize the LBD of SF-1 and have shown that small phospholipid ligands (e.g., phosphatidylinositols) can bind to this structure (fig. 1C) [Krylova et al., 2005; Li et al., 2005; Wang et al., 2005]. These potential ligands can also modulate SF-1 function in vitro and may form an important interface with upstream signaling pathways (e.g., cAMP/diacylglycerol kinase) [Krylova et al., 2005; Li et al., 2007]. The exact in vivo biological role of ligand-mediated SF-1 activation remains to be determined, although a point mutation in the LBD of SF-1 in one patient involves an amino acid (L437) that is likely to be important in receptor-ligand interactions [Lin et al., 2007] (see below). Furthermore, the recent description of putative agonists and antagonists of SF-1 offer a potential opportunity to modulate receptor function in certain situations [Whitby et al., 2006; Del Tredici et al., 2008].
SF-1 Expression and Target Genes
The expression pattern of SF-1 is consistent with its central role in regulating adrenal development, gonad determination and differentiation, and in the hypothalamic-pituitary control of reproduction and metabolism.
In the mouse, Sf-1 is expressed in the early adrenogonadal primordium from 9 dpc and, thereafter, in the developing adrenal gland and gonad [Ikeda et al., 1994; Nef et al., 2005; Val et al., 2007]. In humans, SF-1 expression has been shown in the developing adrenal gland and bi-potential gonad at 32–33 days post conception [Ramayya et al., 1997; Hanley et al., 1999]. Following testis determination (from around 42 days onwards in humans), SF-1 expression is consistently maintained in the somatic cells of the early testis, where it may play a crucial role together with SRY in supporting SOX9 expression [Sekido and Lovell-Badge, 2008]. In Sertoli cells, SF-1 activates expression of Müllerian inhibiting substance (MIS, anti-Müllerian hormone (AMH)) from around 7 weeks gestation, which leads to the regression of Müllerian structures in the developing male fetus. In Leydig cells, SF-1 activates the expression of steroidogenic enzyme systems from 8 weeks gestation, which results in androgenization of the external genitalia. In females, persistent expression of SF-1 has been reported in early ovarian development in humans [Hanley et al., 1999], whereas Sf-1 expression may decline in the mouse. However, SF-1 is detectable in somatic cells (granulosa and theca cells) of the adult ovary [Murayama et al., 2008]. The exact role SF-1 plays in the ovary is unclear, as the related nuclear receptor LRH-1 (NR5A2) may also have a role in regulating aromatization and oestrogen synthesis.
In addition to its expression in the adrenal gland and gonad, SF-1 is also expressed in the developing ventromedial hypothalamus (VMH), pituitary gonadotropes, and spleen [Luo et al., 1994; Shinoda et al., 1995; Kurrasch et al., 2007]. Extensive evidence now exists that SF-1 can regulate an array of target genes involved in the development and function of many of these tissues (table 1), either through a direct effect on the proximal promoter of these genes [Cammas et al., 1997; Caron et al., 1997; Sugawara et al., 1997; Yu et al., 1998; Wang et al., 2001] or through an influence on more distant regulatory regions or enhancer elements [Sekido and Lovell-Badge, 2008]. Thus, SF-1 might be considered a ‘master-regulator’ of many aspects of adrenal, gonadal, and reproductive development and function, and relatively small changes in SF-1 activity could have clinically significant effects on these different endocrine systems.
Table 1.
A selection of SF-1 target genes involved in endocrine development and function
| Gonad/ reproduction |
Steroidogenesis/ adrenal |
Metabolism/ CNS |
|---|---|---|
| NR0B1 (DAX1) | MC2R (ACTHR) | POU5F1 |
| VNN1 | CYP11A1 | NR0B2 (SHP) |
| SOX9 | CYP11B2 | SCARB1 |
| FATE1 | STAR | AKR1B7 |
| ZNF461 (GIOT-1) | HSD3B2 | NPC1 |
| AMH (MIS) | SULT2A1 | NOS1 |
| AMHR2 | ADCY4 | CNR1 |
| INSL3 | NOV (CCN3) | BDNF |
| GnRH/GnRHR | CYB5A | UCN3 |
| LHB/LHCGR | CYP19 | CRHR2 |
| FSHB/FSHR | Hydroxylases | |
| INHA | ||
| OXT | ||
| CXorf6 (MAML2) |
Furthermore, much less is known about the regulation of SF-1 expression itself. Despite reports of NR5A1 promoter or enhancer regulation by factors such as WT1 [Wilhelm and Englert, 2002], SOX proteins, M33 [Katoh-Fukui et al., 2005], Pbx-Hox [Zubair et al., 2006], Pitx2 [Shima et al., 2008], and USF2 [Utsunomiya et al., 2008], or by CpG island methylation [Xue et al., 2007], many of the key mechanisms influencing SF-1 expression remain to be elucidated. Further research in this area could also have important implications for understanding the role of SF-1 in human disease.
Nr5a1 Knockout Mice
The role of NR5A1 in embryogenesis was demonstrated clearly following the generation of Nr5a1 (FtzF1) knockout mice in the mid-1990s [Luo et al., 1994; Sadovsky et al., 1995; Shinoda et al., 1995]. Despite using different targeting strategies, these animals were reported to have a similar phenotype by several different groups. Mice homozygous for deletion of Nr5a1 (Nr5a1−/−) exhibited complete adrenal and gonadal agenesis. Whilst some evidence of early urogenital ridge formation was seen in Nr5a1 knockout embryos (e10.5), the progenitor cells rapidly regressed through apoptosis (e11.5–12). Consequently, all animals had severe adrenal failure, a female phenotype, and persistent Müllerian structures in males. In addition to these features, Nr5a1 knockout mice have abnormal development of the ventromedial hypothalamus, reduced gonadotropin secretion (which responds to some degree to GnRH stimulation), and small spleens. Moreover, increased anxiety is seen in CNS-specific Nr5a1 knockout mice [Zhao et al., 2008], and late-onset obesity is seen in null mice that are rescued by adrenal transplantation [Majdic et al., 2002]. This latter phenotype is likely to result from the effects of VMH dysregulation on metabolic processing and locomotor activity.
Although heterozygous Nr5a1 animals (Nr5a1+/−) were originally thought to be unaffected, more detailed studies have also revealed a subtle phenotype in these animals [Bland et al., 2004]. These features include smaller testes, abnormal adrenal architecture, and impaired adrenal stress response. Notably, differences in adrenal development between heterozygous animals and their wild-type littermates were more striking during embryonic development than postnatally. Up-regulation of related transcription factors (such as nerve growth factor induced B, NGFI-B) might be one mechanism that can compensate for haploinsufficiency of Nr5a1 in heterozygous animals and partly ‘rescue’ adrenal function by the time the animals are born [Bland et al., 2004]. It is unclear whether similar mechanisms might act in the fetal gonad, although NGFI-B is not highly expressed here. Thus, a disparity between the ability of the adrenal gland and gonad to compensate for partial loss of Nr5a1 function might be one mechanism explaining why a gonadal phenotype is more marked than an adrenal phenotype in humans with heterozygous NR5A1 changes (see below).
Human Phenotypes Associated with NR5A1 Changes
Prompted by the phenotype of Nr5a1 knockout mice, initial studies to identify NR5A1 mutations in humans focused on patients with primary adrenal failure, 46,XY gonadal dysgenesis and Müllerian structures. This combination of clinical features is rare, but in 1999 the first human NR5A1 mutation was identified in an individual with this phenotype (OMIM: 184757) [Achermann et al., 1999]. Whereas the homozygous (Nr5a1−/−) mouse has complete agenesis of the adrenal gland and gonads, this patient had decompensating primary adrenal failure presenting after birth and small intra-abdominal gonads removed in early adolescence that contained immature seminiferous tubules (fig. 2A). Thus, the patient's phenotype was milder than that observed in the knockout mouse, where no adrenal glands or gonads are identified. Mutational analysis revealed a de novo heterozygous mutation (p.G35E) in the primary DNA-binding domain (P-box) of NR5A1 in this patient (fig. 3A–C). Despite extensive efforts, no additional changes were found on the other allele. Functional studies show that the p.G35E change significantly impairs the ability of SF-1 to bind to and activate the promoters of several known SF-1 target genes (fig. 4), although partially preserved activation of some targets with ‘perfect’ response elements is observed [Achermann et al., 1999; Ito et al., 2000; Lin et al., 2007]. Although a significant dominant negative effect was not seen, co-transfection of increasing amounts of mutant p.G35E SF-1 is able to reduce (but not abolish) wild-type SF-1 function, suggesting that a competitive effect might occur [Achermann et al., 1999; Ito et al., 2000]. Furthermore, more significant disruption of wild-type action by the mutant protein might occur in more complex biological systems (e.g., synergistic activation of target promoters by SF-1 and partner transcription factors such as GATA-4) [Tremblay and Viger, 2003]. On balance, therefore, it is possible that this p.G35E P-box change reduces SF-1 function below haploinsufficiency (50%) but – consistent with the patient's phenotype – does not completely eliminate it.
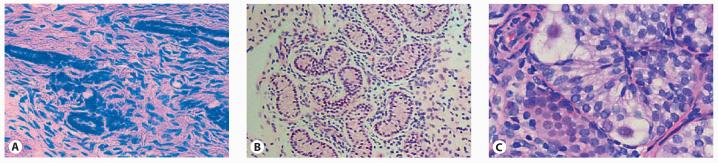
Fig. 2.
Gonadal histology in several patients with NR5A1 (SF-1) mutations (haematoxylin & eosin). A Gonad removed in early adolescence from an individual with an adrenal and gonadal phenotype (G35E) showing scattered seminiferous tubules and fibrous tissue (original magnification ×400). B Gonadal biopsy taken during orchidopexy at 6 years of age in a boy with a milder phenotype (L437Q) showing hyalinized seminiferous tubules and a predominant Sertoli-Cell-Only phenotype (original magnification ×100). C Histology following gonadectomy at 4 months of age in a patient with clitoromegaly (G91S) showing relatively intact seminiferous tubules containing germ cells (original magnification ×400). Reproduced with permission from Achermann et al. [1999] and Lin et al. [2007].
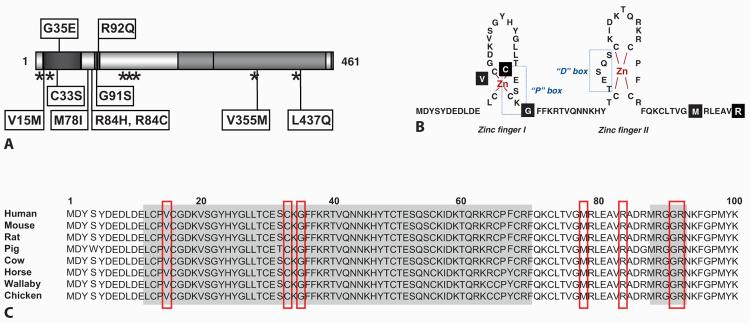
Fig. 3.
A Cartoon of NR5A1 showing the positions of missense (labeled) and nonsense/frameshift (asterisks) mutations in NR5A1. Those changes associated with a combined adrenal and gonadal phenotype are shown above; those associated with variants of 46,XY DSD but normal adrenal function are shown below. B Overview of the zinc-finger region of the DNA-binding domain (DBD) of SF-1 showing the positions of missense mutations in black boxes. C These missense changes affect highly conserved amino acids in this region. Modified with permission from Lin et al. [2007].
Fig. 4.
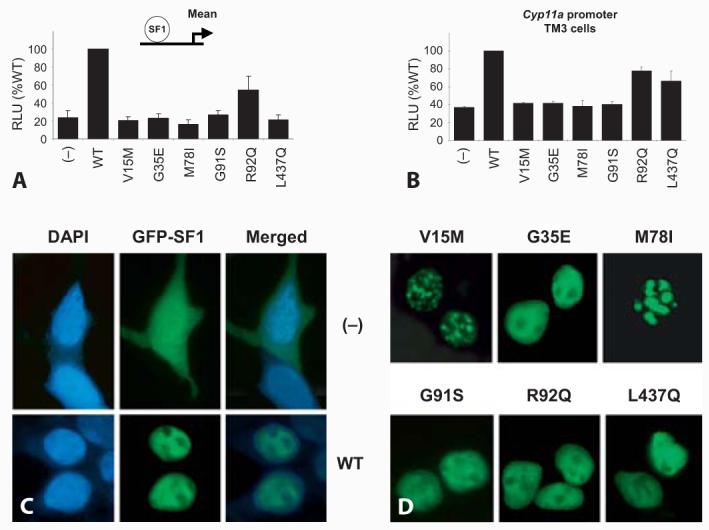
Functional analysis of wild-type (WT) SF-1 and SF-1 mutants associated with 46,XY DSD. A Mean transcriptional activity following studies of 5 different promoters (Cyp11a, MIS, Cyp19, Insl3, LHβ) in transient transfection assays using tsa201 (human embryonic kidney) cells. Most mutants found in patients are present in a heterozygous state and show impaired function. The R92Q change is found in a homozygous state and has partial function. B The L437Q mutation associated with penoscrotal hypospadias has partial function in a TM3 Leydig cell line. C–D GFP-tagged SF-1 shows normal nuclear localization in most cases. However, 2 point mutants (V15M, M78I) show aggregation in subnuclear foci. Modified with permission from Lin et al. [2007].
Further support for a dose-dependent effect of SF-1 on adrenal and testis development came with the report of a patient with a similar phenotype (primary adrenal failure, 46,XY DSD, Müllerian structures) in 2002 [Achermann et al., 2002; Jameson, 2004]. This child was born into a highly consanguineous family and was found to harbor a homozygous p.R92Q mutation in the ‘A-box’ motif of the accessory DBD of NR5A1 (fig. 3A–C). As described above, this region is involved in stabilizing binding by monomeric nuclear receptors. Functional studies show that the p.R92Q mutation only partially reduces SF-1 activity and binding to about 30–40%, although variable activation of different promoters is seen (fig. 4) [Achermann et al., 2002; Lin et al., 2007]. Since this region is within the accessory DNA-binding domain, it seems that a homozygous change affecting both alleles is necessary for the full expression of the phenotype observed in this patient. Of note, 3 heterozygous relatives (both parents and a sibling) were clinically normal, with no evidence of adrenal insufficiency or reproductive dysfunction.
The question therefore arose whether milder or variant changes in NR5A1 could be associated with different adrenal or reproductive phenotypes. Several case reports and cohort studies have now been published that go some way to addressing this issue.
SF-1 and Adrenal Failure
Given the central role that SF-1 plays in adrenal development and steroidogenesis, a number of studies have looked for NR5A1 changes in children and adults with primary adrenal insufficiency. To date, only one NR5A1 mutation has been reported (p.R255L) in a 46,XX girl who presented at 2 years of age with primary adrenal failure [Biason-Lauber and Schoenle, 2000]. Ovarian function at this age is difficult to assess but appeared to be intact; it remains to be seen whether this patient will have sufficient ovarian function for normal pubertal development and folliculogenesis. In another study, no NR5A1 mutations were identified in a small cohort of girls diagnosed with adrenal hypoplasia, nor in women who presented in adulthood with antibody negative Addison's disease [Lin et al., 2006]. Similarly, no significant NR5A1 changes were found in boys who were diagnosed with adrenal hypoplasia who had been pre-screened and found to be negative for DAX1 (NR0B1) mutations [Lin et al., 2006]. Thus, it seems that NR5A1 mutations are not likely to be a common cause of primary adrenal failure in boys without reproductive dysfunction or 46,XY DSD.
SF-1 and 46,XY DSD
The next major development in human SF-1 biology came in 2004 when Correa et al. [2004] reported a heterozygous frameshift mutation (c.1058_1065delAGCTGGTG) in a 46,XY patient who presented in adulthood with clitoromegaly, primary amenorrhea/absent uterus, impaired breast development, hypertension, and moderate obesity. Adrenal function appeared normal in this case [Correa et al., 2004]. This finding was supported soon afterwards by 2 further case reports of heterozygous NR5A1 mutations in patients with 46,XY DSD. Mallet et al. [2004] reported a heterozygous nonsense mutation (p.C16X) in a 46,XY newborn with ambiguous genitalia, mildly dysgenetic testes, a uterus, and normal adrenal function. Similarly, Hasegawa et al. [2004] identified a heterozygous single base pair deletion in exon 2 (c.18delC), resulting in a premature stop codon at position 74, in a 27 year old 46,XY patient with ambiguous genitalia, mildly dysgenetic testes, absent uterus, and normal adrenal function. Taken together these reports suggest that heterozygous disruptive changes in NR5A1 can present with a phenotype of 46,XY DSD.
Within the past year, 2 larger cohort studies and 2 case reports have identified a further 11 individuals or families with heterozygous NR5A1 changes and a range of 46,XY DSD phenotypes but normal adrenal function [Coutant et al., 2007; Lin et al., 2007; Reuter et al., 2007; Köhler et al., 2008]. The prevalence of NR5A1 changes in these cohorts has been approximately 15% (4/30) [Lin et al., 2007; Köhler et al., 2008]. The range of mutations and spectrum of phenotypic features described to date are shown in table 2 and figure 2B, C. In most cases, the degree of underandrogenization was greater than the degree of testicular dysgenesis, suggesting that impaired androgen synthesis contributes a significant element to the phenotype. Müllerian structures may be present or absent, and Wolffian structures are often seen. In 2 cases, double heterozygous inheritance of a disruptive mutation with a potentially hypomorphic polymorphism (p.G146A) may have resulted in a more severe clinical phenotype [Reuter et al., 2007; Köhler et al., 2008].
Table 2.
Molecular and clinical features of 46,XY individuals with mutations in SF-1 (NR5A1)
| Mutation |
Adr | External genitalia |
Testis | Uterus | Reference | ||
|---|---|---|---|---|---|---|---|
| Substitution | Form | Inheritance | |||||
| p.G35E | hetero | de novo | + | female | severe | + | Achermann et al. [1999] |
| p.R92Q | homo | recessive | + | female | NK | + | Achermann et al. [2002] |
| c.424_427dup + p.G146A | hetero + SNP | NK | − | female | severe | + | Köhler et al. [2008] |
| p.M78I | hetero | SLD | − | female | mild | (+) | Lin et al. [2007] |
| p.V15M | hetero | de novo | − | female | mild | − | Lin et al. [2007] |
| p.R84C+ p.G.146A | hetero + SNP | SLD+ SNP | − | ambiguous | mild | − | Reuter et al. [2007] |
| p.C91S | hetero | SLD | − | ambiguous | mild | (+) | Lin et al. [2007] |
| p.C16X | hetero | de novo | − | ambiguous | mild | + | Mallet et al. [2004] |
| c.18delC | hetero | de novo | − | ambiguous | mild | − | Hasegawa et al. [2004] |
| c.1058_1065del | hetero | de novo | − | ambiguous | regressa | − | Correa et al. [2004] |
| p.C33S | hetero | de novo | − | ambiguous | mild | − | Köhler et al. [2008] |
| p.R84H | hetero | de novo | − | ambiguous | normal | − | Köhler et al. [2008] |
| p.Y138X | hetero | de novo | − | ambiguous | mild | − | Köhler et al. [2008] |
| c.536delC | hetero | SLD | − | ambiguous | normal | − | Coutant et al. [2007] |
| c.1277dupT | hetero | SLD | − | ambiguous | normal | − | Köhler et al. [2008] |
| p.L437Q | hetero | de novo | − | hypospadias | mild | − | Lin et al. [2007] |
| p.V355M | hetero | SLD | − | micropenis | regress | − | Philibert et al. [2007] |
Gonadal tissue not found at laparoscopy at age 31 years.
Modified with permission from Köhler et al. [2008]. hetero = Heterozygous; homo = homozygous; SNP = single nucleotide polymorphism; NK = not known; SLD = sex-limited dominant; Adr = adrenal failure; + = present; − = absent; (+) = remnant present; ambiguous = clitoromegaly or ambiguous genitalia.
Several of these NR5A1 changes are heterozygous nonsense or frameshift mutations which might affect RNA stability through nonsense-mediated decay or produce a truncated non-functional protein (p.Y138X, c.424_427dupCCCA, c.536delC, c.1277dupT). However, several novel heterozygous missense mutations in NR5A1 have also been reported, which are providing insight into critical domains for SF-1 function (fig. 3A). Most of these missense mutations lie within the primary or accessory DNA-binding domains of NR5A1 and affect highly-conserved amino acid residues (p.V15M, p.C33S, p.M78I, p.R84H, p.R84C, p.G91S) (fig. 3B, C). As expected, these changes interfere with DNA binding in electromobility shift assays and impair transcriptional activation in transient gene expression assays using known NR5A1 target promoter constructs (fig. 4). No dominant negative effects were seen for these point mutants when wild-type and mutant NR5A1 were co-transfected into neutral cells, or when mutant NR5A1 was transfected into NR5A1 expressing cells, suggesting that haploinsufficiency of NR5A1 may be sufficient to cause the phenotype. Furthermore, several point mutants (e.g., p.V15M, p.C33S, p.M78I) showed focal aggregation into sub-nuclear bodies, which has been seen previously with certain naturally occurring mutations in other nuclear receptors (e.g., androgen receptor, glucocorticoid receptor) (fig. 4C, D) [Lin et al., 2007; Köhler et al., 2008]. The exact biological consequence of this phenomenon is unclear. Finally, the p.L437Q change in the LBD of NR5A1 involves an amino-acid residue that is predicted to contribute to the ligand-binding interface of SF-1. This patient is notable as he has a milder phenotype (penoscrotal hypospadias), is raised male, and may have impaired gonadotropin secretion in adolescence (fig. 2C). This mutation has partial residual function in Leydig and Sertoli cell lines (fig. 4B), which is consistent with the less severe phenotype in this patient [Lin et al., 2007].
Although most NR5A1 mutations appear to arise de novo, another interesting feature that has emerged from this recent work is that approximately one third of heterozygous NR5A1 changes can be inherited from the mother in a sex-limited dominant fashion [Coutant et al., 2007; Lin et al., 2007; Reuter et al., 2007; Köhler et al., 2008]. That is, a woman can carry the heterozygous change without having ovarian dysfunction, but pass it on to sons who are affected. This situation is highlighted in the family reported by Coutant et al. [2007] in which a heterozygous c.536delC change was inherited from the mother to two 46,XY children with ambiguous genitalia and who were diagnosed with partial androgen insensitivity syndrome. The fact that sex-limited dominant inheritance can mimic an X-linked disorder suggests that NR5A1 mutations should be sought in cases of potential partial androgen insensitivity syndrome when several 46,XY offspring are affected but the androgen receptor sequence is normal. Furthermore, the long-term effects of heterozygous NR5A1 mutations in females are not known, so ovarian function should be monitored with time in this cohort.
In addition to these specific NR5A1 changes, genomic disruption of the locus containing NR5A1 (9q33.3) has also been reported in association with human phenotypes. For example, haploinsufficiency of NR5A1 and LMX1B has been described in a patient with genitopatellar syndrome, whereas somatic duplication of NR5A1 is seen in some pediatric adrenal tumors [Figueiredo et al., 2005; Schlaubitz et al., 2007].
SF-1 and Other Reproductive Phenotypes
Given the potential dose-dependent effects of SF-1 on reproductive function, it might be predicted that milder functional changes in SF-1 could be associated with less severe reproductive phenotypes.
In a recent study of 24 patients with bilateral anorchia (vanishing testis syndrome) with or without micropenis, a heterozygous p.V355M in NR5A1 was found in one of a pair of dizygotic twin brothers [Philibert et al., 2007]. This p.V355M did not significantly affect protein expression, nuclear localization or DNA binding, but did reduce SF-1-dependent transcriptional activation by about half, most likely through its effect on the helical conformation of the LBD. Unfortunately, long-term follow up data were not available for the twin brother, who also harbored the p.V355M change. Nevertheless, this study suggests that rare variants in NR5A1 may disrupt its function significantly enough to be a susceptibility factor for milder reproductive phenotypes.
A common non-synonymous polymorphism in NR5A1 (p.G146A) has also been shown to have mildly altered functional activity in a number of studies, with approximately 80% of wild-type SF-1-mediated transcriptional activation in some assay systems [WuQiang et al., 2003]. A link between this polymorphism and micropenis or cryptorchidism has been proposed in 2 population-based association studies [Wada et al., 2005, 2006]. Thus, milder changes in NR5A1 may also be important susceptibility factors for a range of reproductive phenotypes in the population, perhaps together with other oligogenic influences.
Conclusion
The past decade has seen considerable progress in our understanding of the role of SF-1 in adrenal and reproductive function. Identifying patients with naturally occurring NR5A1 mutations is providing important insight into the role of this nuclear receptor in endocrine development and disease. Despite initial efforts aimed at identifying NR5A1 changes in individuals with combined adrenal and gonadal phenotypes, it is now emerging that changes in NR5A1 are a relatively common cause of 46,XY disorders of sex development. Whether this cohort of patients will develop adrenal insufficiency with time remains to be seen.
Acknowledgements
JCA is a Wellcome Trust Senior Research Fellow in Clinical Science (079666).
References
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87:1829–1833. doi: 10.1210/jcem.87.4.8376. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67:1563–1568. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18:941–952. doi: 10.1210/me.2003-0333. [DOI] [PubMed] [Google Scholar]
- Cammas FM, Pullinger GD, Barker S, Clark AJ. The mouse adrenocorticotropin receptor gene: cloning and characterization of its promoter and evidence for a role for the orphan nuclear receptor steroidogenic factor 1. Mol Endocrinol. 1997;11:867–876. doi: 10.1210/mend.11.7.9938. [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ. Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol. 1997;11:138–147. doi: 10.1210/mend.11.2.9880. [DOI] [PubMed] [Google Scholar]
- Correa RV, Domenice S, Bingham NC, Billerbeck AE, Rainey WE, et al. A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab. 2004;89:1767–1772. doi: 10.1210/jc.2003-031240. [DOI] [PubMed] [Google Scholar]
- Coutant R, Mallet D, Lahlou N, Bouhours-Nouet N, Guichet A, et al. Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome. J Clin Endocrinol Metab. 2007;92:2868–2873. doi: 10.1210/jc.2007-0024. [DOI] [PubMed] [Google Scholar]
- Del Tredici AL, Andersen CB, Currier EA, Ohrmund SR, Fairbain LC, et al. Identification of the first synthetic steroidogenic factor 1 inverse agonists: pharmacological modulation of steroidogenic enzymes. Mol Pharmacol. 2008;73:900–908. doi: 10.1124/mol.107.040089. [DOI] [PubMed] [Google Scholar]
- Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol. 2002;22:7193–7203. doi: 10.1128/MCB.22.20.7193-7203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, et al. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, et al. Expression of steroidogenic factor 1 and Wilms' tumour 1 during early human gonadal development and sex determination. Mech Dev. 1999;87:175–180. doi: 10.1016/s0925-4773(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Fukami M, Sato N, Katsumata N, Sasaki G, et al. Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab. 2004;89:5930–5935. doi: 10.1210/jc.2004-0935. [DOI] [PubMed] [Google Scholar]
- Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993;268:7494–7502. [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17:1476–1483. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yu RN, Jameson JL. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol. 1998;12:290–301. doi: 10.1210/mend.12.2.0059. [DOI] [PubMed] [Google Scholar]
- Ito M, Achermann JC, Jameson JL. A naturally occurring steroidogenic factor-1 mutation exhibits differential binding and activation of target genes. J Biol Chem. 2000;275:31708–31714. doi: 10.1074/jbc.M002892200. [DOI] [PubMed] [Google Scholar]
- Jameson JL. Of mice and men: The tale of steroidogenic factor-1. J Clin Endocrinol Metab. 2004;89:5927–5929. doi: 10.1210/jc.2004-2047. [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, et al. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood. 2005;106:1612–1620. doi: 10.1182/blood-2004-08-3367. [DOI] [PubMed] [Google Scholar]
- Köhler B, Lin L, Ferraz-de-Souza B, Wieacker P, Heidemann P, et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29:59–64. doi: 10.1002/humu.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, et al. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol. 2004;18:2451–2462. doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AE, Rusten M, Hoivik EA, Vikse EL, Hansson ML, et al. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol Endocrinol. 2008;22:91–104. doi: 10.1210/me.2006-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol Cell Biol. 2007;27:6669–6685. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, et al. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, et al. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. J Clin Endocrinol Metab. 2006;91:3048–3054. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, et al. Heterozygous mis-sense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92:991–999. doi: 10.1210/jc.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TH, Zhang Y, Matulis CK, Weck J, Zhang Z, et al. Sequence-specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol Endocrinol. 2006;20:831–843. doi: 10.1210/me.2005-0384. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M, Morel Y. Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab. 2004;89:4829–4832. doi: 10.1210/jc.2004-0670. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267:17913–17919. [PubMed] [Google Scholar]
- Murayama C, Miyazaki H, Miyamoto A, Shimizu T. Involvement of Ad4BP/SF-1, DAX-1, and COUP-TFII transcription factor on steroid production and luteinization in ovarian theca cells. Mol Cell Biochem. 2008;314:51–58. doi: 10.1007/s11010-008-9764-y. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Oba K, Yanase T, Nomura M, Morohashi K, Takayanagi R, Nawata H. Structural characterization of human Ad4bp (SF-1) gene. Biochem Biophys Res Commun. 1996;226:261–267. doi: 10.1006/bbrc.1996.1343. [DOI] [PubMed] [Google Scholar]
- Park YY, Park KC, Shong M, Lee SJ, Lee YH, Choi HS. EID-1 interacts with orphan nuclear receptor SF-1 and represses its transactivation. Mol Cells. 2007;24:372–377. [PubMed] [Google Scholar]
- Philibert P, Zenaty D, Lin L, Soskin S, Audran F, et al. Mutational analysis of steroidogenic factor 1 (NR5a1) in 24 boys with bilateral anorchia: a French collaborative study. Hum Reprod. 2007;22:3255–3261. doi: 10.1093/humrep/dem278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayya MS, Zhou J, Kino T, Segars JH, Bondy CA, Chrousos GP. Steroidogenic factor 1 messenger ribonucleic acid expression in steroidogenic and nonsteroidogenic human tissues: Northern blot and in situ hybridization studies. J Clin Endocrinol Metab. 1997;82:1799–1806. doi: 10.1210/jcem.82.6.3967. [DOI] [PubMed] [Google Scholar]
- Reuter AL, Goji K, Bingham NC, Matsuo M, Parker KL. A novel mutation in the accessory DNA-binding domain of human steroidogenic factor 1 causes XY gonadal dysgenesis without adrenal insufficiency. Eur J Endocrinol. 2007;157:233–238. doi: 10.1530/EJE-07-0113. [DOI] [PubMed] [Google Scholar]
- Rice DA, Mouw AR, Bogerd AM, Parker KL. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol. 1991;5:1552–1561. doi: 10.1210/mend-5-10-1552. [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA. 1995;92:10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaubitz S, Yatsenko SA, Smith LD, Keller KL, Vissers LE, et al. Ovotestes and XY sex reversal in a female with an interstitial 9q33.3–q34.1 deletion encompassing NR5A1 and LMX1B causing features of Genitopatellar syndrome. Am J Med Genet A. 2007;143:1071–1081. doi: 10.1002/ajmg.a.31685. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Shima Y, Zubair M, Komatsu T, Oka S, Yokoyama C, et al. Pituitary homeobox 2 regulates Adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol Endocrinol. 2008;22:1633–1646. doi: 10.1210/me.2007-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Kiriakidou M, McAllister JM, Kallen CB, Strauss JF., 3rd Multiple steroidogenic factor 1 binding elements in the human steroidogenic acute regulatory protein gene 5′-flanking region are required for maximal promoter activity and cyclic AMP responsiveness. Biochemistry. 1997;36:7249–7255. doi: 10.1021/bi9628984. [DOI] [PubMed] [Google Scholar]
- Swift S, Ashworth A. The mouse Ftzf1 gene required for gonadal and adrenal development maps to mouse chromosome 2. Genomics. 1995;28:609–610. doi: 10.1006/geno.1995.1204. [DOI] [PubMed] [Google Scholar]
- Taketo M, Parker KL, Howard TA, Tsukiyama T, Wong M, et al. Homologs of Drosophila Fushi-Tarazu factor 1 map to mouse chromosome 2 and human chromosome 9q33. Genomics. 1995;25:565–567. doi: 10.1016/0888-7543(95)80059-u. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J Biol Chem. 2003;278:42637–42642. doi: 10.1074/jbc.M305485200. [DOI] [PubMed] [Google Scholar]
- Ueda H, Sonoda S, Brown JL, Scott MP, Wu C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990;4:624–635. doi: 10.1101/gad.4.4.624. [DOI] [PubMed] [Google Scholar]
- Ueda H, Sun GC, Murata T, Hirose S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol Cell Biol. 1992;12:5667–5672. doi: 10.1128/mcb.12.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, et al. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol. 2008;22:904–914. doi: 10.1210/me.2006-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Wada Y, Okada M, Hasegawa T, Ogata T. Association of severe micropenis with Gly146Ala polymorphism in the gene for steroidogenic factor-1. Endocr J. 2005;52:445–448. doi: 10.1507/endocrj.52.445. [DOI] [PubMed] [Google Scholar]
- Wada Y, Okada M, Fukami M, Sasagawa I, Ogata T. Association of cryptorchidism with Gly146Ala polymorphism in the gene for steroidogenic factor-1. Fertil Steril. 2006;85:787–790. doi: 10.1016/j.fertnstert.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, et al. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA. 2005;102:7505–7510. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Jeffs B, Ito M, Achermann JC, Yu RN, et al. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci USA. 2001;98:7988–7993. doi: 10.1073/pnas.141543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby RJ, Dixon S, Maloney PR, Delerive P, Goodwin BJ, et al. Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J Med Chem. 2006;49:6652–6655. doi: 10.1021/jm060990k. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992;256:107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
- Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL. Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J Mol Endocrinol. 1996;17:139–147. doi: 10.1677/jme.0.0170139. [DOI] [PubMed] [Google Scholar]
- WuQiang F, Yanase T, Wei L, Oba K, Nomura M, et al. Functional characterization of a new human Ad4BP/SF-1 variation, G146A. Biochem Biophys Res Commun. 2003;311:987–994. doi: 10.1016/j.bbrc.2003.10.096. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92:3261–3267. doi: 10.1210/jc.2007-0494. [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Jameson JL. The murine Dax-1 promoter is stimulated by SF-1 (steroidogenic factor-1) and inhibited by COUP-TF (chicken ovalbumin upstream promoter-transcription factor) via a composite nuclear receptor-regulatory element. Mol Endocrinol. 1998;12:1010–1022. doi: 10.1210/mend.12.7.0131. [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, et al. CNS-Specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22:1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]