Abstract
We prospectively examined vBMD and structural bone parameters assessed by QCT among participants of the InCHIANTI study over a 6-yr follow-up. Periosteal apposition occurred both in men and women. Endocortical resorption causes bone loss in older women despite periosteal apposition.
Introduction
To address the hypothesis that age-related changes in BMD and bone geometry may be different in men and women, we prospectively examined volumetric BMD (vBMD) and structural bone parameters assessed by QCT among participants of the InCHIANTI study over a 6-yr follow-up.
Materials and Methods
Three hundred forty-five men and 464 women 21–102 yr of age from the InCHIANTI study, a population-based study in Tuscany, Italy, were included. Tibial QCT bone parameters were measured at enrollment (1998–2000) and at 3- (2001–2003) and 6-yr (2004–2006) follow-ups.
Results
Periosteal apposition occurred both in men and women. The annual rate of bone periosteal apposition was higher in younger than in older men, whereas in women, the rate of apposition was homogenous across age groups. The age-related medullary expansion, expression of endocortical resorption, was significantly higher in women compared with men. In women, but not in men, accelerated endocortical resorption not sufficiently balanced by periosteal apposition caused accelerated loss in cortical bone mass. The cross-sectional moment of inertia decreased progressively over the life span in both sexes.
Conclusions
Endocortical resorption causes bone loss in older women despite periosteal apposition. Obtaining a balance between endocortical resorption and periosteal apposition should be the target for interventions aimed to decrease bone loss and prevent osteoporosis in older women.
Key words: longitudinal study, osteoporosis, periosteal apposition, endocortical resorption, BMD, bone geometry
INTRODUCTION
The tissues that compose the human skeleton are continuously renovated through a dynamic equilibrium between bone formation and resorption. The continued bone resorption and formation is a unique feature of the bone tissue in the entire human body and produces a constant turnover during the life span. It is currently hypothesized that a disequilibrium between periosteal apposition and endocortical resorption is one of the main underlying mechanism that results in osteoporosis,(1) a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of bone fracture.(2) Bone strength reflects the integration of two main features: BMD and bone quality.(3) DXA-BMD is expressed as grams of mineral per area (g/cm2), whereas QCT-BMD is expressed as grams of mineral per volume (g/cm3). In adults of a given age, BMC, which takes into account the total amount of bone material, is determined by the peak bone mass that occurs between 20 and 30 yr of age and the velocity of age-associated bone loss.(3) Bone quality refers to the material properties factors, including bone geometrical structure, damage accumulation (e.g., microfractures), and the amount of the mineralized bone tissue.(3)
The study of bone architecture and specific assessments of trabecular and cortical tissue has provided information on different structural changes in bone mineral distribution and structure that occur with aging in men and women.(4) However, virtually all studies that specifically addressed age-related differences in BMD and bone geometry in men and women were cross-sectional.(5) Therefore, the sequences of events that occur in trabecular and cortical BMD and geometry over the aging process are still debated. Previous studies have not addressed longitudinal changes in BMD and bone geometry in a large population-based sample.
Cross-sectional studies suggested that the rate of periosteal apposition with aging is different between sexes, with a steeper increase of total bone area in men than in women, at both central and peripheral sites.(4) However, whether the imbalance between bone modeling and remodeling with aging is determined by an increased bone resorption (estimated by the increase in medullary area) or insufficient periosteal apposition is unknown. Understanding the mechanism underlying the imbalance of bone turnover with aging may help to develop new therapeutic opportunities for the prevention of osteoporosis and to better monitor the effect of currently available treatments.
To address the hypothesis that changes in BMD and bone geometry during the life span are caused by an imbalance between apposition and resorption and that this imbalance occurs by different mechanisms in men and women, we examined 6-yr longitudinal changes in volumetric BMD (vBMD) and structural bone parameters assessed by tibial QCT among participants of the InCHIANTI study, a population-based study of older adults living in the Chianti region of Tuscany, Italy.
MATERIALS AND METHODS
Population sample
InCHIANTI is a population-based cohort study performed in two Italian towns located in the Chianti countryside: Greve in Chianti (11,709 inhabitants; rural area) and Bagno a Ripoli (village of Antella, 4704 inhabitants; just outside the urban area of Florence). The study population consisted of a random sample of the population ≥65 yr of age living in the two catchments areas, and 30 men and 30 women randomly selected in each decade between 20 and 70 yr. A detailed description of the design and data collection methods of InCHIANTI has been previously published.(6) Of the 1530 subjects originally sampled, 1453 (94%) agreed to participate in the study. Of these, 1232 participants underwent a pQCT examination at enrollment. For this study, we analyzed data from 1173 participants (540 men and 633 women), after exclusion of subjects (n = 59) who were taking medications or had conditions known to interfere with bone metabolism (e.g., primary hyperparathyroidism and Paget's disease). This analysis was limited to participants who were seen at enrollment and at the 3- (2001–2003) and 6-yr (2004–2006) follow-up visits. Of 1173 participants who had images at enrollment, there were 926 who also had images at the 3-yr follow-up visit and 809 who had images at the 6-yr follow-up visit. Of 364 participants who were not seen at the 6-yr follow-up visit, 176 had died, 208 refused to participate in one or more of the follow-ups, and 20 moved out of the study area and could not be contacted.
pQCT examination were performed at the 3- and 6-yr follow-up according to the same protocols used at baseline. The National Institute on Research and Care of the Elderly (INRCA) Institutional Review Board (IRB00001456) approved the study protocol, which complied with the principles stated in the Declaration of Helsinki. Participants consented to participate and to have their blood samples analyzed for scientific purposes. For those unable to fully consent, surrogate consents were obtained from close relatives.
Lower leg PQCT
The analyses were based on measurements of the right tibia using pQCT (XCT 2000; Stratec Medizintechnik; distributed by the UNITREM Company, Rome, Italy) as previously described.(7) pQCT was adopted because central CT equipment was not available at the study sites and to limit the amount of radiation exposure, allowing longitudinal measures. pQCT of the left tibia (n = 20) was performed in participants that reported right femoral (n = 12), tibial (n = 2), or ankle (n = 2) fractures or other disease (n = 4) interfering with the right leg musculo-skeletal system (e.g., right hemiplegia). The most common reason for missing pQCT evaluation was poor mobility or transportation problems. The length of the tibia was measured as the distance between the medial knee joint cleft and the medial malleolus. Transverse scans (2.1 mm thick) were obtained at 4% (trabecular bone) and 38% (cortical bone) of the tibial length from the tibio-talar joint cleft using a 0.5-mm voxel size. The cross-sectional images were analyzed using the Geanie2.1 software that identifies cortical and trabecular bone (BonAlyse, Jyvaskyla, Finland). Areas >710 mg/cm3 were considered cortical and areas between 180 and 710 mg/cm3 were considered trabecular. Beam hardening (attenuation of the low-energy photons) by soft tissues surrounding bone was corrected using the Stratec QCT software.(8)
The following bone parameters were derived from the pQCT images, as reported elsewhere(9):
Total vBMD at 4% (vBMD; mg/cm3).
Trabecular BMD (vBMDt; mg/cm3): assessed as the average density of the trabecular bone area detected at the 4% site. Cortical bone was excluded from this measurement.
Tibial total bone area at 38% (tCSA; mm2) is the subperiosteal area defined as the total area within the periosteum, including cortical and medullary areas. tCSA was obtained using a threshold of 180 mg/cm3 to separate bone from surrounding soft tissues.
Cortical bone area at 38% (CSA) is the bone CSA (mm2) within the internal and external margin of the cortical ring, using an inner threshold of 710 mg/cm3 to separate cortical from medullary area.
Medullary area (mm2) is the difference between total and cortical bone areas. It includes the marrow space and areas of the inner cortex trabecularized by endocortical resorption that have a cortical apparent vBMD <710 mg/cm3. Therefore, an age-related expansion in medullary area is sensitive to endocortical resorption.
Cross-sectional moment of inertia (average of maximum and minimum moment of inertia; g/cm) is calculated as a density weighted moment of inertia at the 38% site. It provides a valid estimate of resistance to bending.(10)
The precision error of the XCT2000 is <1% for vBMD and for cortical bone area(11) and between 1% and 3% for composite geometry parameters.(11)
Other measures
Demographic characteristics, such as education level, smoking, and menopausal status, were recorded during the home interview and the structured medical examination.(6) Weight was measured to the nearest 0.1 kg using a high-precision mechanical scale with the participant wearing light clothes and without shoes. Height without shoes was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as kilograms per square meter.
The level of physical activity in the year before the interview was classified on an ordinal scale based on responses to a modified standard questionnaire(12) into (1) hardly any physical activity; (2) mostly sitting (occasionally walking, easy gardening); (3) light exercise (no sweat) 2–4 h/wk; (4) moderate exercise (sweat) 1–2 h/wk (level 4); (5) moderate exercise >3 h/wk; (6) intense exercise (at the limits) >3 times/wk. For analytical purposes, we grouped the participants as follows: 1–3, inactive or having light physical activity; 4–5, having moderate physical activity; 6, having intense activity.
Alcohol consumption was assessed by administering the food frequency questionnaire originally created for the European Prospective Investigation into Cancer and nutrition (EPIC) study.(13)
Statistical analysis
Variables are reported as means (SDs) for continuous variables or percentages for dichotomous variables. To minimize the interference of secularization on measures of bone geometry, bone areas and cross-sectional moment of inertia were normalized by tibial length according to Ruff and Hayes.(14) The cross-sectional relationships between age at enrollment and bone parameters were examined separately in men and women by scatter plots and visually summarized by locally weighted regression smoothers.
Mean values of the bone parameters for each baseline and each follow-up survey according to age group were estimated from generalized estimating equations (GEEs), which are considered the ideal method to estimate population-averaged effects.(15) Longitudinal trajectories and their 95% CIs for each bone parameter were also examined.
We examined age-associated longitudinal changes of bone parameters in men and women, testing analytically models that adequately met the visual cross-sectional relationships between age and bone parameters. In details, when a linear relationship between age and a specific bone parameters was observed from cross-sectional data, we fitted only GEE equations where age at baseline and time (expressed as years since baseline), the interaction terms “age × time,” and “age × time × sex” were independent variables. When a nonlinear cross-sectional relationship was observed between age at baseline and each specific bone parameters in men and women, we fitted GEEs where age at baseline, time, a term for time in years (squared time2), and the interaction terms “age × time2,” “sex × time2,” and “age × sex × time2” were independent variables. The term for time indicates the mean annual linear decline in bone parameters for average participants. The quadratic term for time (i.e., time2) allows the rate of decline in bone parameters to be nonlinear. Finally, the “age × sex × time2” interaction term, as reported elsewhere,(16) tests the hypothesis that the effect of time on the longitudinal change in bone parameters is affected by the initial age differently in men and women. A line plot of the age-related trajectories of the bone parameters between ages 20 and 100 yr and their 95% CIs were estimated from these models, separately in men and women.
The SAS 8.2 statistical package was used for all analyses (SAS Institute, Cary, NC, USA).
RESULTS
The characteristics of the study population as a whole and limited to participants evaluated both at the 3- and 6-yr follow-ups are shown in Table 1. Men had a higher educational level, were more physically active, had higher alcohol intake, and were more likely to be smokers than women in both the study population evaluated at enrollment and in those re-evaluated at the 6-yr follow-up study.
Table 1.
Characteristics of the Study Population Evaluated at Enrollment (1998–2000) and at 3- (2001–2003) and 6-yr (2004–2006) Follow-Ups
| Characteristics |
All participants (n = 1173) |
Participants who were re-evaluated at the 3- and 6-yr follow-ups (n = 809) |
||||
| Men | Women | p | Men | Women | p | |
| Age (yr) | 66.9 ± 15.6 | 68.6 ± 15.6 | 0.05 | 63 ± 15.2 | 64.4 ± 15.3 | 0.18 |
| Education (yr) | 7.5 ± 4.4 | 6.0 ± 4.0 | <0.0001 | 8.1 ± 4.5 | 6.8 ± 4.3 | <0.0001 |
| Current smokers (%) | 138 (25.6) | 86 (13.6) | <0.0001 | 113 (29.7) | 61 (14.2) | <0.0001 |
| Body mass index (kg/m2) | 27.1 ± 3.4 | 27.3 ± 4.7 | 0.46 | 27.1 ± 3.3 | 27.1 ± 4.6 | 0.99 |
| Alcohol intake (g/d) | 24.4 ± 19.6 | 9.6 ± 7.6 | <0.0001 | 25.6 ± 18.8 | 9.9 ± 7.5 | <0.0001 |
| Physical activity | ||||||
| Low level | 46 (8.5) | 130 (20.5) | <0.0001 | 17 (4.9) | 47 (12.6) | <0.0001 |
| Medium level | 420 (77.8) | 475 (75.0) | 272 (77.7) | 306 (82.0) | ||
| High level | 74 (13.7) | 28 (4.4) | 61 (17.4) | 20 (5.4) | ||
| Postmenopausal (%) | — | 545 (88.1) | — | — | 309 (84.43) | |
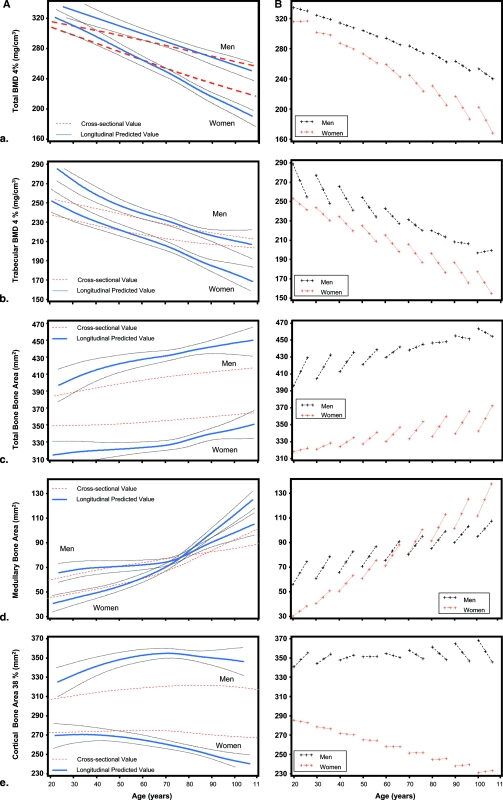
Figures 1A–1E use an analogous graphical and statistical approach to examine five different bone parameters (vBMD, vBMDt, tCSA, medullary area, CSA). In general, rate of decline in bone parameters estimated from cross-sectional data substantially underestimates the true rate of decline indicated by the longitudinal data, and age-related changes are not linear. The left panel shows age-related trajectories of the bone parameters during the life span estimate from the cross-sectional and longitudinal data and their 95% CIs. The right panels show longitudinal changes in bone parameters during the 6-yr follow-up, according to baseline age decade, from 20 to 100 yr, in men and women.
FIG. 1.
(Right panels) Longitudinal changes in total BMD (vBMD) (A), trabecular BMD (vBMDt) (B), total bone area (tCSA) (C), medullary bone area (D), and cortical bone area (E) during the 6-yr follow-up according to baseline age decade, from 20 to 100 yr, in men and women. (Left panels) Age-related trajectories of the bone parameters during life span estimates from the cross-sectional (red line) and longitudinal data (blue line) and their 95% CIs (black lines).
Women showed a steeper decline of total vBMD than men, which becomes more evident after 65 yr of age (Fig. 1A). The sex difference in the rate of decline is confirmed by the significant “age × time × sex” interaction term in the final model (p = 0.02; Table 2, model 1). Women showed a steeper decline in vBMDt than men, and such a decline did not seem to be linear, with a rate of decline that slightly accelerates after 65 yr of age (Fig. 1B). The different rate of decline is statistically significant as indicated by a significant “age × time × sex” (p = 0.0015; Table 2, model 1) and “age × sex × time2” interaction terms (p = 0.0017; Table 2, model 2). For example, in women, each year of follow-up is associated with a loss in trabecular vBMD of 5.6 mg/cm3, with a significantly 0.05-mg/cm3 higher loss in women than in men for each year of follow-up.
Table 2.
Association Between Age and Sex and the pQCT Bone Parameters Over Time
|
pQCT bone parameters |
||||||
| Total vBMD (4%) (mg/cm3) | p | Trabecular vBMD (4%) (mg/cm3) | p | Total bone area 38% (mm2) | p | |
| Model 1 | ||||||
| Intercept | 344.08 | 272.27 | 312.1 | |||
| Age* | −1.42 ± 0.10 | <0.0001 | 0.95 ± 0.12 | <0.0001 | 0.30 ± 0.12 | 0.009 |
| Time† | 1.62 ± 0.94 | 0.08 | −1.56 ± 1.38 | 0.26 | −0.40 ± 1.76 | 0.82 |
| Age × time | −0.07 ± 0.03 | <0.0001 | −0.02 ± 0.02 | 0.32 | 0.054 ± 0.028 | 0.05 |
| Sex‡ | 10.78 ± 9.7 | 0.27 | 39.53 ± 11.4 | 0.0005 | 66.98 ± 14.72 | <0.0001 |
| Age × sex | 0.40 ± 0.15 | 0.006 | −0.20 ± 0.17 | 0.25 | 0.54 ± 0.22 | 0.01 |
| Sex × time | −2.01 ± 1.38 | 0.15 | −5.61 ± 1.98 | 0.005 | 7.71 ± 2.69 | 0.004 |
| Age × time × sex | 0.06 ± 0.02 | 0.02 | 0.10 ± 0.03 | 0.0015 | −0.14 ± 0.042 | 0.0007 |
| Model 2 | ||||||
| Intercept | 262.74 | |||||
| Age | 0.87 ± 0.12 | <0.0001 | ||||
| Time | 16.16 ± 3.95 | <0.0001 | ||||
| Age × time | 0.16 ± 0.09 | 0.06 | ||||
| Age × time × time | 0.03 ± 0.02 | 0.12 | ||||
| Time × time | 2.95 ± 0.56 | <0.0001 | ||||
| Sex | 42.70 ± 12.50 | 0.0006 | ||||
| Sex × age | 0.28 ± 0.19 | 0.13 | ||||
| Sex × time | 16.99 ± 5.89 | 0.004 | ||||
| Sex × age × time | 0.39 ± 0.11 | 0.0003 | ||||
| Sex × time × time | 2.09 ± 0.80 | 0.009 | ||||
| Sex × age × time × time | 0.05 ± 0.07 | 0.0017 | ||||
* Age = age at enrollment.
† Time = years since follow-up (range, 0–6 yr).
‡ Sex = men: 1, women: 0.
As expected, we observed an age-related increase in tCSA in both sexes, which is evident from both the cross-sectional and longitudinal data (Fig. 1C). In men, the increase in tCSA is particularly evident until ∼60 yr of age, whereas in women, tCSA increase becomes progressively steeper from young to old age. A significant “age × time × sex” interaction term showed that women gain more bone (expression of periosteal apposition) than men at older ages (Table 2, model 1).
The widening of medullary area (expression of the endocortical resorption) with age was considerably steeper in women than in men. In fact, whereas at younger ages the medullary area is larger in men (as an expression of larger bones), there is a crossover at 70–75 yr of age so that after 75 yr of age, women have larger medullary area than men (Fig. 1D). There is a significant “age × sex × time2” interaction (p = 0.04).
In younger men, the cortical bone area increases slightly, with a rate of increase that slows down with age, flattens at ∼50 yr of age, and slightly declines at older ages. Women showed a slight, but constant decline in cortical bone area across the entire age range (Fig. 1E). Thus, older women attempt to compensate for the fast endocortical resorption, but such a compensation is inadequate (Table 3, model 1).
Table 3.
Association Between Age and Sex and the pQCT Bone Parameters Over Time
|
pQCT bone parameters |
||||||
| Medullary bone area 38% (mm2) | p | Cortical bone area 38 (mm2) | p | Cross-sectional moment of inertia 38% (g/cm) | p | |
| Model 1 | ||||||
| Intercept | 298.99 | 294.54 | 1322.01 | |||
| Age* | 1.02 ± 0.07 | <0.0001 | −0.60 ± 0.11 | <0.0001 | −1.81 ± 0.68 | 0.008 |
| Time† | 0.60 ± 1.03 | 0.56 | −7.55 ± 2.27 | 0.001 | −25.46 ± 13.24 | 0.05 |
| Age × time | 0.038 ± 0.02 | 0.02 | 0.11 ± 0.03 | 0.0007 | 0.33 ± 0.19 | 0.08 |
| Sex‡ | 36.35 ± 6.52 | <0.0001 | 48.69 ± 13.08 | 0.0002 | 804.17 ± 141.25 | <0.0001 |
| Age × sex | −0.53 ± 0.10 | <0.0001 | 0.81 ± 0.19 | <0.0001 | 1.22 ± 2.33 | 0.59 |
| Sex × time | 2.73 ± 1.39 | 0.04 | 8.14 ± 3.62 | 0.02 | −7.72 ± 32.97 | 0.82 |
| Age × time × sex | −0.05 ± 0.02 | 0.02 | −0.14 ± 0.05 | 0.006 | −0.08 ± 0.52 | 0.87 |
| Model 2 | ||||||
| Intercept | 11.1 | |||||
| Age | 1.02 ± 0.07 | <0.0001 | ||||
| Time | −0.38 ± 2.48 | 0.88 | ||||
| Age × time | −0.001 ± 0.05 | 0.98 | ||||
| Age × time × time | 0.01 ± 0.008 | 0.39 | ||||
| Time × time | 0.13 ± 0.42 | 0.76 | ||||
| Sex | 40.03 ± 6.73 | <0.0001 | ||||
| Sex × age | 0.58 ± 0.10 | <0.0001 | ||||
| Sex × time | −7.65 ± 3.51 | 0.03 | ||||
| Sex × age × time | 0.08 ± 0.06 | 0.19 | ||||
| Sex × time × time | 1.71 ± 0.62 | 0.006 | ||||
| Sex × age × time × time | −0.02 ± 0.01 | 0.04 | ||||
* Age = age at enrollment.
† Time = years since follow-up (range, 0–6 yr).
‡ Sex = men: 1, women: 0.
An overall view of changes in bone parameters across the entire age range obtained by combining cross-sectional and longitudinal data is shown in Table 4. The reduction in total vBMD from 20 to 100 yr was almost 2-fold higher in women than in men (−36.4% in women versus −18.5% in men), whereas the overall loss in trabecular BMD is similar in the two sexes (−22.8% in women and −20.5% in men). The overall increase in total bone area in men is almost 2-fold higher in men than in women (5.7% in women versus 9.0% in men), and it is suggestive of a continuous periosteal apposition with aging in both sexes. Noteworthy, the increment in medullary area was almost 4-fold higher in women than in men during the entire age range (114% in women versus 30% in men), which is an expression of accelerated endocortical resorption. The cortical bone area, which depends on periosteal apposition, tended to increase slightly but significantly in men, whereas it declined steeply in women (−13.4% in women versus 4.1% in men) as result of the rapid endocortical resorption.
Table 4.
pQCT Measurement at the Tibia Level
| Variable |
Men |
Women |
||||
| Mean ± SD (20–29 yr) | Δ change between 20 and 90 yr |
Mean ± SD (20–29 yr) | Δ change between 20 and 90 yr |
|||
| Absolute | Percentage | Absolute | Percentage | |||
| Total BMD 4% (mg/cm3) | 330.36 ± 3.89 | −61.1 | −18.5 | 309.21 ± 5.90 | −91.5 | −36.4 |
| Trabecular BMD 4% (mg/cm3) | 270.94 ± 12.96 | −55.6 | −20.5 | 241.37 ± 9.66 | −55.0 | −22.8 |
| Total bone area 38% (mm2) | 413.30 ± 10.67 | 37.1 | 9 | 327.27 ± 5.90 | 18.8 | 5.7 |
| Medullary bone area 38% (mm2) | 74.02 ± 7.08 | 22.2 | 30 | 50.28 ± 4.63 | 57.3 | 113.9 |
| Cortical bone area 38% (mm2) | 339.25 ± 4.39 | 14 | 4.1 | 274.60 ± 2.51 | −36.9 | −13.4 |
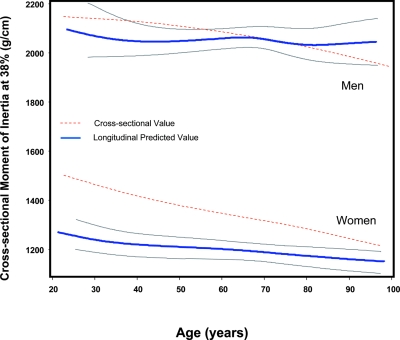
Finally, Fig. 2 shows age-related trajectories of the bone parameters during the life span estimate from the cross-sectional and longitudinal data and their 95% CIs. We found that cross-sectional moment of inertia declined progressively with aging in both sexes, although the magnitude of decline was not statistically significant over the 6-yr follow-up in both sexes (Table 3, model 1).
FIG. 2.
Age-related trajectories of the cross-sectional moment of inertia at 38% during life span estimates from the cross-sectional (red line) and longitudinal data (blue line) and their 95% CIs (black lines).
DISCUSSION
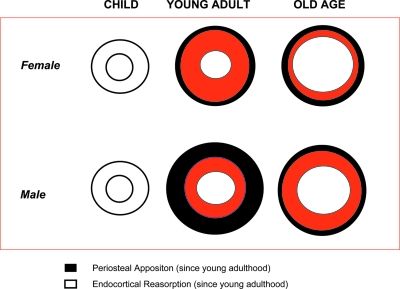
Using longitudinal data collected over a 6-yr follow-up in a representative sample of the population, we found that the shaft of the tibia undergoes periosteal apposition and endocortical resorption over the aging process. Periosteal apposition in men occurs mostly at younger ages, whereas in women, it is evident across the entire age range. However, the rate of endocortical reabsorption in women is much higher than in men and considerably exceeds the effect of periosteal apposition. The cross-sectional moment of inertia—a derived parameter that summarizes how parallel changes in bone material and in bone geometry translate into changes in bone mechanical properties (bone mechanical resistance to bending)(10)—declined over the life span in both sexes. An overall model of change in bone macroarchitecture over the aging process is given in Fig. 3. This model is estimated from scans performed at the 38% of the tibia.
FIG. 3.
Hypothetical schematic model of the changes in bone geometry parameters, at 38% of the tibia level, during life span in men and women. The rate of endocortical reabsorption is much higher in women than in men and considerably exceeds the effect of periosteal apposition, which assumes a different rate in men and women with aging. In fact, whereas periosteal apposition is higher in younger men than in old men, in women, it is higher in older women than young women.
To our knowledge, this is the first large population-based study to document longitudinal changes of BMD and bone geometry across the life span in both sexes. Our results support the hypothesis that the substantial decline of bone strength in aging women is related to a unique endocortical resorption that erodes the cortical bone area internally. This decline in cortical bone accelerates after 50 yr of age. Our findings are consistent with those recently reported by the only other prospective study showing that bone loss in women accelerated after menopause despite periosteal apposition.(5) The occurrence of periosteal apposition with aging in men and women had been suggested by previous cross-sectional studies.(4,10,18,19) However, this is the first longitudinal evidence to show that cross-sectional data cannot capture the heterogeneity of changes in bone parameters over the life span and how they differ between men and women.
Our findings support the notion that there is a substantial loss of bone tissue elastic stability with age and consequent decline in bone toughness, which is mainly because of a reduction in cortical bone.(20) This is consistent with recent evidence that cortical femoral thickness assessed by QCT predicted the risk of fractures, and this reduction of thickness was particularly high in a site where bone was not under loading.(20)
Endocortical resorption seems to be a key process for the development of bone fragility in older age, which could be the result of the loss of estrogen with menopause and is the mechanism by which older women became more susceptible to fracture than men.
In our study, women showed a steeper bone trabecular loss with aging than men, and this is in accordance with observational studies showing that bone strength depends also on trabecular architecture. In osteopenic subjects, the plates become fenestrated, resulting in more rods that deteriorate and become disconnected.(3) It is notable that, in this study, trabecular BMD in men is higher than in women across the life span and declines longitudinally in both sexes, although the rate of decline in women is higher than in men at older ages. This particular behavior of trabecular bone may be linked to age-associated changes in sexual hormones levels.
The strengths of this study are the representative nature of the study population and the fact that the participants were prospectively observed for 6 yr of follow-up. On the other hand, a possible limitation of this study is that the findings were obtained using peripheral bone sites instead of central site such as vertebral of hip structures. However, recent data from the literature have shown that peripheral BMD is highly predictive of fracture risk.(21)
An additional problem is that the oldest subjects, who did not participate in the pQCT evaluation, had greater comorbidity than the subjects who were evaluated. This selective exclusion may have caused an underestimation of the effect of age. This problem may be particularly important when looking at the oldest age groups, because of the small number of participants. This potential limitation should be addressed in future studies.
In conclusion, our study suggests that an imbalance between periosteal apposition and endocortical resorption(17) is the key factor for the development of the bone fragility in older women, with an amplification of endocortical resorption at an older age that causes an acceleration of bone loss after menopause. This phenomenon should be the target for interventions aimed at retarding the progression of bone loss in older adults. Future studies are needed to identify the specific effects on hormones and other factors(22) that potentially influence periosteal apposition(1) and endocortical resorption.
ACKNOWLEDGMENTS
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, and N01-AG-5-0002 and NIA Grant R01 AG027012. This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH. While working on the manuscript, Luigi Ferrucci and Jack M Guralnik were employees of the National Institute of Aging (NIH). The authors thank Dr Cosimo Roberto Russo for providing technical expertise.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Seeman E. The periosteum-a surface for all seasons. Osteoporos Int. 2007;18:123–128. doi: 10.1007/s00198-006-0296-6. [DOI] [PubMed] [Google Scholar]
- 2.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Statement Online. Osteoporosis prevention, diagnosis, and therapy. 2000 Available online at http://consensus.nih.gov/2000/2000Osteoporosis111html.htm. [PubMed]
- 4.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 5.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 7.Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, Guralnik JM, Harris T, Ferrucci L. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 8.Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–326. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 9.Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, Guralnik J, Ferrucci L. Structural adaptations to bone loss in aging men and women. Bone. 2006;38:112–118. doi: 10.1016/j.bone.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Gatti D, Sartori E, Braga V, Corallo F, Rossini M, Adami S. Radial bending breaking resistance derived by densitometric evaluation predicts femoral neck fracture. Osteoporos Int. 2001;12:864–869. doi: 10.1007/s001980170038. [DOI] [PubMed] [Google Scholar]
- 11.Sievanen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I. Peripheral quantitative computed tomography in human long bones: Evaluation of in vitro and in vivo precision. J Bone Miner Res. 1998;13:871–882. doi: 10.1359/jbmr.1998.13.5.871. [DOI] [PubMed] [Google Scholar]
- 12.Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 13.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 14.Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982;217:945–948. doi: 10.1126/science.7112107. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- 16.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 17.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: Failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21:1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 18.Heaney RP, Barger-Lux MJ, Davies KM, Ryan RA, Johnson ML, Gong G. Bone dimensional change with age: Interactions of genetic, hormonal, and body size variables. Osteoporos Int. 1997;7:426–431. doi: 10.1007/pl00004150. [DOI] [PubMed] [Google Scholar]
- 19.Bouxsein ML, Myburgh KH, van der Meulen MC, Lindenberger E, Marcus R. Age-related differences in cross-sectional geometry of the forearm bones in healthy women. Calcif Tissue Int. 1994;54:113–118. doi: 10.1007/BF00296061. [DOI] [PubMed] [Google Scholar]
- 20.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 21.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 22.Lauretani F, Bandinelli S, Russo CR, Maggio M, Di Iorio A, Cherubini A, Maggio D, Ceda GP, Valenti G, Guralnik JM, Ferrucci L. Correlates of bone quality in older persons. Bone. 2006;39:915–921. doi: 10.1016/j.bone.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]