Abstract
Background
Recent studies suggest an association between polyunsaturated fatty acids (PUFAs) and the development of chronic kidney disease. The aim of this study was to examine the relationship between PUFAs and renal function in older adults.
Methods
We performed a cross-sectional and prospective analysis of 931 adults, ≥65 years old, enrolled in the InCHIANTI study, a population-based cohort in Tuscany, Italy. Plasma PUFAs were measured at enrollment, and creatinine clearance was estimated by the Cockcroft-Gault equation at baseline and after 3-year follow-up.
Results
At enrollment, participants with higher creatinine clearance had higher concentrations of HDL cholesterol, total plasma PUFAs, plasma n-3 fatty acid (FA), and plasma n-6 FA and lower triglycerides. From enrollment to the 3-year follow-up visit, creatinine clearance declined by 7.8 (12.2) mL/min (P <0.0001). Baseline total plasma PUFAs, n-3 FA, n-6 FA, and linoleic, linolenic, and arachidonic acids were strong independent predictors of less steep decline in creatinine clearance from baseline to follow-up (P <0.0001, after adjusting for baseline creatinine clearance). After adjusting for baseline creatinine, baseline total plasma PUFAs, n-3 FA, and linoleic, linolenic, and arachidonic acids were negatively associated with creatinine at 3-year follow-up. Participants with higher plasma PUFAs at enrollment had a lower risk of developing renal insufficiency, defined by a creatinine clearance <60 mL/min, during 3-year follow-up.
Conclusion
High PUFA concentrations, both n-3 FA and n-6 FA, may attenuate the age-associated decline in renal function among older community-dwelling women and men.
Chronic kidney disease is emerging as a major public health problem among older adults and can result in end-stage renal disease with need for dialysis or transplantation for kidney failure (1, 2). In the US, an estimated 19 million adults are in the early stage of disease (1). The Prevention of Renal and Vascular End-Stage Disease study in Europe showed that up to 12% of the adult population had some renal impairment (3). Creatinine clearance is widely used to assess chronic kidney disease in clinical practice and large epidemiologic studies (4, 5). The major risk factors for chronic kidney disease are increasing age, hypertension, diabetes, cardiovascular disease, and a family history of the disease (1). Abnormalities in lipids and atherogenic lipoprotein metabolism may contribute to glomerular and interstitial injury and progression of renal disease (4).
Recent studies suggest that there may be an association between polyunsaturated fatty acids (PUFAs)7 and the development of chronic kidney disease (6). PUFA supplementation has been shown to reduce renal inflammation and fibrosis in animal models (6). PUFAs may protect kidney function by modulating the inflammatory response through downregulation of the production of proinflammatory cytokines, cyclooxy-genase-2 activity, and expression of endothelial leukocyte adhesion molecules (7, 8). Accordingly, among older adults, high levels of plasma PUFAs were associated with lower levels of C-reactive protein (CRP) and proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α and higher levels of antiinflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β (9).
We hypothesized that low total plasma PUFA levels were associated with an accelerated decline of kidney function in older adults. To test this hypothesis, we examined the relationship between total plasma PUFA levels and change in creatinine clearance over a 3-year follow-up in the older participants of the InCHIANTI study, a population-based epidemiology study conducted in Tuscany, Italy.
Materials and Methods
The study participants consisted of men and women ages 65 and older who participated in the InCHIANTI study (Aging in the Chianti Area) conducted in 2 small towns in Tuscany, Italy. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethics Committee. The rationale, design, and data collection have been described (10). Briefly, in August 1998, 1270 people ages 65 years and older were randomly selected from the population registry of Greve in Chianti (population 11 709) and Bagno a Ripoli (population 4704); of 1256 eligible subjects, 1155 (90.1%) agreed to participate. Of the 1155 participants, 1055 (91.3%) donated a blood sample. The subjects who did not participate in the blood drawing were generally older and had greater comorbidity than those who participated in the blood drawing (11).
Participants received an extensive description of the project and were enrolled in the study after formal consent. Participants were evaluated again for a 3-year follow-up visit that was conducted from 2001 to 2003 and included a new phlebotomy and laboratory testing.
Demographic information and information on smoking and medication use were collected using standardized questionnaires. Average daily intakes of energy (kcal), carbohydrates, total protein, total lipids, etc. were estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire, previously validated in the InCHIANTI population (12). All participants were examined by a trained geriatrician, and diseases (coronary heart disease, congestive heart failure, hypertension, diabetes, chronic obstructive pulmonary disease, osteoarthritis, and cancer) were ascertained according to standard, preestablished criteria and algorithms based on those used in the Women's Health and Aging Study (13). Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight in kg/(height in m)2. Mini-Mental Status Examination (MMSE) was administered at enrollment (14).
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at −80 °C. Fatty acids (FAs) were measured using a fasting plasma sample. The rationale and methodology of the FA determination have been described (9). The intraassay and interassay CVs for all FAs were on average 1.6% and 3.3%, respectively. The following fatty acid–related variables were selected for analyses: (a) ω-6 fatty acids (n-6, mg/L), linoleic acid (18:2n-6, mg/L), and arachidonic acid (20:4n-6, mg/L); (b) ω-3 fatty acids (n-3, mg/L) and linolenic acid (18:3n-3, mg/L), and (c) total polyunsaturated fatty acids (total plasma PUFAs, mg/L) (9).
Serum total cholesterol was assessed by commercial enzymatic tests (Roche Diagnostics, GmbH) and a Roche-Hitachi 917 analyzer. The lower detection limit was 30 mg/L. The intraassay and interassay CVs were 0.8% and 3.3%, respectively. Serum creatinine was measured by commercial enzymatic assay using a Modular P800 Hitachi Analyzer. The interassay CV was 2.3%, and the detection limit was 1 mg/L. Serum creatinine (Scr) measured by use of a modified Jaffe method was used to estimate creatinine clearance according to the Cockcroft-Gault formula (Ccr) = [(140 − age) × weight/(72 × Scr) × 0.85 (if the subject is female)] where Ccr is expressed in mL/min, age in years, weight in km, and Scr in mg/dL (15).
We found a high correlation between the creatinine clearance estimated by the Cockcroft-Gault formula and that estimated by using the 4-variable Modification of Diet in Renal Disease (MDRD) equation (r = 0.71; P <0.0001) (16).
Urinary protein was measured using a Modular P800 Hitachi Analyzer. Laboratory normal range for proteinuria was 0–150 mg/L.
Statistical Analysis
Variables are reported as means (SD) for normally distributed variables or as percentages. Anthropometric, biochemical, and clinical characteristics of the population at enrollment were stratified across creatinine clearance strata (<30, 30–59, ≥60 mL/min) and compared using ANOVA-based test for trend adjusting for age, sex, and BMI.
Total plasma PUFAs were divided into quintiles (≤34.3, 34.4–36.9, 37.0–39.5, 39.6–42.11, ≥42.14 mg/L). We used test for a linear trend to compare changes in creatinine clearance between enrollment and 3-year follow-up across baseline total plasma PUFA quintiles. We used Pearson correlation to examine the relationship between creatinine clearance, all types of plasma PUFAs, and other variables. The relationship between changes in creatinine and changes in creatinine clearance from baseline to follow-up and all types of plasma PUFA was examined using multivariate linear models adjusted for multiple confounders. We obtained parsimonious models by starting with an initial models that included all variables associated with follow-up creatinine or creatinine clearance at a P level <0.10 (age, sex, BMI, education, cigarette smoking pack-years, MMSE score, energy intake, alcohol, LDL and HDL cholesterol, self-reported cancer, cardiovascular disease, and hypertension) plus baseline creatinine or creatinine clearance.
We used a fully adjusted logistic regression analysis to test the hypothesis that lower plasma PUFA levels were associated with a significantly higher probability of developing renal insufficiency. In this analysis, participants with renal insufficiency at enrollment (defined by a creatinine clearance <60 mL/min) were excluded. The analysis was also repeated using a composite outcome consisting of either the development of renal insufficiency or death during the 3-year follow-up.
All analyses were performed using SAS (v. 8.2, SAS Institute, Inc.) with a statistical significance level set at P <0.05.
Results
Of the 1055 participants who donated a blood sample at baseline, 931 (80.6%) had both plasma PUFAs and serum creatinine measurements available for this analysis. Of these 931 participants, 676 (72.7%) had creatinine clearance measurements available at the 3-year follow-up visit. Of the 255 subjects not included in the longitudinal analyses, 96 died between enrollment and follow-up, 147 refused to participate in the blood drawing and/or interview, and 12 moved out of the study area.
Participants evaluated at baseline with missing creatinine clearance at the 3-year follow-up (n = 255) were significantly older (79.9 vs 73.2 years, P <0.0001), had lower baseline creatinine clearance (55.1 vs 67.1 mL/min, P <0.0001), and had lower total plasma PUFAs (36.9 vs 38.1 mg/L, P = 0.0005) than those included in the present study (n = 709). Of the 676 participants evaluated at the 3-year follow-up visit, 398 had creatinine clearance ≥60 mL/min at enrollment.
The demographic and disease characteristics of the study participants at enrollment, according to creatinine clearance strata (<30, 30–59, ≥60 mL/min), are shown in Table 1. Higher creatinine clearance was associated with younger age, male sex, and lower CRP level and BMI. Persons with higher creatinine clearance also had higher HDL cholesterol, total plasma PUFAs, plasma n-3 FA, and plasma n-6 FA and lower triglycerides. The proportion of persons with proteinuria > 150 mg/L was lower among persons with higher creatinine clearance. The prevalence of congestive heart failure, stroke, and peripheral artery disease was significantly higher among persons with higher creatinine clearance.
Table 1. Characteristics of the study population according to creatinine clearance strata.
| Creatinine clearance | ||||
|---|---|---|---|---|
| <30 mL/min | 30–59 mL/min | >60 mL/min | Pa | |
| n | 27 | 383 | 521 | |
| Age, years | 87.50 (5.00) | 79.65 (7.34) | 71.87 (4.68) | <0.0001 |
| Female, % | 70.4 | 65.8 | 47.22 | <0.0001 |
| Education, years | 4 (3–5) | 5 (3–5) | 5 (5–6) | 0.26 |
| Current smokers, % | 15 | 12.4 | 24.6 | 0.0002 |
| Former smokers, % | 29 | 24.3 | 37.9 | 0.0002 |
| Smoking, pack-years | 0.0 (0.0–25.4) | 0.0 (0.0–8.2) | 0.0 (0.0–28.0) | 0.47 |
| Alcohol, g/day | 10.0 (7.14–28.57) | 10.0 (4.29–20.0) | 13.57 (5.71–22.86) | 0.20 |
| MMSE score | 18.74 (8.20) | 23.31 (5.15) | 25.66 (3.59) | 0.13 |
| BMI, kg/m2 | 24.38 (4.00) | 25.77 (3.68) | 28.59 (3.76) | <0.0001 |
| Energy intake, kcal/day | 1535 (344.29) | 1813.65 (542.70) | 2017.69 (562.32) | 0.90 |
| Carbohydrate intake, g/day | 202.83 (44.84) | 237.28 (76.08) | 259.96 (82.28) | 0.593 |
| Protein intake, g/day | 59.80 (15.29) | 71.73 (20.50) | 78.53 (19.76) | 0.32 |
| Total lipid intake, g/day | 50.99 (14.46) | 61.13 (20.26) | 67.48 (19.31) | 0.75 |
| Saturated FA intake, g/day | 17.68 (5.48) | 21.15 (8.03) | 22.47 (7.69) | 0.44 |
| Monounsatured FA intake, g/day | 24.81 (7.91) | 30.12 (10.55) | 34.14 (10.77) | 0.78 |
| Polynsaturated FA intake, g/day | 5.72 (1.70) | 6.60 (2.11) | 7.43 (2.21) | 0.79 |
| Total cholesterol, mg/L | 2004.1 (277.0) | 2161.2 (425.0) | 2169.5 (390.8) | 0.18 |
| LDL cholesterol, mg/L | 1168.9 (212.4) | 1340.1 (358.0) | 1369.7 (341.1) | 0.07 |
| HDL cholesterol, mg/L | 487.0 (159.2) | 584.3 (169.7) | 544.7 (135.1) | 0.02 |
| Triglycerides, mg/L | 1734.4 (989.2) | 1185.1 (535.5) | 1275.6 (685.0) | 0.0004 |
| Total plasma PUFAs, mg/L | 32.26 (4.80) | 37.34 (4.87) | 37.76 (4.85) | <0.0001 |
| Plasma n-3 FA, mg/L | 2.69 (0.58) | 3.34 (0.97) | 3.29 (0.96) | 0.004 |
| Plasma n-6 FA, mg*10/L | 2.76 (0.45) | 3.26 (0.44) | 3.24 (0.45) | <0.0001 |
| CRP, mg/L | 4.66 (1.76–15.20) | 2.59 (1.30–5.56) | 2.89 (1.36–5.85) | <0.0001 |
| Urine protein, mg/L | 0 (0–5) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.04 |
| Coronary heart disease, % | 10.00 | 5.93 | 5.21 | 0.43 |
| Stroke, % | 15.38 | 6.70 | 4.12 | 0.01 |
| Diabetes, % | 11.11 | 10.98 | 11.89 | 0.67 |
| Hypertension, % | 60.00 | 48.52 | 17.28 | 0.38 |
| Peripheral artery disease, % | 20.00 | 8.09 | 4.98 | 0.004 |
| Congestive heart failure, % | 44.44 | 8.56 | 4.86 | <0.0001 |
| COPD, % | 26.92 | 7.18 | 8.65 | 0.35 |
| Cancer, % | 11.11 | 6.79 | 5.79 | 0.30 |
Continuous variables expressed as mean (SD) or as median (IQR).
From age-, sex-, and BMI-adjusted linear or multinomial logistic regression models as appropriate.
In age- and sex-adjusted analysis, plasma PUFAs were also inversely associated with urine protein excretion at enrollment (β = −0.15, SE = 0.06, P = 0.02) (data not shown).
From enrollment to the 3-year follow-up visit, creatinine clearance declined by 7.8 (12.2) mL/min (P <0.0001). Creatinine clearance at enrollment and the 3-year follow-up were highly correlated (r = 0.76; P <0.0001).
The relationship between total plasma PUFAs at enrollment and mean change in creatinine clearance between baseline and the 3-year visit is shown in Fig. 1. The analysis is adjusted for baseline creatinine clearance. From the lowest to the highest quintile of total plasma PUFAs, the mean declines in creatinine clearance were 8.8, 5.0, 4.8, 3.2, and 1.4 mL/min, (P <0.0001).
Fig. 1.
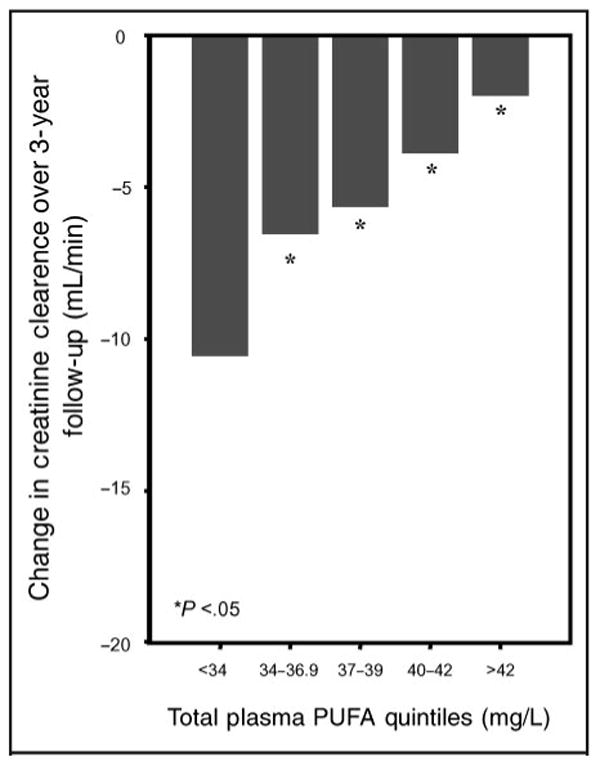
Relationship of total plasma PUFAs (in quintiles; mg/L) at enrollment with change in creatinine clearance between enrollment and 3-year follow-up visit after adjusting for baseline creatinine clearance (* P <0.05 in relation to the lowest quintile). P for linear trend across quintiles <0.0001.
The relationships of total plasma PUFAs, n-3 FA, linolenic acid, n-6 FA, linoleic acid, and arachidonic acid with change in creatinine clearance from baseline to follow-up was examined using multivariate linear regression models adjusted for covariates, including education, cigarette smoking (pack-years), MMSE score, energy intake, alcohol intake, LDL cholesterol, HDL cholesterol, cancer, cardiovascular disease, and hypertension (Table 2). Adjusting for baseline creatinine clearance and other confounders, higher total plasma PUFAs, n-3 FA, linolenic acid, n-6 FA, linoleic acid, and arachidonic acid at enrollment were significantly associated with lower decline in creatinine clearance from baseline to follow-up; participants with higher plasma PUFAs at enrollment had a smaller decline in creatinine clearance from baseline to follow-up.
Table 2. Backward regression models relating change in creatinine clearance over 3-year follow-up and total plasma PUFAs at baseline.
| Dependent change in creatinine clearance | ||
|---|---|---|
| β (SE) | P | |
| Total plasma PUFAs, mg/L | 0.43 (0.11) | <0.0001 |
| Plasma n-3 FA, mg/L | 6.28 (1.29) | <0.0001 |
| Plasma linolenic acid, mg/L | 0.19 (0.05) | 0.0004 |
| Plasma n-6 FA, mg*10/L | 3.62 (1.19) | 0.03 |
| Plasma linoleic acid, mg*10/L | 0.08 (0.03) | 0.004 |
| Plasma arachidonic acid, mg*10/L | 0.24 (0.06) | <0.0001 |
Data adjusted for creatinine clearance at baseline, education, cigarette-smoking pack-years, MMSE score, energy intake, alcohol consumption, LDL cholesterol, HDL cholesterol, self-reported cancer, cardiovascular disease, and hypertension. Covariates were selected using a Pearson correlation coefficient <0.10.
The relationship of total plasma PUFAs, n-3 FA, linolenic acid, n-6 FA, linoleic acid, arachidonic acid, and creatinine at enrollment with change in creatinine from baseline to follow-up was examined using multivariate linear regression models adjusted for covariates, including age, sex, BMI, education, cigarette smoking (pack-years), MMSE score, energy intake, alcohol intake, LDL cholesterol, HDL cholesterol, cancer, cardiovascular disease, and hypertension (Table 3).
Table 3. Backward regression models relating creatinine at baseline, creatinine at the 3-year follow-up study, and plasma PUFAs at baseline.
| Creatinine at baseline | Creatinine at 3-years follow-up studya | |||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| Total plasma PUFAs, mg/L | −0.005 (0.002) | 0.06 | −0.005 (0.002) | 0.03 |
| Plasma n-3 FA, mg/L | −0.02 (0.02) | 0.31 | −0.12 (0.03) | <0.0001 |
| Plasma linolenic acid, mg/L | −0.002 (0.001) | 0.10 | −0.003 (0.001) | 0.002 |
| Plasma n-6 FA, mg*10/L | −0.05 (0.02) | 0.03 | −0.04 (0.02) | 0.11 |
| Plasma linoleic acid, mg*10/L | −0.001 (0.0005) | 0.07 | −0.001 (0.0006) | 0.01 |
| Plasma arachidonic acid, mg*10/L | −0.0001 (0.001) | 0.95 | −0.002 (0.0006) | 0.003 |
Data adjusted for age, sex, BMI, education, cigarette smoking pack-years, MMSE score, energy intake, alcohol, LDL cholesterol, HDL cholesterol, self-reported cancer, cardiovascular disease, and hypertension. Covariates were selected using a Pearson correlation coefficient <0.10.
Also adjusted for creatinine at baseline.
At enrollment, total plasma PUFAs and n-6 FA were significantly associated with lower creatinine. Adjusting for baseline creatinine, higher total plasma PUFAs, n-3 FA, linolenic acid, linoleic acid, and arachidonic acid at enrollment were significantly associated with a smaller decline in creatinine from baseline to follow-up.
Excluding participants with creatinine clearance <60 mL/min at baseline, and adjusting for multiple confounders, those with higher plasma PUFAs at enrollment had a lower risk of developing renal insufficiency, defined by a creatinine clearance <60 mL/min at the 3-year follow-up. Participants with higher plasma N-6 and N-3 fatty acids tended to have a lower risk of developing renal insufficiency (Table 4), although this trend was not statistically significant. N-3 FA concentrations were inversely associated with risk of developing renal insufficiency or dying over the 3-year follow-up (Table 5).
Table 4. Odds of developing renal insufficiency (n = 134) (defined by a creatinine clearance <60 mL/min, among the 398 participants with creatinine clearance >60 mL/min at baseline) over 3-year follow-up according to plasma baseline PUFA level.
| Crude odds ratio (95% CI) | P | Fully adjusted odds ratio (95% CI)a | P | |
|---|---|---|---|---|
| Total plasma PUFAs, mg/L | 0.95 (0.91–0.99) | 0.02 | 0.94 (0.89–0.99 | 0.015 |
| Plasma n-3 FA, mg/L | 0.60 (0.35–1.04) | 0.06 | 0.68 (0.37–1.28) | 0.23 |
| Plasma linolenic acid, mg/L | 0.98 (0.96–1.01) | 0.10 | 0.99 (0.96–1.01) | 0.30 |
| Plasma n-6 FA, mg*10/L | 0.68 (0.42–1.10) | 0.11 | 0.96 (0.88–1.00) | 0.09 |
| Plasma linoleic acid, mg*10/L | 0.99 (0.98–1.01) | 0.59 | 1.00 (0.98–1.02) | 0.98 |
| Plasma arachidonic acid, mg*10/L | 0.99 (0.96–1.02) | 0.44 | 0.98 (0.96–1.02) | 0.49 |
Adjusted for education, cigarette smoking pack-years, MMSE score, energy intake, alcohol, LDL cholesterol, HDL cholesterol, cancer, congestive heart failure, cardiovascular disease, and hypertension.
Table 5. Odds of developing renal insufficiency (defined by a creatinine clearance <60 mL/min, among the 398 participants with creatinine clearance >60 mL/min at baseline) or dying over 3-year follow-up according to plasma baseline PUFA level.
| Crude odds ratio (95% CI) | P | Fully adjusted odds ratio (95% CI)a | P | |
|---|---|---|---|---|
| Total plasma PUFAs, mg/L | 0.95 (0.91–0.99) | 0.02 | 0.98 (0.94–1.03) | 0.36 |
| Plasma n-3 FA, mg/L | 0.47 (0.30–0.74) | 0.001 | 0.53 (0.31–0.88) | 0.02 |
| Plasma linolenic acid, mg/L | 0.96 (0.95–0.98) | 0.002 | 0.97 (0.95–0.99) | 0.008 |
| Plasma n-6 FA, mg*10/L | 0.71 (0.48–1.05) | 0.08 | 0.93 (0.57–1.54) | 0.19 |
| Plasma linoleic acid, mg*10/L | 0.98 (0.97–0.99) | 0.01 | 0.99 (0.98–1.01) | 0.30 |
| Plasma arachidonic acid, mg*10/L | 0.98 (0.96–1.01) | 0.07 | 0.98 (0.95–1.01) | 0.16 |
Adjusted for education, cigarette smoking pack-years, MMSE score, energy intake, alcohol, LDL cholesterol, HDL cholesterol, cancer, congestive heart failure, cardiovascular disease, and hypertension.
Discussion
This study shows that older adults with low total plasma PUFA concentrations have a greater decline in creatinine and creatinine clearance over 3 years of follow-up than those with higher concentrations of total plasma PUFAs. In addition, participants with lower baseline plasma PUFAs and free of renal insufficiency were significantly more likely to develop renal insufficiency at the 3-year follow-up than those with higher plasma PUFAs.
These findings suggest that a higher dietary intake of PUFAs, both n-3 FA and n-6 FA, may be protective against progression to chronic kidney disease, and are consistent with observations from animal models that show that PUFA supplementation reduces progression of renal disease (6). The observation that total plasma PUFAs and also ω-3 fatty and ω-6 fatty acids separately appear to have a beneficial effect on renal function require consideration. In fact, ω-3 polyunsaturated fatty acids are generally considered more beneficial than ω-6 fatty acids (17). However, recent data showed that both ω-6 (18) and ω-3 (19) fatty acids have antiinflammatory properties. PUFAs are present in high concentrations not only in fish oil but also in vegetable oils. For example, large quantities of ω-6 fatty acids are present in sunflower oil, soybean/corn oil, and safflower oil, whereas large quantities of ω-3 fatty acids are present in flax oil and hemp oil.
To our knowledge, this is the first human study that clearly observed a protective effect of PUFA on the age-associated decline of renal function. Strengths of this study are the relatively large sample size and the prospective, longitudinal analysis. The study is limited in that plasma PUFA levels were measured only at enrollment and not at the 3-year follow-up visit. Also, the loss of some respondents to follow-up may have influenced our findings.
The mechanisms by which PUFAs may protect the kidneys from damage in older adults remain unknown. Data from the literature suggest that PUFAs may be antiinflammatory (6). The main histopathological changes associated with the progression of renal disease in older adults are fibrosis, glomerulonephritis, progressive tubulointerstitial injury, and renal fibrosis (6). In addition, experimental data have clearly shown that aging is associated with increased oxidative stress (19), enhanced tubular cell apoptosis (20), and exacerbation of glomerular inflammatory responses induced by glomerular fibrin deposition (21).
Dietary fish oil supplementation has been shown to reduce progression of renal disease among patients with IgA nephropathy (22) and to suppress mesangial cell activation and proliferation in animal models (23). PUFAs may reduce inflammation through several possible pathways, such as reduction of nitric oxide (24), downregulation of TNF-α (25), and modulation of protein kinases (26).
Plasma PUFA concentrations have previously been associated with lower levels of markers of inflammation in the InCHIANTI study (9); in the present analysis, markers of inflammation were not included as confounders in the analysis because proinflammatory cytokines are considered to be in the causal pathway between plasma PUFAs and progression of renal disease (6, 27). A recent double-blind, placebo-controlled trial in 103 middle-aged men and women showed that increased dietary intake of α-linolenic acid lowered CRP levels (28). Another trial involving 60 subjects with active rheumatoid arthritis showed that n-3 FA supplementation decreased CRP (29).
Our findings prompt the hypothesis that a diet rich in PUFAs may be protective against the decline in renal function that is common with aging. A Mediterranean-style diet that is characterized by a relatively high consumption of fish and low consumption of saturated fats has been shown to be protective against cardiovascular disease (30, 31), markers of inflammation (32), and cancer (33). Further work is needed to confirm the association between plasma PUFAs and renal function in other cohorts of older persons and provide enough evidence to translate these findings into clinical trials.
Acknowledgments
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5-0002, and NIA Grant R01 AG027012. The research was also supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Nonstandard abbreviations: PUFA, polyunsaturated fatty acid; CRP, C-reactive protein; IL, interleukin; InCHIANTI, Aging in the Chianti Area; BMI, body mass index; MMSE, Mini-Mental Status Examination; FA, fatty acid; Ccr, Cockcroft-Gault formula; Scr, serum creatinine.
Financial Disclosures: None declared.
References
- 1.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 2.Xue JL, Ma JZ, Louis TA, Collins AJ. Forecast of the number of patients with end-stage renal disease in the United States by the year 2010. J Am Soc Nephrol. 2001;12:2753–8. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 3.de Zeeuw D, Hillege HL, de Jong PE. The kidney, a cardiovascular risk marker, and a new target for therapy. Kidney Int. 2005;68(Suppl 98):S25–9. doi: 10.1111/j.1523-1755.2005.09805.x. [DOI] [PubMed] [Google Scholar]
- 4.Giannelli SV, Patel KV, Windham BG, Pizzarelli F, Ferrucci L, Guralnik JM. Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55:816–23. doi: 10.1111/j.1532-5415.2007.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–9. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 6.Baggio B, Musaachio E, Priante G. Polyunsaturated fatty acids and renal fibrosis: pathophysio-logic link and potential clinical implications. J Nephrol. 2005;18:362–7. [PubMed] [Google Scholar]
- 7.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–58. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 8.de Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005;206:103–16. doi: 10.1007/s00232-005-0783-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–25. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. NIH publ. no. 95-4009. Bethesda, MD: National Institute on Aging; 1995. The Women's Health and Aging Study: health and social characteristics of older women with disability. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Simopoulos AP, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63:119–21. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 18.Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7:531–4. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Zou Y, Yoon S, Lee JH, Kim YK, Yu BP, et al. Vascular aging: molecular modulation of the prostanoid cascade by calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:B876–85. doi: 10.1093/gerona/59.9.b876. [DOI] [PubMed] [Google Scholar]
- 20.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, et al. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60:830–9. doi: 10.1093/gerona/60.7.830. [DOI] [PubMed] [Google Scholar]
- 21.Xi C, Chen X, Sun X, Shi S, Feng Z, Wang J, et al. Effects of alterations of glomerular fibrin deposition on renal inflammation in rats at different age stages. J Gerontol A Biol Sci Med Sci. 2005;60:1099–110. doi: 10.1093/gerona/60.9.1099. [DOI] [PubMed] [Google Scholar]
- 22.Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. N Engl J Med. 1994;331:1194–9. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 23.Grande JP, Walker HJ, Holub BJ, Warner GM, Keller DM, Haugen JD, et al. Suppressive effects of fish oil on mesangial cell proliferation in vitro and in vivo. Kidney Int. 2000;57:1027–40. doi: 10.1046/j.1523-1755.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- 24.Das UN. Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension. Eur J Clin Nutr. 2004;58:195–203. doi: 10.1038/sj.ejcn.1601766. [DOI] [PubMed] [Google Scholar]
- 25.Kielar ML, Jeyarajah DR, Zhou XJ, Lu CY. Docosahexaenoic acid ameliorates urine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase. J Am Soc Nephrol. 2003;14:389–96. doi: 10.1097/01.asn.0000045047.44107.0b. [DOI] [PubMed] [Google Scholar]
- 26.de Jonge HW, Dekkers DH, Lamers JM. Polyunsaturated fatty acids and signalling via phospholipase C-beta and A2 in myocardium. Mol Cell Biochem. 1996;157:199–210. doi: 10.1007/BF00227899. [DOI] [PubMed] [Google Scholar]
- 27.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–80. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 28.Bemelmans WJ, Lefrandt JD, Feskens EJ, van Haelst PL, Broer J, Meyboom-de Jon B, et al. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr. 2004;58:1083–9. doi: 10.1038/sj.ejcn.1601938. [DOI] [PubMed] [Google Scholar]
- 29.Sundrarjun T, Komindr S, Archararit N, Dahlan W, Puchaiwatananon O, Angthararak S, et al. Effects of n-e fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. J Int Med Res. 2004;32:443–54. doi: 10.1177/147323000403200501. [DOI] [PubMed] [Google Scholar]
- 30.de Lorgeril M, Salen P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006;9:118–23. doi: 10.1079/phn2005933. [DOI] [PubMed] [Google Scholar]
- 31.Zatonski WA, Willett W. Changes in dietary fat and declining coronary heart disease in Poland: population based study. BMJ. 2005;331:187–8. doi: 10.1136/bmj.331.7510.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–4. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]


