Abstract
Endometrial cytokine expression is poorly understood. T-Bet and GATA-3 regulate cytokine expression in T-lymphocytes. Previous work has demonstrated expression of T-Bet in human endometrium. Changes in human endometrial T-Bet and GATA-3 mRNA and protein expression during the normal menstrual cycle were characterized. Human endometrium from each phase of the menstrual cycle underwent real-time reverse-transcriptase polymerase chain reaction and immunohistochemistry to examine expression and localization. T-Bet and GATA-3 mRNA were increased in the late secretory phase. Progesterone receptor (PR) mRNA was increased during the proliferative and early secretory phases. T-Bet and GATA-3 proteins localized cytoplasmically in the late secretory phase. PR protein displayed nuclear localization and maximal immunostaining during the early secretory phase. T-Bet and GATA-3 are expressed in endometrial epithelium cyclically during the menstrual cycle. T-Bet and GATA-3 are both upregulated during the late secretory phase and in the same cell types. The expression patterns of T-Bet and GATA-3 oppose PR, suggesting antagonistic function and/or regulation between PR and T-Bet/GATA-3.
Keywords: T-Bet, GATA-3, progesterone receptor, human endometrium, reproductive mmunology.
Epithelial cells throughout the body function as a physical barrier to pathogen invasion. They also provide a chemical defense by recognizing and initiating immune responses to potential pathogens. Epithelial immune responses must be tightly regulated to prevent inappropriate immune responses to commensal or beneficial microbes.A major part of immune response by epithelia is the production and secretion of cytokines.
Endometrial epithelial cells are a rich source of cytokine production. Endometrial cytokines regulate local immune response but also act to regulate menstruation and embryo implantation.The regulation of cytokines in the endometrium is complex. This regulation must support immunological defense against pathogens, permit immunological tolerance to an invading semiallogeneic trophoblast, and enable the shedding and apoptosis that occurs during menstruation. However, the critical mechanisms regulating endometrial cytokine expression remain poorly understood.
In T-lymphocytes, 2 transcription factors—T-box expressed in T cell (T-Bet) and GATA-3—have a profound effect on the overall patterns of cytokine expression. For example, T-Bet is required for T cells to differentiate into a TH1 cytokine expression pattern (interleukin [IL]–2, IL-3, interferon γ, and tumor necrosis factor–α).1 In contrast, GATA-3 is required for T cells to differentiate into a TH2 cytokine expression pattern (IL-4, IL-5, IL-6, IL-10, and IL-13).2 The expression of TH1 and TH2 cytokines is usually mutually exclusive and generally thought to have opposing immunological function. It has been suggested that polarization of cytokine expression away from the generally proinflammatory TH1 response and toward the generally anti-inflammatory TH2 response is essential for pregnancy maintenance and is altered in recurrent miscarriage.3–12 Although there clearly is a bias toward TH2 cytokine expression during pregnancy, the relative roles of eliminating TH1 cytokine expression and promoting TH2 response is debated.3–12 Our recent finding of T-Bet expression in endometrial epithelium, a rich source of cytokine expression, suggests a possible role for T-Bet and GATA-3 in the regulation of endometrial cytokine expression and thus in pregnancy tolerance.13
Cytokines also play direct roles in the promotion of embryo implantation. For example, Stewart et al14 showed that female mice lacking the expression of the cytokine leukemia inhibitory factor (LIF) are completely unreceptive to the implantation of embryos. As might be expected of a factor critical for implantation, endometrial epithelial LIF expression is cyclic and regulated by reproductive steroid hormones.15 We recently demonstrated reproductive hormone regulation of T-Bet expression,13 echoing an earlier study showing effects of low-dose estrogen on T-Bet expression and/or activity in the brain.16 In vitro evidence also supports estradiol upregulation of GATA-3 and downregulation of T-Bet mRNA.17 We hypothesize that estrogen and progesterone regulation of T-Bet and GATA-3 would in turn regulate local cytokine expression, resulting in changes in endometrial function. We also hypothesize that T-Bet and GATA-3 would be subject to opposing cyclic regulation since expression of each directly inhibits expression of the other in T lymphocytes.18 Thus, we seek to characterize endometrial T-Bet and GATA-3 RNA and protein expression in each phase of the normal menstrual cycle using quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) and immunohistochemistry.
MATERIALS AND METHODS
Endometrial Specimen Collection and Preservation
Endometrial specimens were collected from healthy volunteers under a protocol approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. All women were between 19 and 34 years of age and were not taking any medications known to affect reproductive hormone production or action. Endometrial sampling was done using a Pipelle sampler. Samples were divided into portions and flash frozen in liquid N2 or placed in formalin. The formalin-fixed samples were embedded in paraffin and sectioned for immuno-histochemistry and staining with hematoxylin and eosin (H&E). The flash-frozen samples were used to generate RNA for real-time RT-PCR.
Cycle Day Identification
Two methods were used to determine the secretory phase day of each sample. Prior to biopsy, urinary luteinizing hormone (LH) surges were monitored by the subjects using home test kits. Postbiopsy, H&E-stained sample morphologies were blindly analyzed by a pathologist using microscopy.
RNA Isolation and Quantification
Endometrial total RNA was isolated from 21 frozen tissue samples using the RNAqueous-4PCR Kit (Ambion, Foster City, CA) and the manufacturer’s suggested conditions. RNA was quantitated using RiboGreen (Molecular Probes, Carlsbad,CA) with a ribosomal RNA standard curve. First-strand cDNA was synthesized from 100 ng of total RNA using the Roche 1st Strand cDNA Synthesis Kit for RT-PCR.
For T-Bet, each sample of cDNA was diluted 1:25 and plated in triplicate. Detection used the following primers and probe (Synthegen, Houston, TX): forward primer, 5′-AAGTTTA ATCAGCACCAGACAGAG-3′; reverse primer, 5′-GCCACAGTAAATGACAGGAATGG-3′; and probe, 5′-AACATCCGCCGTCCCTGCTTGGTG-3′. Primers and probes for cyclophilin (PPIA), progesterone receptor, and GATA-3 were obtained in a predesigned mix for each gene (Gene Expression Assays; Applied Biosystems,Foster City,CA).The efficiency of each primer–probe set was run on each analysis day and used serial dilutions of peripheral blood mononuclear cell cDNA. The total reaction volume for all real-time PCR experiments was 25 µL. All reactions were performed in 96-well plates on a Stratagene MX3000 device for 40 two-step cycles (95°C for 20 seconds and then 60°C for 1 minute) and used a commercially prepared master mix (ABI).
The efficiency of all PCR reactions ranged from 97% to 103%.The average cycle number at which the TaqMan fluorescence became detectable above the threshold (Ct) was 31.4 (T-Bet), 27.8 (GATA-3), 23.0 (progesterone receptor [PR]), and 19.6 (PPIA). Ct values were converted to relative expression using the ΔΔCt method, allowing normalization to both the housekeeping gene cyclophilin (PPIA) and a single sample in the early secretory phase (LH2) for T-Bet, GATA-3, and the late secretory phase for PR.
Tissue Preparation and Immunolocalization
Paraffin-embedded tissue samples (n = 8) were sectioned and mounted onto slides. Duplicate samples were subjected to antigen retrieval using boiling (100°C) citrate buffer (pH = 6.0) for two 5-minutes intervals. Following antigen retrieval, the slides were immersed in 0.3% H2O2 + methanol for 30 minutes to quench endogenous peroxidase activity. The T-Bet antibody (4B10-sc21749; Santa Cruz Biotechnology, Santa Cruz, CA), GATA-3 antibody (MAB260501, clone-291106; R&D Systems, Minneapolis, MN), PR antibody (A/B,VP-p977;Vector Laboratories, Burlingame, CA), ER (positive control) antibody (NCL-ER-6F11; Novocastra, Norwell, MA), and IgG isotype (negative control) antibody (MAB002; R&D Systems) were diluted 1:20, 1:200, 1:50, 1:40, and 1:50, respectively.The sections were incubated with primary antibodies overnight at 4°C. The secondary antibody, biotinylated goat antimouse IgG (BA-9200;Vector Laboratories) was diluted 1:100 and incubated for 1 hour at room temperature. The stain was then developed using an ABC kit (Vector Laboratories). The slides were rinsed and counterstained with 5% hematoxylin (DAKO, Glostrup, Denmark).
RESULTS
Healthy, regularly menstruating volunteers had an endometrial biopsy timed by daily urinary LH surge measurements. An aliquot was placed in formalin for later immunohistochemistry, and the remaining tissue was flash frozen in liquid nitrogen. RNA was extracted from frozen tissue, and relative expression of T-Bet and GATA-3 mRNA at each phase of the menstrual cycle was determined using real-time RT-PCR. The analysis of mRNA expression was based on 3 proliferative samples (cycle days 1–14), 6 early secretory samples (post–LH surge days 1–4), 9 midsecretory samples (post–LH surge days 5–9), and 3 late secretory samples (post–LH surge days 10–14). The relative expression of PR, which is expected to fall in the mid and late secretory phases,19 was used as a control, and PPIA was chosen as a housekeeping control gene because it varied only minimally in this sample set and in other endometrial sample sets in our experience (data not shown).
Formalin-fixed sections were stained with H&E, and histological dating was performed using the criteria of Noyes et al.20 LH surge and histological dating showed the same trends. However, if the sample was within 1 day of being in a different cycle phase, histological determination was used to allow reassignment to 1 day later or earlier and thus to the other cycle phase.
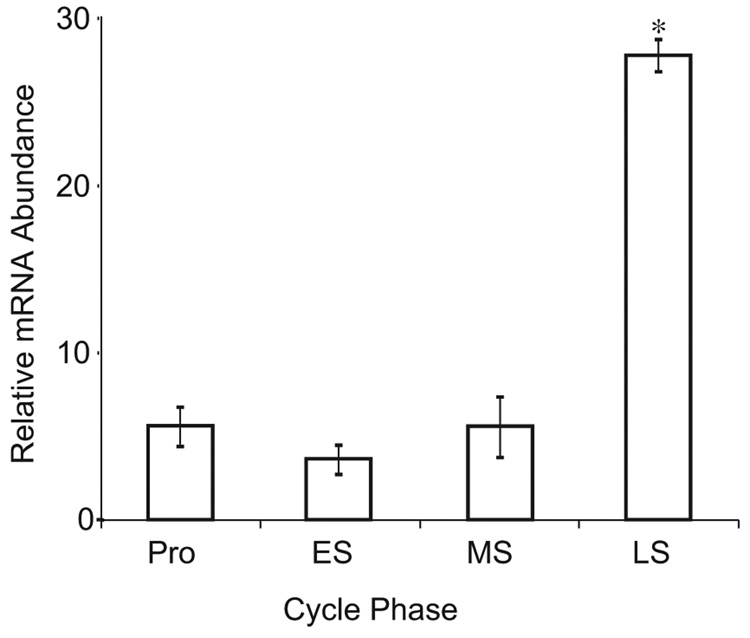
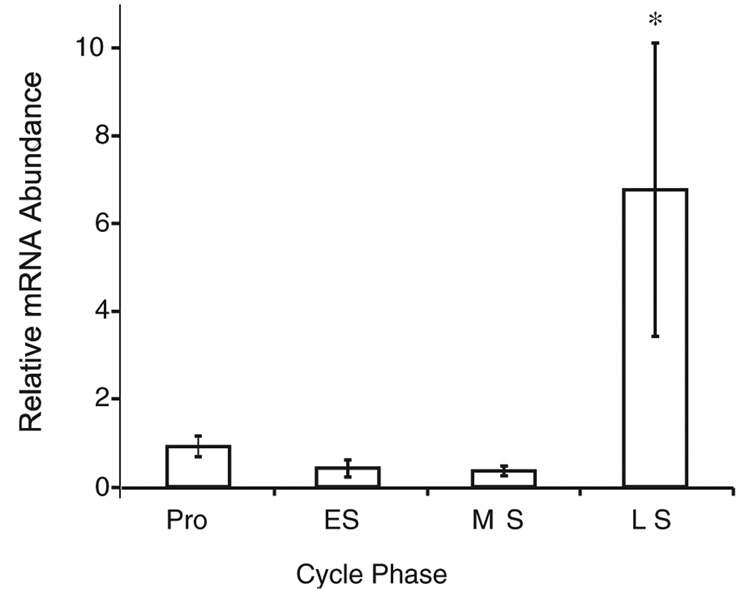
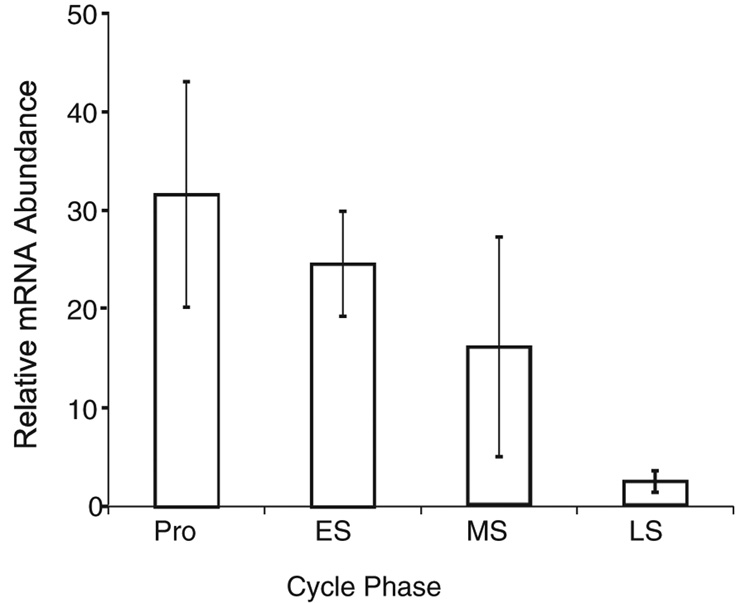
A 25-fold increase in T-Bet (P <.01; Figure 1) and a 6-fold increase in GATA-3 (P <.01; Figure 2) were observed during the late secretory as compared with the early secretory phase. The same samples demonstrated a 30-fold decrease in PR between the early and late secretory phases (Figure 3). Thus, a specific upregulation of mRNA for both T-Bet and GATA-3 is seen (Figure 1–Figure 3).
Figure 1.
Relative expression of T-Bet mRNA across the menstrual cycle. Relative mRNA expression was calculated after real-time reverse-transcriptase polymerase chain reaction using the ΔΔCt method, with cyclophilin as a housekeeping control gene. Data are expressed as the mean ± standard deviation of samples grouped by menstrual cycle phase. *P <.01 as compared with all other phases using 1-way analysis of variance with the Tukey post hoc test. Pro indicates proliferative; ES, early secretory; MS, midsecretory; LS, late secretory.
Figure 2.
Relative expression of GATA-3 mRNA across the menstrual cycle. Data generation, representation, and analysis are identical to that in Figure 1. Pro indicates proliferative; ES, early secretory; MS, midsecretory; LS, late secretory.
Figure 3.
Relative expression of progesterone receptor mRNA across the menstrual cycle. Data representation and analysis are identical to that in Figure 1. Pro indicates proliferative; ES, early secretory; MS, midsecretory; LS, late secretory.
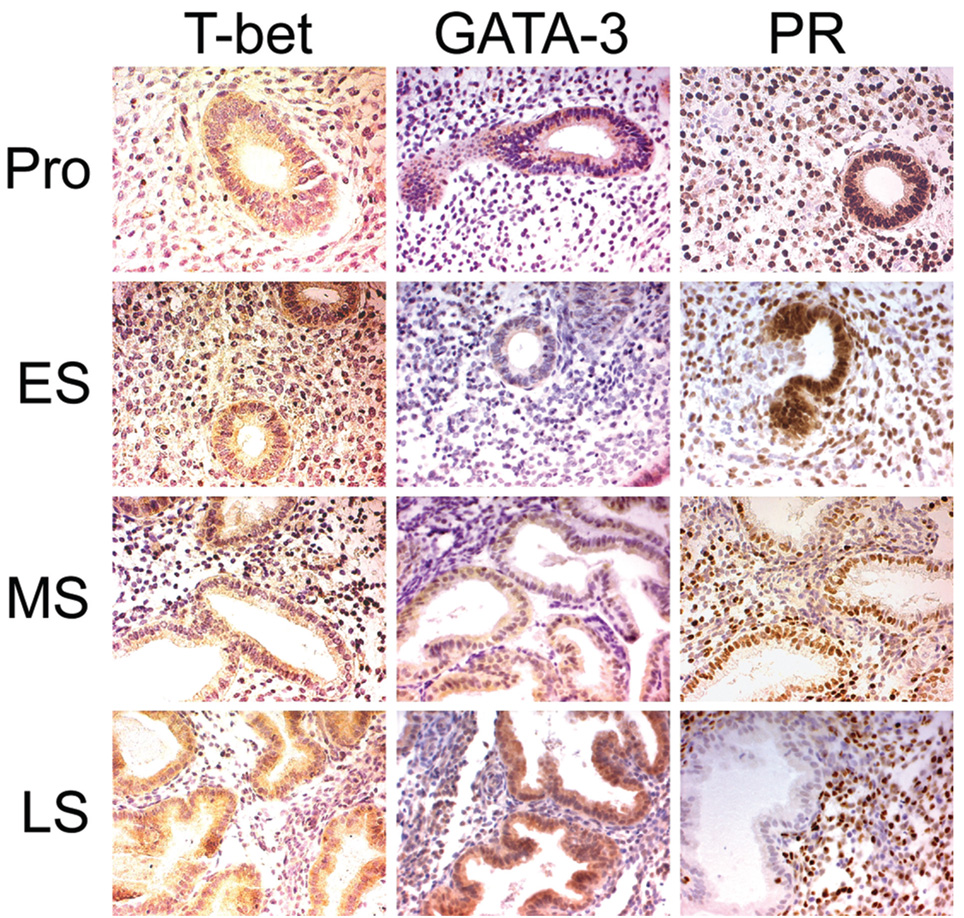
After identifying the changes in mRNA levels in whole endometrial tissue,we next sought to identify the cell types expressing T-Bet and GATA-3 protein and whether relative protein expression was predicted by mRNA levels. Immunohistochemical localization of T-Bet, GATA-3, and PR was evaluated at each phase of the menstrual cycle. Several samples from all phases of the menstrual cycle are represented in Figure 4.
Figure 4.
Immunohistochemistry. Proliferative (Pro), cycle day 5; early secretory (ES), luteinizing hormone (LH) day 1; midsecretory (MS), LH day 8; late secretory (LS), LH day 12. Magnification 20×
In proliferative samples, both T-Bet and GATA-3 proteins were detected and localized primarily to the cytoplasm of epithelial cells. In contrast, PR was detected in the nuclei of both the epithelial and stromal cells of proliferative samples. T-Bet was also found in a small number of strongly staining smaller cells, likely lymphocytes. In samples from the early secretory phase, GATA-3 and T-Bet staining was faint and not always detectable, but PR staining was prominent. A few stromal cells were darkly stained for T-Bet. Samples from the midsecretory phase (the implantation window) showed a somewhat reduced intensity of nuclear PR staining and increased cytoplasmic staining for T-Bet and GATA-3 in glandular epithelial cells. In the late secretory phase, PR protein was, as expected, undetectable in the glandular epithelium but clearly detectable in some stromal cells.19 Late secretory immunostaining for both T-Bet and GATA-3 protein expression was markedly elevated in the cytoplasm of the glandular epithelium. Thus, the intensity of immunohistochemical staining for T-Bet, GATA-3, and PR generally parallels the mRNA changes. Furthermore, the cell types expressing GATA-3 and T-Bet appear to be primarily endometrial epithelium, with a small number of stromal cells (lymphocytes) also positive for T-Bet staining, especially in the proliferative and early secretory phases.
DISCUSSION
Cytokines play key roles in reproductive, immune, and menstrual functions of human endometrium. This study was designed to measure the endometrial expression of 2 transcription factors, T-Bet and GATA-3, known to be critical regulators of cytokine expression in lymphocytes. We have demonstrated that T-Bet and GATA-3 mRNA and protein are cyclically expressed in human endometrium, predominantly in the epithelial cells. Furthermore, expression of both was shown to be maximal in the late secretory phase, and both proteins were primarily localized to the cytoplasm of endometrial epithelial cells.
The cyclical changes in T-Bet and GATA-3 expression strongly suggest regulation by reproductive steroid hormones. T-Bet promoter activity in an endometrial cell line has been previously shown to be stimulated by treatment with progesterone in vitro, in a time-dependent oscillation.13 In the same set of experiments, progesterone treatment also stimulated stat5 binding to the T-Bet promoter, possibly via an autocrine feedback loop involving IL-15 activation of stat5. Since endometrial IL-15 expression is increased in the late secretory phase by the action of progesterone on endometrial stromal cells,21 a paracrine effect of IL-15 may underlie the cyclic expression of T-Bet in the endometrial epithelium. Interestingly, endometrial stromal expression of IL-15 is also induced by co-culture with trophoblast,22 suggesting a mechanism for possible increased endometrial expression of T-Bet during embryo implantation.
Another potential mechanism of steroid action on T-Bet and GATA-3 expression is via the nuclear factor κB (NF-κB) pathway. Both T-Bet and GATA-3 expression can be stimulated by NF-κB.23–25 Furthermore, NF-κB activity can be inhibited by both estrogen and progesterone. 26–28 Since estrogen and progesterone and specific subtypes of the estrogen and progesterone receptors are low in the late luteal phase, falling estrogen and progesterone actions may disinhibit NF-κB, leading to increased T-Bet and GATA-3 expression.
In lymphocytes, T-Bet expression and GATA-3 expression are generally mutually exclusive, and the functions of these 2 transcription factors are often antagonistic. 1,18 Despite the antagonism, common factors have been identified that have similar effects on expression of both T-Bet and GATA-3. For instance, in classical immune cells, transforming growth factor–β (TGF-β) has been shown to inhibit both the expression of T-Bet29,30 and differentiation of TH1-type cells31,32 and the expression of GATA-3 and differentiation TH2-type cells.32 Thus, our finding that both GATA-3 and T-Bet are maximally expressed in endometrial epithelia during the late secretory phase, while unexpected, may reflect control through signaling pathways similar to that associated with TGF-β. Interestingly, several TGF-β isoforms are strong inhibitors of trophoblast outgrowth and proliferation.33,34 Although the reason for this double-barreled immune response in the late secretory phase involving both T-Bet and GATA-3 remains unclear, both factors can activate the expression of COX-2, IL-8, monocyte chemotactic protein, and IL-2, all of which play functional roles in immune cell migration, inflammation, and tissue breakdown during menstruation.
Another unexpected finding was that both T-Bet and GATA-3 proteins are immunolocalized to the cytoplasm of the glandular epithelium rather than the nucleus, where both transcription factors are thought to function. This cytoplasmic localization suggests either an alternative, non-transcriptional function or a requirement for activation prior to entering the nucleus. Mariani et al,35 in developing a model of cross-regulation of T-Bet and GATA-3 in T lymphocytes, proposed 3 possible mechanisms of inhibition: sequestration by a binding protein, repression of basal transcription, and repression of autoactivation. One recent study showed that GATA-3 nuclear translocation is dependent on its phosphorylation on serine residues by p38 MAPK36; however, the factors determining T-Bet nuclear translocation remain unreported.
T-Bet was also immunolocalized to the nucleus of a small number of stromal cells during the proliferative and early secretory phase.This expression pattern is in contrast to the cytoplasmic expression seen in the glandular cells. These cells are likely lymphocytes or uterine natural killer cells, both of which are known to express T-Bet.37
This article localizes T-Bet and GATA-3 to human endometrial epithelial cells histologically and supports previously published endometrial data.13 Expression of T-Bet and GATA-3 has been characterized in other epithelial tissues, including bronchi and bowel, and alterations in these epithelia-expressed transcription factors have been linked to human disease. GATA-3 is underexpressed while T-Bet is overexpressed in the gut mucosa of celiac disease patients.38 GATA-3 is upregulated in human bronchial and nasal mucosa in asthmatic subjects compared with controls.39 However, in each of these cases, the alterations in transcription factor expression patterns that result in functional impairment are presumed or demonstrated to be limited to resident lymphocytes, not epithelial cells. Lymphocyte expression may, in fact, represent only part of the pathogenesis.
Because of the prominent role of cytokines in implantation, menstruation, and endometriosis, it is tempting to speculate that T-Bet and GATA-3 play a role in endometrial function and dysfunction. However, T-Bet has not been linked to endometrial dysfunction in any article to date, nor has any mention of T-Bet appeared in the various microarray studies examining the physiology and patho-physiology of human endometrium.GATA-3 was shown to be upregulated in eutopic endometrial stromal cells selected by laser capture microdissection from patients with endometriosis.40 However, GATA-3 microarray data were not confirmed by an alternative method, and no other microarray data have mentioned GATA-3. Thus, it is likely that these cycle-regulated proteins have important effects on endometrial function, but their role remains speculative.
CONCLUSION
Our data demonstrate GATA-3 and T-Bet expression in human endometrium and suggest hormonal regulation of expression. Furthermore, we demonstrate a somewhat unexpected coexpression of these antagonistic transcription factors and unconventional tissue and subcellular localization. Further studies are required to understand the role of these transcription factors in the physiology and pathophysiology of human endometrium.
Acknowledgments
We gratefully acknowledge the financial support of the Holderness Foundation and the University of North Carolina Nova Carta Foundation. This research was also supported by the National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD035041 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. We also gratefully acknowledge the outstanding technical assistance of Lingwen Yuan, PhD, Xi Yang, and Gail Grossman.
Footnotes
REFERENCES
- 1.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation:new players and new insights. J Clin Immunol. 2003;23(3):147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res. 2003;28(1):25–37. doi: 10.1385/IR:28:1:25. [DOI] [PubMed] [Google Scholar]
- 3.Chaouat G, Zourbas S, Ostojic S, et al. A brief review of recent data on some cytokine expressions at the maternofoetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. 2002;53(1–2):241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 4.Haynes MK, Smith JB. Can Th1-like immune responses explain the immunopathology of recurrent spontaneous miscarriage? J Reprod Immunol. 1997;35(1):65–71. doi: 10.1016/s0165-0378(97)00036-3. [DOI] [PubMed] [Google Scholar]
- 5.Hill JA, III, Choi BC. Immunodystrophism: evidence for a novel alloimmune hypothesis for recurrent pregnancy loss involving Th1-type immunity to trophoblast. Semin Reprod Med. 2000;18(4):401–405. doi: 10.1055/s-2000-13730. [DOI] [PubMed] [Google Scholar]
- 6.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273(24):1933–1936. [PubMed] [Google Scholar]
- 7.Piccinni MP. T-cell cytokines in pregnancy. Am J Reprod Immunol. 2002;47(5):289–294. doi: 10.1034/j.1600-0897.2002.01104.x. [DOI] [PubMed] [Google Scholar]
- 8.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4(9):1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 9.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by hormone-controlled Th1- and Th2-type cytokines. Immunol Res. 1996;15(2):141–150. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 10.Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196(2):122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 11.von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6(7):627–634. doi: 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- 12.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 13.Kawana K, Kawana Y, Schust DJ. Female steroid hormones utilize Stat protein-mediated athways to modulate the expression of T-Bet in epithelial cells: a mechanism for local immune regulation in the human reproductive tract. Mol Endocrinol. 2005;19(8):2047–2059. doi: 10.1210/me.2004-0489. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 15.Hambartsoumian E, Taupin JL, Moreau JF, Frydman R, Chaouat G. In-vivo administration of progesterone inhibits the secretion of endometrial leukaemia inhibitory factor in vitro. Mol Hum Reprod. 1998;4(11):1039–1044. doi: 10.1093/molehr/4.11.1039. [DOI] [PubMed] [Google Scholar]
- 16.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 17.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor α (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17β-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175(9):5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 18.Chen GY, Osada H, Santamaria-Babi LF, Kannagi R. Interaction of GATA-3/T-Bet transcription factors regulates expression of sialyl Lewis X homing receptors on Th1/Th2 lymphocytes. Proc Natl Acad Sci U S A. 2006;103(45):16894–16899. doi: 10.1073/pnas.0607926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young L. Endometriosis in Clinical Practice. Normal Cycling Endometrium: Molecular, Cellular, and Histologic Perspectives. New York, NY: Taylor & Francis; 2004. [Google Scholar]
- 20.Noyes RW, Hertig AW, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1(1):3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Dunn CL, Critchley HO, Kelly RW. IL-15 regulation in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87(4):1898–1901. doi: 10.1210/jcem.87.4.8539. [DOI] [PubMed] [Google Scholar]
- 22.Popovici RM, Betzler NK, Krause MS, et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147(12):5662–5675. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- 23.Corn RA, Aronica MA, Zhang F, et al. T cell-intrinsic requirement for NF-κ B induction in postdifferentiation IFN-γ production and clonal expansion in a Th1 response. J Immunol. 2003;171(4):1816–1824. doi: 10.4049/jimmunol.171.4.1816. [DOI] [PubMed] [Google Scholar]
- 24.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-κ B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2(1):45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 25.McCracken SA, Hadfield K, Rahimi Z, Gallery ED, Morris JM. NF-κB-regulated suppression of T-Bet in T cells represses Th1 immune responses in pregnancy. Eur J Immunol. 2007;37(5):1386–1396. doi: 10.1002/eji.200636322. [DOI] [PubMed] [Google Scholar]
- 26.Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-κB signaling. Sci STKE. 2005;2005(288):pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 27.Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121(1):3–19. doi: 10.1530/rep.0.1210003. [DOI] [PubMed] [Google Scholar]
- 28.King AE, Critchley HO, Kelly RW. The NF-κB pathway in human endometrium and first trimester decidua. Mol Hum Reprod. 2001;7(2):175–183. doi: 10.1093/molehr/7.2.175. [DOI] [PubMed] [Google Scholar]
- 29.Lin JT, Martin SL, Xia L, Gorham JD. TGF-β 1 uses distinct mechanisms to inhibit IFN-γ expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-Bet. J Immunol. 2005;174(10):5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 30.Park IK, Letterio JJ, Gorham JD. TGF-beta1 inhibition of IFN-γ-induced signaling and Th1 gene expression in CD4(+) T cells is Smad3 independent but MAP kinase dependent. Mol Immunol. 2007;44(13):3283–3290. doi: 10.1016/j.molimm.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195(11):1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-βinhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165(9):4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 33.Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138(11):4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- 34.Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-βsuperfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132(2):217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- 35.Mariani L, Löhning M, Radbruch A, Höfer T. Transcriptional control networks of cell differentiation: insights from helper T lymphocytes. Prog Biophys Mol Biol. 2004;86(1):45–76. doi: 10.1016/j.pbiomolbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Maneechotesuwan K, Xin Y, Ito K, et al. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178(4):2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 37.Tayade C, Fang Y, Black GP, V AP, Jr, Erlebacher A, Croy BA. Differential transcription of Eomes and T-Bet during maturation of mouse uterine natural killer cells. J Leukoc Biol. 2005;78(6):1347–1355. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- 38.Monteleone I, Monteleone G, Del Vecchio Blanco G, et al. Regulation of the T helper cell type 1 transcription factor T-Bet in coeliac disease mucosa. Gut. 2004;53(8):1090–1095. doi: 10.1136/gut.2003.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura Y, Hoshino M. TH2 cytokines and associated transcription factors as therapeutic targets in asthma. Curr Drug Targets Inflamm Allergy. 2005;4(2):267–270. doi: 10.2174/1568010053586273. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki S, Canis M, Vaurs-Barrière C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril. 2005;84 suppl 2:1180–1190. doi: 10.1016/j.fertnstert.2005.04.041. [DOI] [PubMed] [Google Scholar]