Abstract
The establishment and persistence of many chronic infections have been demonstrated to depend on restraint of the vigor of the anti-microbial immune responses by CD4+CD25+ regulatory T (Treg) cells. In HIV-infected individuals, Treg cells suppress both HIV-specific and general CD4+ and CD8+ T cell responses. Increases of CD4+CD25+ Treg cell function during viral infections might be mediated by host-derived pro-inflammatory molecules or directly by viral infection or binding. We examined the effect HIV has upon binding to CD4+CD25+ Treg cells by exposing human purified CD4+CD25+ T cells from healthy donors to HIV-1 in vitro and assessing their Treg-associated functional marker profile and suppressive activities. We found that HIV-1 binding increased their suppressor activities by 2- to 5-fold, which was accompanied by enhanced expression of Treg-associated functional markers sCTLA-4, glucocorticoid-induced tumor necrosis factor receptor and FoxP3. Moreover, HIV-1 binding extended the survival of CD4+CD25+ Treg cells and up-regulated the expression of homing receptors CD62L and integrin α4β7, which in turn would result in Treg cells migrating more rapidly to the peripheral lymph nodes and mucosal lymphoid tissues where anti-HIV immune responses are occurring. Importantly, CD4+CD25+ Treg cells exposed to HIV were not susceptible to homing-induced apoptosis like are other resting CD4+ cells following HIV-1 binding. We show that CD4+CD25+ Treg cells respond directly to HIV-1 itself through HIV gp120 interactions with CD4 molecules. Collectively, our findings explain a mechanism that contributes to the abnormal accumulation of intensified Treg cells in lymphoid and mucosal tissues in HIV patients, resulting in impairment of immune responses which would greatly help HIV persistence.
Keywords: CD4+CD25+ regulatory T cells, HIV-1, homing, suppressive function
Introduction
Persistent HIV infection is characterized by a slow, progressive depletion of CD4+ T cells and increasing immune dysfunction (1–4). Persistence of HIV is associated with impaired anti-HIV immune responses, as well as many other factors. HIV-specific immune responses become impaired early after infection, even before the loss of responses directed against other antigens (5, 6). HIV-specific CD4+ T cell proliferation is undetectable soon after primary infection (7). Decreased cytolytic function, inappropriate maturation and limited proliferation have been associated with the inability of CD8+ T cells to control chronic viral replication, although HIV-specific CD8+ T cell responses play an important role in limiting acute viral spread (8). The molecular basis for such impaired responses has not been elucidated fully, and it is likely that multiple mechanisms, including both HIV-specific and non-specific, are involved (9–11). In this regard, the roles of host-mediated immunosuppressive mechanisms activated in the face of persistent HIV replication have not been well delineated.
CD4+CD25+ regulatory T (Treg) cells, a subset of CD4+ lymphocytes with constitutive immunosuppressive activity, were first described in the context of autoimmune disorders (12, 13). Treg cells have been grossly grouped into natural and adaptive, based on their development, antigenic specificity, mechanisms of action and dependence on TCR and co-stimulatory signaling (14, 15). CD4+CD25+ Treg cells are capable of recognizing self-antigens in autoimmune disorders and non-self-antigens in infectious diseases and restrain inappropriate or excessive immune activation (16, 17). Phenotypically, CD4+CD25+ Treg cells have been characterized as expressing CD25 (12), CTLA-4 (18), glucocorticoid-induced tumor necrosis factor receptor (GITR) (19), CD86 (20) and the most specific marker identified so far, FoxP3 (a transcription factor) (21). They also harbor high levels of cyclic adenosine monophosphate (cAMP) (22). However, it is less clear what the precise roles are of these molecules in CD4+CD25+ Treg cell function, although cAMP was reported as a key component of Treg-mediated suppression (17, 22). The establishment and persistence of many chronic infections have been recently demonstrated to depend on restraint of the vigor of the anti-microbial immune responses by CD4+CD25+ Treg cells (23–25). Similarly, CD4+CD25+ Treg cells have been implicated in potential suppression of HIV-specific CD4+ and CD8+ T cell responses in HIV-infected individuals (26–28). In addition, chronic HIV infection changes CD4+CD25+ Treg cell tissue distribution with a marked increase of CD4+CD25+ Treg cells in peripheral lymph nodes (LNs) and mucosal lymphoid tissues, where most HIV replication occurs (29–31). Taken together, these studies suggest that CD4+CD25+ Treg cells could play a major role in the induction/maintenance of an environment that would favor HIV survival and persistence by impairing protective immune responses (26–29).
CD4+CD25+ Treg cells may themselves be infected by HIV because they express HIV's primary receptor CD4, as well as its co-receptors for entry: CXCR4/CCR5. Studies by Oswald-Richter et al. (32) showed that both natural CD4+CD25+ Treg cells and conventional CD4+ T cells transduced with FoxP3 to generate functional Treg cells are easily infected by HIV. However, it is not clear whether such HIV-infected CD4+CD25+ Treg cells are killed by virus replication or remain effective as regulators. Nilsson et al. (30) and Epple et al. (31) observed that the numbers of FoxP3+ T cells increased in LNs and duodenal mucosa of HIV+ subjects and there was increased expression of Treg-associated functional markers in total CD4+ cells, respectively. In addition, it was reported that the activity of Treg cells from LNs was better in comparison to peripheral blood (33). However, the mechanisms underlying the accumulation and altered activity of Treg in LNs and mucosal lymphoid tissues (e.g. distribution altered, new Treg cells induced or existing Treg cells become long lived or expanded) are not clear. The present study presents evidence that HIV direct binding to CD4+CD25+ Treg cells enhances their function, survival abilities and their rates of homing to peripheral LNs and mucosal lymphoid tissues, and thereby explains the accumulation of Treg cells in lymphoid tissues that are intensified in their function in HIV-infected subjects.
Materials and methods
Mice
Female scid mice (allele: Prkdcscid) were purchased from the Jackson Laboratories and housed under specific pathogen-free conditions and used for experiment at 6 weeks of age according to protocols approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX, USA).
Flow cytometry
Phenotypes of T cells were analyzed on a FACS Scan instrument (BD Bioscience, San Diego, CA, USA). FITC- or PE-conjugated mAbs specific for CD25, CD69, CD38, CD44, CD62L, CLA, CD49d (α4, GeneTex, Inc., San Antonio, TX, USA), β7, GITR, surface CTLA-4 (sCTLA-4), Bcl-2, Bcl-x, FoxP3 (Clone: hFOXY or PCH101, eBioscience, San Diego, CA, USA) and TRAIL were used. FITC- or PE-labeled mouse IgG1, IgG2a and IgG2b isotype controls were applied as needed. Unless specified, all mAbs were obtained from BD Biosciences.
Purification of CD4+CD25+ T cells
Blood was obtained from HIV-negative healthy human volunteers or from buffy coats (Gulf Coast Regional Blood Center, Houston, TX, USA). PBMC were isolated from heparinized venous blood by centrifugation through Lymphocyte Separation Medium (Mediatech Inc., Herndon, VA, USA) and washed twice with PBS. Conventional CD4+ T cells were purified from PBMC by negative selection using magnetic beads (Dynal, Lake Success, NY, USA). Briefly, PBMC were incubated with appropriate amounts of antibody cocktail (anti-CD14, anti-CD8, anti-CD19, anti-CD56 and anti-glycophorin A) for 30 min on ice. After incubation with goat anti-mouse IgG (GAM)-coated beads for 30 min at room temperature with gentle shaking, the cells were recovered after the magnetic bead-positive cells were removed using a magnetic separator. Subsequently, CD4+CD25+ T sub-populations were obtained by positive selection using anti-CD25-coupled magnetic beads (Dynal). CD4+CD25− T sub-populations were obtained by depletion of CD25+ cells from conventional CD4+ T cells using anti-CD25-coupled magnetic beads. CD25+ cells in CD4+CD25+ and CD4+CD25− T sub-populations were 90–95% and 0.5–1%, respectively, as analyzed by flow cytometry. Cells positive for CCR5 and CXCR4 in CD4+CD25+ T sub-populations were >52 and 75%, respectively. In homing assays, CD4+CD25+ T cells were expanded in vitro as described (34) and displayed the same phenotype and suppressive activities (data not shown).
HIV-1 culture and cell exposure
X4 virus HIV-1213, previously isolated from PBMC of an infected individual, biologically cloned as described (35), was propagated in CEM cells. R5 virus HIV-1BaL was propagated in VB cells. Virus was collected following acute infection when cells were ∼100% HIV antigen positive and concentrated by ultracentrifugation and re-suspended in RPMI 1640 cell culture medium (Gibco, Carlsbad, CA, USA) supplemented with 10% human AB serum, penicillin–streptomycin, HEPES buffer and L-glutamine. Uninfected CEM or VB culture supernatants were collected with or without concentration as mock controls. All the HIV-1 preparations were tested and found free of bacterial endotoxin.
CD4+CD25− or CD4+CD25+ T cells were exposed overnight to HIV-1213 or HIV-1BaL at a multiplicity of infection (MOI) equal to 1 in the presence of recombinant IL-2 (10 U ml−1). They were then washed and further cultured for indicated experiments unless specified. Recombinant IL-2 was required in the culture of CD4+CD25+ T cells since they would lose their suppressive ability if cultured in vitro for >3 days in the absence of IL-2 (data not shown). In some experiments, HIV-1213 and HIV-1BaL were UV inactivated before infection as described (35). UV-inactivated virus was tested for its ability to productively infect cells and was found to be unable to replicate. CD4+CD25− or CD4+CD25+ T cells exposed to UV-inactivated HIV were also checked by PCR for HIV-1 gag region and p24 ELISA (Beckman Coulter Inc., Fullerton, CA, USA) as described (36).
Cross-linking (XL) of CD4 (XLCD4), CD62L (XLCD62L) or α4β7 (XLα4β7)
Cells were incubated with mouse anti-human CD4 mAb (clone: RPA-T4), mouse anti-human CD62L (clone: Dreg56) and mouse anti-human α4 mAb (clone: 9F10) plus rat anti-human β7 mAb (clone: FIB504) (2 μg 10−6 cells) for 30 min on ice, respectively. After three washes, cells were cultured in 96-well plates coated with GAM or GAM plus goat anti-rat IgG (Sigma, St Louis, MO, USA). For the coating of GAM, or GAM plus goat anti-rat IgG to the plates, 50 μl of antibodies in coating buffer (50 μg ml−1 in 30 mmol l−1 NaHCO3, 15 mmol l−1 Na2CO3, pH 9.6) were added to each well, incubated at 4°C overnight and washed five times with PBS. XLCD4 was also performed by plate-coated native gp160 from HIV-1213 as described (36). HIV-1213 gp160 was purified using affinity chromatography from SF-9 cells infected with gp160-expressing baculovirus by Bio-Molecular Tech., Inc. (Frederick, MD, USA).
Assessment of cell death and survival
Cells were cultured for 3 days in medium only or in the presence of recombinant IL-2 with or without anti-CD3 antibody. Cells were harvested and viable cells were counted by Trypan Blue exclusion in triplicate samples. Results were expressed as percentage of recovered viable cells at day 3 relative to day 0. In some experiments, cells undergoing apoptosis were detected by annexin V-PE staining, and necrotic cells were determined by 7-aminoactinomycin D (7-AAD) staining according to protocols provided by BD Biosciences. Apoptotic cells were gated on annexin V+ 7-AAD cells.
Proliferation assays and IL-2 ELISA
CD4+CD25− T cells (5 × 104) and autologous, mitomycin C-treated (50 μg ml−1, 30 min), T-depleted PBMC (5 × 105) were cultured in the absence or presence of increasing numbers of CD4+CD25+ T cells for 5 days in 96-well, flat-bottom plates. Anti-CD3 (1 μg ml−1) was added to the culture for stimulation in the presence or absence of anti-IL-10 (25 μg ml−1; BD Biosciences) or anti-transforming growth factor (TGF)-β (25 μg ml−1; R&D Systems). In some experiments, CD4+CD25– T cells were pre-stimulated with plate-coated anti-CD3 (2 μg ml−1) plus anti-CD28 (1 μg ml−1) for 1 day, washed and then co-cultured with CD4+CD25+ T cells or CD4+CD25− T cells (HIV or mock-exposed) at various ratios for 5 days. A total of 1 μCi [3H]thymidine was added 18 h before harvest and incorporated radioactivity was determined on a beta emission reader. The percent of inhibition was calculated as: [counts per minute (c.p.m.) in CD4+CD25− T cell culture − c.p.m. in CD4+CD25− and CD4+CD25+ T cell co-culture]/c.p.m. in CD4+CD25− T cell culture. Supernatants were collected from the cultures of CD4+CD25+ and/or CD4+CD25− T cells at 24 h. The levels of IL-2 in the supernatants were determined by specific ELISA using paired mAbs for IL-2 along with the appropriate cytokine controls.
Determination of FoxP3 expression
Total RNA was extracted from cells using RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's manual. Both cDNA synthesis and PCR amplification were performed using SuperScriptTM III One-step reverse transcription (RT)–PCR kit (Invitrogen, Carlsbad, CA, USA) and gene-specific primers as following:
FoxP3: 5′- GAAACAGCACATTCCCAGAGTTC-3′ and 5′-ATGGCCCAGCGG ATGAG-3′, GAPDH: 5′-CCACATCGCTCAGACACCAT-3′ and 5′-GGCAACAAT ATCCACTTTACCAGAGT-3′. The quantity of mRNA was determined based on the signal densities as determined with an AlphaImager 2200 optical densitometer (Alpha Innotech Corporation, San Leandro, CA, USA). Sample loading was normalized to the amount of the housekeeping gene coding for GAPDH.
Homing assay
The in vitro expanded CD4+CD25+ Treg cells were labeled with 51Cr as described previously (37). Then, these cells were transferred intravenously into scid mice (1 × 107 cells per mouse). Three and 15 h post-transfer, mice were sacrificed and superficial inguinal LNs, GALT and spleen were collected. Radioactivity was determined on a gamma counter (Beckman Coulter, Inc.).
Statistical analysis
Differences between experimental groups were examined using the Student's t-test. A difference in mean values was considered significant when the P value was <0.05 or very significant when the P value was <0.01.
Results
Resting CD4+CD25+ T cells exposed to HIV-1 resulted in abortive infection
Previous studies have shown that resting CD4+ lymphocytes exposed to HIV resulted in abortive infection, in which the virus binds, enters and partially or completely reverse transcribes proviral DNA, but this DNA does not integrate. Rather, HIV replicates in activated T cells, leading to production of progeny viruses (productive infection) (38–40). It has been reported that HIV can replicate in activated CD4+CD25+ Treg cells (activated via TCR signals) (32, 41). Therefore, we asked whether HIV can replicate in resting CD4+CD25+ T cells. Table 1 showed that supernatant from resting CD4+CD25+ T cells exposed to either HIV-1213 or HIV-1BaL scored undetectable in p24 ELISA for up to 5 days, indicating HIV-1 cannot productively infect non-stimulated CD4+CD25+ Treg cells. Thus, any effects on the cells by exposure to HIV must be due to the virus binding to its receptors.
Table 1.
Levels of HIV-1 replication in CD4+CD25+ cells
| CD4+CD25+ | CD4−CD25+ | H-9/VB | |
| 3 day post-exposure | |||
| Mock | - | - | - |
| HIV-1213 | - | - | 227 ± 31 |
| HIV-1BaL | - | - | 192 ± 24 |
| 5 day post-exposure | |||
| Mock | - | - | - |
| HIV-1213 | - | - | 897 ± 98 |
| HIV-1BaL | - | - | 792 ± 85 |
CD4+CD25+or CD4+CD25− cells were exposed to HIV-1213 or HIV-1BaL at a MOI equal to 1 as described in Methods. Cell lines H-9 and VB cells were exposed to HIV-1213 or HIV-1BaL (MOI: 1), respectively. Supernatants from the cell cultures were collected at day 3 and day 5 and the level of HIV p24 (ng ml−1) was measured by ELISA. The sensitivity of HIV p24 ELISA was 8 pg ml−1 (‘-’ indicates p24-undetectable). Results represent the mean ± SD of three independent assessments.
HIV-1 exposure enhances the expression of Treg-associated functional markers but not regular T-cell activation markers in CD4+CD25+ T cells
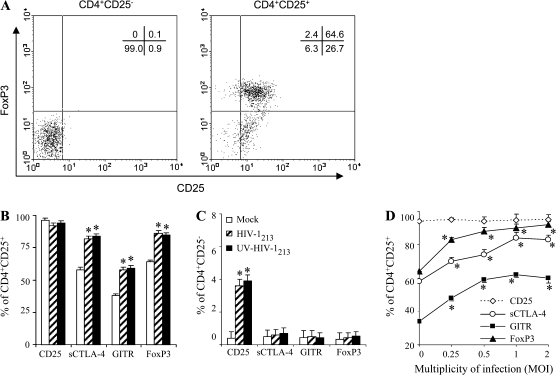
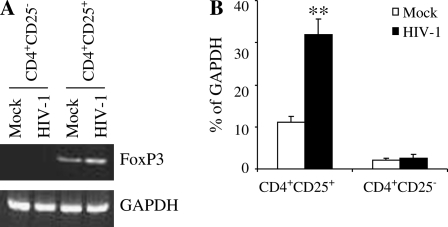
Studies of lymphocytes of HIV-infected subjects have shown an increase in the frequencies of cells expressing certain activation markers, leading to the concept that HIV causes ‘global activation’ of the immune system (42, 43). Some of this is certainly due to HIV antigen activation of T cells via their TCR, but some of it appears atypical with certain other activation markers not appropriately expressed (e.g. CD26). It has been shown that HIV binding, per se, up-regulates some of these markers and it is due to signals induced through CD4 molecules (36, 44, 45). Furthermore, HIV binding to CD4 lymphocytes, most of which are resting and not permissive for virus replication (i.e. abortive infection), occurs at a much greater frequency than the frequency of productive infection. Thus, to investigate whether HIV-1 binding directly affects CD4+CD25+ Treg cell function, we performed flow cytometric analyses of Treg-associated functional markers and regular T-cell activation markers in CD4+CD25+ T cells after exposure to either infectious or UV-inactivated HIV-1213 in vitro. Increased numbers of cells expressing sCTLA-4, GITR and FoxP3 in HIV-1213- or UV-inactivated HIV-1213-exposed CD4+CD25+ T cells were observed as compared with mock controls (Fig. 1A). HIV-1213 exposure significantly enhanced the expression of Treg-associated functional markers even at MOI equal to 0.25 (Fig. 1D), although the effect was more pronounced at higher MOIs. The expressions of sCTLA4, GITR and FoxP3 were barely detected in CD4+CD25− T cells exposed to either HIV-1 or mock control (Fig. 1C). Interestingly, HIV-1 exposure induced increased levels of CD25 expression on CD4+CD25− T cells (Fig. 1C) although the mean fluorescence intensity (MFI) is much lower than that of CD4+CD25+ T cells (CD4+CD25− T cells: MFI: 22 ± 3 versus CD4+CD25+ T cells: MFI: 55 ± 4, P < 0.01). In contrast, comparable expression of CD25 was found on HIV-1-exposed CD4+CD25+ T cells and mock control (Fig. 1B). As shown in Table 2, CD4+CD25+ T cells expressed extremely low levels of the activation markers CD38, HLA-DR or CD69, and HIV-1 exposure had no effect on the expression of these molecules on these cells. However, HIV-1-exposed CD4+CD25− T cells expressed higher levels of CD38 and HLA-DR as compared with those of mock controls on day 3 (Table 2). HIV-1-exposed CD4+CD25− T cells also displayed transient increases of CD69 expression on day 1 (Table 2), which returned to normal levels by day 2–3 (data not shown). As shown in Fig. 2(A and B), RT–PCR data confirmed that HIV-1 exposure significantly enhanced the expression of FoxP3 mRNA in CD4+CD25+ T cells (P < 0.01), although FoxP3 mRNA was not detectable in CD4+CD25− T cells either exposed to HIV-1 or not.
Fig. 1.
Expression of Treg-associated functional makers in CD4+CD25+ T cells after HIV-1 exposure in vitro. CD4+CD25+ or CD4+CD25− T cells were purified as described in Methods and stained for CD25 and FoxP3. Data are representative of many purifications (A). CD4+CD25+ or CD4+CD25− T cells were exposed to HIV-1213 (UV-inactivated or not) or mock controls as described in Methods. Three days later, CD4+CD25+ (B) or CD4+CD25− T cells (C) were harvested and conducted surface and intracellular staining for various molecules. Data are the mean ± SD percent of CD4+CD25+ or CD4+CD25− T cells positive for the indicated molecules. Representative analyses out of three independent assessments are shown. Asterisks indicate a statistically significant difference (*P < 0.05) as compared with the mock controls. A dose–response curve is shown for the expression of Treg-associated molecules in CD4+CD25+ T cells after exposure to various concentrations of HIV-1213 (MOI from 0.25 to 2) (D).
Table 2.
The effect of HIV-1 binding on expression of activation markers on CD4+CD25+ cells
| CD4+CD25+ |
CD4+CD25− |
|||
| Mock | HIV-1 | Mock | HIV-1 | |
| CD69 | 1.1 ± 0.4 | 1.2 ± 0.5 | 0.5 ± 0.4 | 9.4 ± 1.1* |
| CD38 | 1.8 ± 0.6 | 2.1 ± 0.6 | 0.4 ± 0.4 | 34.3 ± 2.5** |
| HLA-DR | 1.3 ± 0.6 | 1.5 ± 0.5 | 0.6 ± 0.4 | 5.4 ± 0.4* |
CD4+CD25+ or CD4+CD25− cells were exposed to HIV-1213 or mock controls as described in Methods. Cells were harvested at 1 day (CD69) or three days (CD38 and HLA-DR) and stained for the indicated surface activation markers. The percentage of positive cells is shown. Results represent the mean ± SD of three independent assessments. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01) as compared with the mock controls.
Fig. 2.
FoxP3 mRNA expression in CD4+CD25+ T cells upon HIV-1 exposure in vitro. Total RNA was extracted from CD4+CD25+ and CD4+CD25− T cells, respectively, and RT–PCR amplification was performed as described in Methods. Results of a representative assay are shown (A). Quantities of FoxP3 mRNA detected was normalized and expressed relative to the amount of GAPDH and representative analysis out of three independent assessments is shown (B). Asterisks indicate a statistically significant difference (**P < 0.01) compared with any other controls.
HIV-1 exposure enhances survival of CD4+CD25+ T cells
Human anergic/suppressive CD4+CD25+ Treg cells are thought to be a highly differentiated and an apoptosis-prone population (46). Therefore, we asked whether HIV-1 infection would impact the survival of CD4+CD25+ T cells. Fig. 3(A and B) showed that after 3 days of culture, there was not a significantly lower recovery of live cells in HIV-1-exposed CD4+CD25+ T cells as compared with mock controls. In addition, HIV-1-exposed CD4+CD25+ T cells did not display significant activation-induced apoptosis (data not shown). Rather, we found that in the presence of IL-2, HIV-1 exposure increased live-cell recovery significantly, regardless of the presence of anti-CD3 mAb. Of note, CD4+CD25+ T cells did not proliferate after HIV-1 exposure, even in the presence of anti-CD3 mAb. Under the same conditions, IL-2 supplement resulted in vigorous proliferation of CD4+CD25+ T cells; however, there was no difference between HIV-1 exposure and mock controls after adding anti-CD3 and IL-2 (Fig. 3C). These results suggest that HIV-1 exposure increases the survival of CD4+CD25+ T cells, but not through stimulating proliferation. However, in the presence of IL-2, HIV-1 exposure did not significantly alter live-cell recovery of CD4+CD25− T cells (Fig. 3D) but did significantly decreased the recovery of live CD4+CD25− T cells after anti-CD3 stimulation (Fig. 3E). Decreased survival of CD4+CD25− T cells was associated with their proliferation stimulated by the anti-CD3 and IL-2 (Fig. 3F). Furthermore, flow cytometric analyses showed that IL-2 supplement increased Bcl-2 expression (IL-2: MFI = 168 ± 13; control: MFI = 105 ± 9, P < 0.05), and HIV-1 exposure significantly enhanced Bcl-2 expression in the presence of IL-2. These results suggest that HIV-1 enhances the survival of CD4+CD25+ T cells by synergizing with IL-2 to up-regulate the anti-apoptotic molecule Bcl-2. However, HIV-1 exposure did not show any impact on the expression of Bcl-x and TRAIL (Fig. 3G).
Fig. 3.
Effect of HIV-1 exposure CD4+CD25+ T cells on cell recovery, proliferation and expression of anti-apoptotic molecules. Purified CD4+CD25+ (A and B) or CD4+CD25− T cells (D and E) were exposed to HIV-1 or mock control overnight, washed and cultured for an additional 3 days in medium only or in medium supplemented with recombinant IL-2 (10 U ml−1) in the presence of anti-CD3 (1 μg ml−1) or not. Cells were harvested and viable cells were counted by Trypan Blue exclusion in triplicate samples. Results are expressed as percentage of recovered viable cells on day 3 relative to day 0. Proliferation of CD4+CD25+ (C) or CD4+CD25− cells (F) was determined by an 18 h [3H]thymidine incorporation assay from 3-day culture in the presence of anti-CD3 (solid bar) or not (open bar). (G) HIV-1-exposed (solid lines) or mock-treated CD4+CD25+ cells (dashed lines) from cultures with or without recombinant IL-2 were stained for intracellular Bcl-2, Bcl-x or surface TRAIL. Histograms correspond to flow cytometric analyses for the indicated molecules. Representative analyses out of three independent assessments are shown.
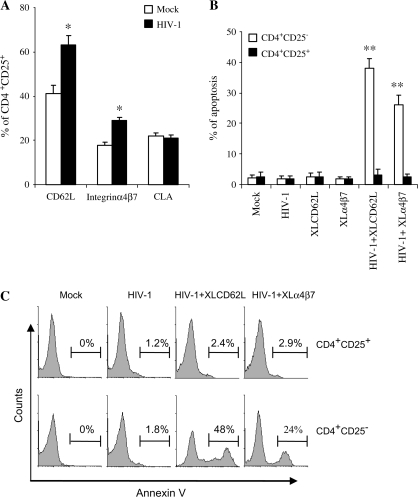
HIV-1 exposure enhances homing of CD4+CD25+ T cells in vivo but confers resistance to apoptosis induced by signals through homing receptors in vitro
We previously showed that HIV-1 binding to resting CD4+CD25− T cells induces expression of the homing receptor CD62L, enhances their homing to the peripheral LNs and that subsequent cross-linking of the homing receptors resulted in Fas/Fas ligand-mediated apoptosis (47–49). To test whether HIV-1 exposure enhances the homing of CD4+CD25+ T cells, we measured the expression of homing receptors CD62L and α4β7 on the HIV-exposed CD4+CD25+ T cells and analyzed their homing activities in vivo. As shown in Fig. 4(A), HIV-1 binding increased the expression of homing receptors CD62L and α4β7, but not that of CLA (Fig. 4A). Furthermore, HIV-1 exposure enhanced the homing of CD4+CD25+ T cells to peripheral LNs (at 3 and 15 h) and to mucosal LNs and tissues (at 15 h) in vivo (Table 3). In this study, we also found that cross-linking integrin α4β7 on HIV-1-exposed regular CD4+CD25− T cells in vitro induced apoptosis (Fig. 4B and C). However, cross-linking of the homing receptors CD62L or integrin α4β7 on HIV-1-exposed CD4+CD25+ T cells in vitro did not induce such apoptosis (Fig. 4B and C). Thus, if homing-induced apoptosis of HIV-signaled normal resting CD4+ T lymphocytes is an important mechanism of depletion (47–49), then that HIV-exposed Treg cells may not die after homing would clearly exacerbate the immune dysfunction occurring in HIV-infected people.
Fig. 4.
HIV-1 exposure enhanced the homing of CD4+CD25+ cells but cross-linking CD62L (XLCD62L) or α4β7 (XLα4β7) failed to induce HIV-1-exposed CD4+CD25+ cells to undergo apoptosis. (A) CD4+CD25+ cells were exposed to HIV-1213 or mock controls as described in Methods. Three days later, CD4+CD25+ were harvested and expression of homing receptors CD62L and α4β7 was analyzed by flow cytometry. Representative analyses out of three independent assessments are shown. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01) as compared with the mock controls. (B) CD4+CD25− T cells or CD4+CD25+ cells were exposed to HIV-1213 (MOI: 1) overnight and cross-linking of CD62L or XLα4β7 was performed. Three days later, cells were harvested and stained for annexin V and 7-AAD. Apoptotic cells (excluding necrotic cells) after XLCD62L were gated on the annexin V+ and 7-AAD− cells. Apoptotic cells (excluding necrotic cells) after XLα4β7 were gated on the annexin V+, 7-AAD− and CD62L− cells. The basal levels of necrotic cells, ∼5%, were not altered by exposure to HIV or/and XLCD62L or XLα4β7. Data are shown as the % of cells in apoptosis. Results represent the mean ± SD of three experiments. Asterisks indicate a statistically significant difference (**P < 0.01) compared with controls. (C) Representative histograms correspond to flow cytometric analyses are shown.
Table 3.
The effect of HIV-1 binding on homing of CD4+CD25+ cells in vivo
| PLN | MLN | Duodenum | Spleen | |
| 3 h post-transfer | ||||
| Mock | 626 ± 72 | 1679 ± 579 | 467 ± 56 | 5958 ± 1916 |
| HIV-1 | 947 ± 99* | 1978 ± 627 | 604 ± 48 | 6181 ± 1871 |
| 15 h post-transfer | ||||
| Mock | 1395 ± 156 | 11 400 ± 1823 | 2563 ± 217 | 41 889 ± 4 327 |
| HIV-1 | 2078 ± 301** | 20 504 ± 2753** | 4289 ± 415** | 24 608 ± 1515** |
Human CD4+CD25+ cells were exposed to HIV-1213 or mock controls for 24 h as described in Methods. After labeled with 51Cr, cells were injected intravenously into scid mice (1 × 107 cells per mouse, five mice per group). Mice were sacrificed at indicated time points of post-transfer, and peripheral (superficial inguinal) lymph nodes (PLN), mesenteric lymph nodes (MLN), duodenum and spleen were collected. Radioactivity was determined on a gamma counter and is presented as c.p.m. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01) as compared with the mock controls.
Exposure of CD4+CD25+ T cells to HIV-1 also enhances their suppressive abilities
Next, we investigated whether HIV-1 binding affects the effector functions of CD4+CD25+ T cells. We assessed the suppressive activity of CD4+CD25+ T cells by testing their ability to inhibit the proliferation of autologous CD4+CD25− T cells after anti-CD3 mAb stimulation in the presence of antigen-presenting cells (APCs). CD4+CD25− T cells, in contrast to CD4+CD25+ T cells, showed a vigorous proliferative response that was inhibited up to 62% at a 1:1 ratio upon co-culture of the two cell populations (Fig. 5A). However, exposure to HIV-1 (either HIV-1213 or HIV-1BaL) resulted in even higher levels of suppressive activity (up to 2- to 5-fold) (Fig. 5A). As expected, CD4+CD25+ T cell suppressive function enhanced by HIV-1 is not IL-10- or TGF-β-dependent because neither anti-IL-10 and/or anti-TGF-β could block it, although HIV-1 exposure increased IL-10 production by CD4+CD25+ T cells (data not shown). It has been demonstrated that HIV-1 or some of its components could reduce IL-2 production and survival (50) and inhibit proliferation (51) of CD4 T cells. To test whether the residual virus or its components on the surface of CD4+CD25+ T cells directly contribute to the inhibited proliferation of autologous CD4+CD25− T cells in our experiments, we conducted the experiments using HIV-1-exposed CD4+CD25− T cells as effector cells in place of HIV-1-exposed CD4+CD25+ T cells. In order to avoid interference with the proliferation of CD4+CD25− T cells (effector cells) in the presence of anti-CD3 and APCs, we pre-activated CD4+CD25− T cells (target cells) by plate-coated anti-CD3 plus anti-CD28, then co-culture with HIV-1-exposed CD4+CD25− T cells or controls and measured their proliferation. As shown in Fig. 5(B), neither mock-exposed CD4+CD25− T cells nor HIV-1213-exposed CD4+CD25− T cells displayed suppressive abilities as compared with CD4+CD25+ T cells. These results show that neither HIV-1-exposed CD4+CD25− T cells nor possibly existing virus or virus components have suppressive abilities.
Fig. 5.
HIV-1 binding enhances the suppressive function of CD4+CD25+ cells. (A) Purified CD4+CD25+ cells were exposed to HIV-1 or mock control overnight and washed. CD4+CD25– T cells (5 × 104) and autologous, mitomycin C-treated (50 μg ml−1, 30 min), T-depleted PBMC (5 × 105) were cultured in the absence or presence of increasing numbers of CD4+CD25+ T cells for 5 days in 96-well, flat-bottom plates. Anti-CD3 (1 μg ml−1) was added to the culture for stimulation. (B) CD4+CD25− T cells (target cells) were pre-stimulated with plate-coated anti-CD3 (2 μg ml−1) plus anti-CD28 (1 μg ml−1) for 1 day, washed and then co-cultured with CD4+CD25+ T cells or CD4+CD25− T cells (effector cells, HIV-1 or mock-exposed) at indicated ratio for 5 days. A total of μCi [3H]thymidine was added 18 h before harvest and incorporated radioactivity was determined on a beta emission reader. (C) Supernatants were collected at 24 h and levels of IL-2 were determined by ELISA. All data are presented as means ± SD of triplicate wells. One of three independent experiments is shown. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01) compared with any other controls.
Down-regulation of IL-2 in the target cells by CD4+CD25+ T cells is enhanced by HIV-1 exposure
Activated Treg cells suppress the proliferation of other T cells through down-regulation of IL-2 in the target cells (52). To verify this mechanism in the co-culture of CD4+CD25+/CD4+CD25− T cells, we measured IL-2 production in the co-culture supernatant. As shown in Fig. 5(C), culture supernatants from either HIV-1213- or HIV-1BaL-exposed CD4+CD25+ T cells co-cultured with CD4+CD25− T cells contained lower levels of IL-2 over the ratios of 1:4 through 1:32 than those of mock-treated CD4+CD25+ and CD4+CD25− T cell co-cultures. These results suggest that HIV-1 binding enhances the Treg function of interferring with the IL-2 production in target cells.
Involvement of HIV gp120-CD4 interaction in increased FoxP3 expression
To determine whether HIV gp120 binding to CD4, per se, was involved in inducing increased FoxP3 expression, we added soluble recombinant CD4 (sCD4) before HIV-1 exposure of CD4+CD25+ T cells and found that sCD4 almost completely abrogated HIV-1213- or HIV-1BaL-mediated increases in FoxP3 expression (Fig. 6A). Then, experiments examining CD4 signaling alone were performed. XLCD4 with anti-CD4 mAb and goat anti-mouse IgG or with plate-coated gp160 on the CD4+CD25+ T cells resulted in higher expression of FoxP3 (Fig. 6A). However, anti-CD4 mAb alone without cross-linking could not alter the expression of FoxP3 (data not shown). These results suggest that CD4 cross-linking, per se, is required to induce FoxP3 up-regulation. XLCD4 either with anti-CD4 mAb plus GAM or with plate-coated gp160 on the CD4+CD25+ T cells also enhanced their suppressive activity and survival (Fig. 6B and C). Finally, as shown in Fig. 6(A–C) UV-inactivated HIV-1213 or HIV-1BaL had the same effects on FoxP3 expression, survival and suppressive activities of CD4+CD25+ T cells as live viruses. All these data demonstrate that HIV-1 induces enhanced suppressive properties and other changes of CD4+CD25+ T cells via HIV gp120 binding to CD4 molecules and that active virus replication is not required.
Fig. 6.
Involvement of gp120-CD4 interactions in enhancement of FoxP3 expression, suppressive activities and cell recovery of CD4+CD25+ cells upon exposure to HIV-1. Purified CD4+CD25+ cells were exposed to HIV-1 with or without sCD4, UV-inactivated HIV-1, anti-CD4 plus GAM or plated-coated gp160. Three days later, (A) cells were harvested and intracellular FoxP3 was stained as described in Fig. 1, (B) suppressive activities were determined as in Fig. 4 or (C) cell recovery were measured as described in Fig. 3. Asterisks indicate a statistically significant difference (*P < 0.05, **P < 0.01) as compared with the mock controls.
Discussion
Here, we provide evidence that HIV-1 binding to CD4 directly enhances CD4+CD25+ Treg cell suppressive function, induces them to home to peripheral lymphoid tissues and mucosal tissues, where virus replication is occurring, and enhances their survival. These data indicate that HIV-1 manipulates CD4+CD25+ Treg cell activities to contribute to diminution of T cell responses in vivo, and thereby favoring HIV survival and persistence.
Although CD4+CD25+ T cells represent 5–10% of the CD4+ cell population, in general, in rodents (12,53–55), they are ∼2% of CD4+ cells in peripheral blood of humans (56, 57) and only CD4+CD25brigh+/high or CD4+Fox3+ cells seem to represent the Treg cells (21). Alteration of the distribution of Treg cells in vivo is often associated with disease (58). Expression of CD25 on CD4+ cells in the peripheral blood of HIV+ patients has been reported to be decreased compared with healthy individuals (59). Whether these reduced CD4+CD25+ cells are Treg or activated CD4+ T cells, or both, are not known because CD25 is also expressed on antigen-activated CD4+ cells (60). Nevertheless, decreases in Treg cells in the peripheral blood are associated with HIV disease progression (29, 32, 61). Oswald-Richter et al. (32) reported increased death of HIV-infected Treg cells in vitro, compared with non-Treg cells. However, higher numbers of Treg cells were detected in peripheral and mucosal lymphoid tissues of HIV-infected patients, arguing against increased killing of Treg cells in the compartments where most viral replication is occurring (29–31). Consistent with a previous report (30), our in vitro data showed that HIV-1 binding could directly enhance the survival of CD4+CD25+ cells, which is accompanied by increased Bcl-2 expression (Fig. 3A, B and G). In addition, the increased levels of TGF-β found in HIV+ patients could also promote Treg survival in vivo (62). FoxP3 over-expression in HEK293T and purified CD4+ T cells results in suppression of HIV-1 promoter transcription (63). Therefore, limiting HIV-1 transcription by increased FoxP3 in HIV-1-infected Treg cells may be one of the mechanisms for Treg cells to avoid HIV-1 replication-mediated cell death in vivo. Furthermore, CD4+CD25+ cells are not susceptible to activation-induced apoptosis (46), and such a phenotype may be responsible for the inability of HIV-1 binding to induce the expression of regular T-cell activation markers such as CD69, CD38 and HLA-DR on these cells.
In previous studies, we demonstrated that cross-linking of the homing receptor CD62L on HIV-1-exposed resting (e.g. CD25−) CD4+ T cells resulted in Fas/Fas ligand-mediated apoptosis (49). However, cross-linking of the homing receptors CD62L or integrin α4β7 on HIV-1-exposed CD4+CD25+ cells could not induce such apoptotic signals, although HIV-1 binding up-regulated the expression of these homing receptors and enhanced their homing (Fig. 4 and Table 3). Taken together, it appears that HIV-1 cannot induce the death of CD4+CD25+ Treg cells directly or indirectly, and thus this mechanism cannot explain the observed decrease in Treg cells in the peripheral blood. Rather, it is likely that their decrease in peripheral blood reflects their accelerated exit and re-distribution to LNs and gut, rather than an overall decrease. In support of this, the number of Treg cells is greatly increased in peripheral LNs (29, 30) and mucosal lymphoid organs (31), while they are decreased in the circulating T cell pool in HIV-infected people (29). It should be recognized, however, that although several groups have reported a decrease in CD4+CD25+ Treg cells in the peripheral blood of HIV patients (29, 32, 61), other groups demonstrated that a slight elevation in CD4+CD25+ Treg cells occurred in the circulating T cell pool (28, 31). One explanation may be the difference in selection of Treg-associated functional markers and/or the variation of selected HIV-infected subjects. Another explanation is that in the peripheral blood of advanced HIV patients, the relative proportion of Treg cells is probably elevated because of the depletion of non-Treg CD4+ cells, although their absolute number is not increased (31).
It is possible that in HIV+ patients, increases in cells having Treg-associated functional markers FoxP3, sCTLA-4 and GITR may also derive from the presence of activated non-Treg cells since these molecules can be up-regulated briefly during antigen activation of non-Treg cells (64, 65). However, results from our and other's studies do not favor this possibility because increased expression of these molecules was not accompanied by concomitant expression of other typical activation markers (CD38, CD69 and HLA-DR) (Table 1 and Figs. 2–3) (30, 31). Furthermore, although HIV-1 binding could partially activate CD4+CD25− cells, as judged by induction of CD38, CD69 and HLA-DR, as well as CD25, HIV-1-exposed CD4+CD25− cells lack concomitant FoxP3 expression and only express CD25low (Figs. 1–2). This suggests that HIV-1-activated CD4+CD25− cells are phenotypically different from FoxP3+CD25high Treg cells. Consistent with our data, several other studies have not detected increased FoxP3 expression in activated CD4+CD25− cells (66, 67). CD4+CD25+ Treg cells also can be distinguished from conventional CD4+ T cells in that they are deficient in IL-2 production and anergic to TCR stimulation. However, this unresponsive state can be overridden by the addition of IL-2 (55, 68) (Fig. 3C). The CD4+CD25+ Treg cells suppress T cell proliferation by inhibiting IL-2 production in the target cells (52), and in agreement with these observations, the enhanced suppressive effect of CD4+CD25+ Treg cells by HIV-1 binding correlated with their enhanced ability to suppress IL-2 production by target cells (Fig. 5B).
Our data suggest that HIV gp120-CD4 interactions initiate the effects on Treg cells, since XLCD4 by anti-CD4 mAb plus GAM or plate-coated gp160, also induced increased FoxP3 expression, survival rate and suppressive activity of Treg cells. Addition of sCD4 blocked FoxP3 induction following HIV-1 exposure (Fig. 6). Following HIV infection in vivo, the likelihood of CD4 engagement occurring is extremely high, since the relatively rare virus-producing cells are present in the lymphoid tissues in close proximity to large numbers of CD4+ cells, and binding of cell surface-expressed gp120 will occur with surrounding cells (69–71). However, in contrast to the report by Nilsson et al. (30), in which engagement of CD4 by anti-CD4 antibody alone was enough to drive FoxP3 induction, we found that only XLCD4 by anti-CD4 plus GAM or plate-coated gp160, rather than CD4 engagement by mAb alone (data not shown), was necessary to drive these effects in Treg cells. Such CD4-gp120-dependent mechanisms also suggest that active virus replication in the effected cells seems not to be required. This hypothesis is also favored by our finding that UV-inactivated HIV-1 showed similar effects on Treg cells as live HIV-1 (Fig. 6A–C). Of note, both the X4 strain HIV-1213 and the R5 strain HIV-1BaL caused increases in FoxP3 expression, survival rate and suppressive activity of Treg cells. Therefore, it appears that the mechanisms described here are not restricted to certain HIV-1 strains or limited by co-receptor involvement.
In numerous studies, it has been difficult to distinguish the relative contribution of loss of CD4+ cell numbers from loss of CD4+ T cell function for the progressive immunodeficiency that is characteristic of AIDS. FIV-infected cats do not suffer a dramatic loss of CD4+ cells until late in infection (72), but any loss in CD4+ cell numbers appears to more than offset by activation of suppressor function (73). CD4+CD25+ Treg cells in vivo can be activated by many types of pathogens, including bacteria, viruses, fungi and intracellular parasites, and play an important role in immunosuppression (74–76). It has been demonstrated that CD4+CD25+ cells regulate the magnitude of Th immune responses and affect the course of infection and development of memory immunity to Pneumocystis carinii and Candida albicans (77). Several studies showed that CD4+CD25+ cells accumulate at the sites of infection and suppress the ability of immune system to eliminate parasite infections or to mediate pathogenesis (76, 78). Removal of CD4+CD25+ cells from PBMC in vitro results in increased anti-HIV CD4+ T cell responses (26). In vitro HIV-specific proliferation and cytokine production of CD4+ and CD8+ T cells in most asymptomatic HIV-infected individuals is substantially suppressed by CD4+CD25+ cells (28). It has been demonstrated that CD4+ Th-dependent immune dysfunction develops before a significant decrease in CD4+ cell numbers occurs (2). Furthermore, it has been shown that, as CD4+ cells disappear from blood, the net number of CD4+ cells is not reduced as much; in fact, it usually increases in LNs above normal levels contributing to lymphodenopathy. Only in late disease do CD4+ cells disappear in LNs (79, 80). Therefore, early immune dysfunction might be attributed to activated Treg cell-mediated immunosuppression, especially in the early stages of HIV infections. In support of this, Kinter et al. (28) reported that in most healthy HIV-infected individuals, CD4+CD25+ Treg cells abrogated HIV-specific CD4 and CD8 T cell responses. In the present study, we provide evidence that HIV-1 binding up-regulates Treg-associated functional molecules, including sCTLA-4, GITR and FoxP3 and directly enhances CD4+CD25+ Treg cell function. It also induces their enhanced migration to lymphoid tissues where most HIV-infected cells reside and where they survive to a greater degree. Consequently, they accumulate there at enhanced rates and function better as observed in vivo by Kinter et al. (33). These mechanisms can explain HIV-induced immunosuppression even in the absence of extensive CD4+ T cell depletion.
In summary, our data show that HIV-1 binding to CD4+CD25+ Treg cells enhances their survival and function and induces their accumulation in lymphoid tissues. These cells then, in turn, may allow HIV-1-infected cells to escape immune control, providing another underlying mechanism for persistence of virus replication and disease development. These findings may have potential for novel therapeutic approaches improving the immunocompromised environment in HIV-infected patients through counteracting Treg cell function.
Funding
National Institutes of Health (AI054291 and Al51177) to M.W.C.
Acknowledgments
We thank Drs Samuel Baron and Rolf Konig for critical reading of the manuscript.
Glossary
Abbreviations
- 7-AAD
7-aminoactinomycin D
- APC
antigen-presenting cell
- cAMP
cyclic adenosine monophosphate
- c.p.m.
counts per minute
- GAM
goat anti-mouse IgG
- GITR
glucocorticoid-induced tumor necrosis factor receptor
- LN
lymph node
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- RT
reverse transcription
- sCD4
soluble recombinant CD4
- TGF
transforming growth factor
- Treg
regulatory T
References
- 1.Antonen J, Ranki A, Valle SL, et al. The validity of immunological studies in human immunodeficiency virus infection: a three-year follow-up of 235 homo- or bisexual persons. Acta. Pathol. Microbiol. Immunol. Scand. [C] 1987;95:275. doi: 10.1111/j.1699-0463.1987.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Stocks NI, Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Invest. 1989;84:1892. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedema F. Immunological abnormalities in the natural history of HIV infection: mechanisms and clinical relevance. Immunodefic. Rev. 1992;3:173. [PubMed] [Google Scholar]
- 4.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 2002;8:319. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 5.Pontesilli O, Carlesimo M, Varani AR, et al. HIV-specific lymphoproliferative responses in asymptomatic HIV-infected individuals. Clin. Exp. Immunol. 1995;100:419. doi: 10.1111/j.1365-2249.1995.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalod M, Dupuis M, Deschemin JC, et al. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J. Clin. Invest. 1999;104:1431. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 8.Arrode G, Finke JS, Zebroski H, Siegal FP, Steinman RM. CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. Eur. J. Immunol. 2005;35:159. doi: 10.1002/eji.200425744. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 10.Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002;23:580. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 2001;14:753. doi: 10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151. [PubMed] [Google Scholar]
- 13.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212. [PubMed] [Google Scholar]
- 14.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 15.Bach JF. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 2003;3:189. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Regulatory T cells: mediating compromises between host and parasite. Nat. Immunol. 2003;4:10. doi: 10.1038/ni0103-10. [DOI] [PubMed] [Google Scholar]
- 17.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 19.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, et al. GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft-versus-host disease. J. Exp. Med. 2004;200:149. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 2004;172:2778. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007;204:1303. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 24.Thompson C, Powrie F. Regulatory T cells. Curr. Opin. Pharmacol. 2004;4:408. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005;6:353. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 26.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 28.Kinter AL, Hennessey M, Bell A, et al. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 2004;200:331. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson J, Boasso A, Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 2005;174:3143. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epple HJ, Loddenkemper C, Kunkel D, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 32.Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl Acad. Sci. USA. 2007;104:3390. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 35.Cloyd MW, Moore BE. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology. 1990;174:103. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- 36.Ji J, Sahu GK, Braciale VL, Cloyd MW. HIV-1 induces IL-10 production in human monocytes via a CD4-independent pathway. Int. Immunol. 2005;17:729. doi: 10.1093/intimm/dxh252. [DOI] [PubMed] [Google Scholar]
- 37.Scott-Algara D, Vuillier F, Cayota A, Dighiero G. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin. Exp. Immunol. 1992;90:181. doi: 10.1111/j.1365-2249.1992.tb07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cloyd MW, Chen JJ, Adeqboyega P, Wang L. How does HIV cause depletion of CD4 lymphocytes? A mechanism involving virus signaling through its cellular receptors. Curr. Mol. Med. 2001;1:545. doi: 10.2174/1566524013363320. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Robb CW, Cloyd MW. HIV induces homing of resting T lymphocytes to lymph nodes. Virology. 1997;228:141. doi: 10.1006/viro.1996.8397. [DOI] [PubMed] [Google Scholar]
- 40.Zack JA, Haislip AM, Krogstad P, Chen IS. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J. Virol. 1992;66:1717. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antons AK, Wang R, Oswald-Richter K, et al. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J. Immunol. 2008;180:764. doi: 10.4049/jimmunol.180.2.764. [DOI] [PubMed] [Google Scholar]
- 42.Peakman M, Mahalingam M, Pozniak A, McManus TJ, Phillips AN, Vergani D. Markers of immune cell activation and disease progression. Cell activation in HIV disease. Adv. Exp. Med. Biol. 1995;374:17. doi: 10.1007/978-1-4615-1995-9_2. [DOI] [PubMed] [Google Scholar]
- 43.Mahalingam M, Peakman M, Davies ET, Pozniak A, McManus TJ, Vergani D. T cell activation and disease severity in HIV infection. Clin. Exp. Immunol. 1993;93:337. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirmule N, Kalyanaraman VS, Pahwa S. Signals transduced through the CD4 molecule on T lymphocytes activate NF-kappa B. Biochem. Biophys. Res. Commun. 1994;203:498. doi: 10.1006/bbrc.1994.2210. [DOI] [PubMed] [Google Scholar]
- 45.Hivroz C, Mazerolles F, Soula M, et al. Human immunodeficiency virus gp120 and derived peptides activate protein tyrosine kinase p56lck in human CD4 T lymphocytes. Eur. J. Immunol. 1993;23:600. doi: 10.1002/eji.1830230303. [DOI] [PubMed] [Google Scholar]
- 46.Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 2001;31:1122. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Chen JJ, Gelman BB, Konig R, Cloyd MW. A novel mechanism of CD4 lymphocyte depletion involves effects of HIV on resting lymphocytes: induction of lymph node homing and apoptosis upon secondary signaling through homing receptors. J. Immunol. 1999;162:268. [PubMed] [Google Scholar]
- 48.Chen JJ, Huang JC, Shirtliff M, et al. CD4 lymphocytes in the blood of HIV+ individuals migrate rapidly to lymph nodes and bone marrow: support for homing theory of CD4 cell depletion. J. Leukoc. Biol. 2002;72:271. [PubMed] [Google Scholar]
- 49.Ji J, Chen JJ, Braciale VL, Cloyd MW. Apoptosis induced in HIV-1-exposed, resting CD4+ T cells subsequent to signaling through homing receptors is Fas/Fas ligand-mediated. J. Leukoc. Biol. 2007;81:297. doi: 10.1189/jlb.0506338. [DOI] [PubMed] [Google Scholar]
- 50.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 51.Locher CP, Witt SA, Kassel R, Dowell NL, Fujimura S, Levy JA. Differential effects of R5 and X4 human immunodeficiency virus type 1 infection on CD4+ cell proliferation and activation. J. Gen. Virol. 2005;86:1171. doi: 10.1099/vir.0.80674-0. [DOI] [PubMed] [Google Scholar]
- 52.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 54.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J. Immunol. 2000;165:3105. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 55.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:1285. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 57.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 58.Sather BD, Treuting P, Perdue N, et al. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zola H, Koh LY, Mantzioris BX, Rhodes D. Patients with HIV infection have a reduced proportion of lymphocytes expressing the IL-2 receptor p55 chain (TAC, CD25) Clin. Immunol. Immunopathol. 1991;59:16. doi: 10.1016/0090-1229(91)90078-o. [DOI] [PubMed] [Google Scholar]
- 60.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 61.Apoil PA, Puissant B, Roubinet F, Abbal M, Massip P, Blancher A. FOXP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J. Acquir. Immune Defic. Syndr. 2005;39:381. doi: 10.1097/01.qai.0000169662.30783.2d. [DOI] [PubMed] [Google Scholar]
- 62.Hu R, Oyaizu N, Than S, Kalyanaraman VS, Wang XP, Pahwa S. HIV-1 gp160 induces transforming growth factor-beta production in human PBMC. Clin. Immunol. Immunopathol. 1996;80:283. doi: 10.1006/clin.1996.0125. [DOI] [PubMed] [Google Scholar]
- 63.Grant C, Oh U, Fugo K, et al. Foxp3 represses retroviral transcription by targeting both NF-kappa B and CREB pathways. PLoS Pathog. 2006;2:e33. doi: 10.1371/journal.ppat.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J. Clin. Invest. 2003;112:1437. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mantel PY, Ouaked N, Ruckert B, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J. Immunol. 2006;176:3593. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 66.Lundgren A, Stromberg E, Sjoling A, et al. Mucosal FOXP3-expressing CD4+CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 2005;73:523. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 68.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+CD25+ suppressor T cells. Immunol. Rev. 2001;182:58. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 69.Oh SK, Cruikshank WW, Raina J, et al. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J. AIDS. 1992;5:251. [PubMed] [Google Scholar]
- 70.Amadori A, De Silvestro G, Zamarchi R, et al. CD4 epitope masking by gp120/anti-gp120 antibody complexes. A potential mechanism for CD4+ cell function down-regulation in AIDS patients. J. Immunol. 1992;148:2709. [PubMed] [Google Scholar]
- 71.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-gamma and tumor necrosis factor-alpha secretion. Blood. 1994;84:2622. [PubMed] [Google Scholar]
- 72.English RV, Nelson P, Johnson CM, Nasisse M, Tompkins WA, Tompkins MB. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J. Infect. Dis. 1994;170:543. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- 73.Vahlenkamp TW, Tompkins MB, Tompkins WA. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J. Immunol. 2004;172:4752. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 74.Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 2002;32:1282. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 75.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl Acad. Sci. USA. 2001;98:9226. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 77.Montagnoli C, Bacci A, Bozza S, et al. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 2002;169:6298. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 78.Ji J, Masterson J, Sun J, Soong L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J. Immunol. 2005;174:7147. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janossy G, Pinching AJ, Bofill M, et al. An immunohistological approach to persistent lymphadenopathy and its relevance to AIDS. Clin. Exp. Immunol. 1985;59:257. [PMC free article] [PubMed] [Google Scholar]
- 80.Mangkornkanok-Mark M, Mark AS, Dong J. Immunoperoxidase evaluation of lymph nodes from acquired immune deficiency patients. Clin. Exp. Immunol. 1984;55:581. [PMC free article] [PubMed] [Google Scholar]