Abstract
GM-CSF plays an essential role in the differentiation of dendritic cells (DCs). Our studies have shown that GM-CSF treatment can induce semi-mature DCs and CD4+CD25+ regulatory T cells (Tregs) and suppress ongoing autoimmunity in mouse models. In this study, we examined the differences in the potential of GM-CSF to exert tolerogenic function on CD8a+ and CD8a− sub-populations of DCs in vivo. We show that GM-CSF modulates CD8a−, but not CD8a+ DCs in vivo, by inhibiting the surface expression of activation markers MHC II and CD80 and production of inflammatory cytokines such as IL-12 and IL-1β. Self-antigen [mouse thyroglobulin (mTg)] presentation by GM-CSF-exposed CD8a− DCs to T cells from mTg-primed mice induced a profound increase in the frequency of forkhead box P3 (FoxP3)-expressing T cells compared with antigen presentation by GM-CSF-exposed CD8a+ DCs and control CD8a+ and CD8a− DCs. This tolerogenic property of GM-CD8a− DCs was abrogated when IL-12 was added. GM-CSF-exposed CD8a− DCs could also induce secretion of significantly higher amounts of IL-10 by T cells from mTg-primed mice. Importantly, adoptive transfer of CD8a− DCs from GM-CSF-treated SCID mice, but not untreated mice, into wild-type CBA/J mice prevented the development of experimental autoimmune thyroiditis (EAT) in the recipient animals upon immunization with mTg. Collectively, our results show that GM-CSF renders CD8a− DCs tolerogenic, and these DCs induce Foxp3+ and IL-10+ Tregs.
Keywords: autoimmunity, dendritic cells, GM-CSF, regulatory T cells, thyroiditis, tolerance
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that initiate and regulate immune responses against foreign as well as self-antigens (1, 2). The strength and type of immune response evoked by the DCs are determined by a variety of factors including nature of the antigen, the type and maturation status of DCs and their ability to produce certain cytokines (3–8). For example, antigen presentation by immature DCs can lead to tolerance through the induction of regulatory T cells (Tregs), anergy or deletion of responding T cells, whereas antigen presentation along with optimum co-activation stimuli can result in an effective immune response (9–12). DCs can be broadly classified into CD11c+CD8a+ and CD11c+CD8a− subtypes, and both are capable of inducing effective T cell response as well as tolerance depending on their maturation status. In addition, it has been widely considered that CD8a+ DCs preferentially elicit a Th1 type response, whereas CD8a− DCs promote a Th2 response (13–17).
Several cytokine factors can influence the differentiation and the maturation status of DCs. GM-CSF, G-CSF and Flt3-L are widely used for modulating DC function (18–22). GM-CSF has been routinely used in clinics post-chemo/radiotherapy for repopulating myeloid cells in cancer patients (23, 24). This cytokine has also been widely used for generating DCs from bone marrow and peripheral precursor cells. In recent studies, we demonstrated the ability of GM-CSF to induce and maintain semi-mature tolerogenic status of DCs in vivo (25–27). In addition, we also demonstrated that GM-CSF treatment could suppress autoimmune thyroiditis and myasthenia gravis by inducing CD4+CD25+ Tregs (25–27). Similarly, therapeutic potential of GM-CSF in type 1 diabetes (T1D) was demonstrated in a non-obese diabetic (NOD) mouse model by another group (28).
In this study, we characterized the effects of GM-CSF on CD8a+ and CD8a− DC sub-populations and tested their ability to induce Tregs. Our results clearly show that GM-CSF exerts tolerogenic effect primarily on CD8a−, but not CD8a+ DCs, by retaining them in a semi-mature status. Moreover, we show that upon adoptive transfer, CD8a− DCs from GM-CSF-treated mice facilitate induction of mouse thyroglobulin (mTg)-specific forkhead box P3 (FoxP3)+ and IL-10+ Tregs that suppress mTg immunization-induced experimental autoimmune thyroiditis (EAT).
Materials and methods
Mice
Six- to eight-week old female CBA/J and CB17-Prkdcscid/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in the Biological Resources Laboratory at the University of Illinois (Chicago, IL, USA) and provided food and water ad libitum. Animals were cared for in accordance with the guidelines set forth by the University of Illinois Animal Care and Use Committee.
GM-CSF and antibodies
Recombinant mouse-GM-CSF was purchased from Invitrogen (Carlsbad, CA, USA). Either FITC- or APC-conjugated anti-CD11c (N4181), PE-labeled H-2kd (11-5.2), CD8a (53-6.7), CD25 (3C7), CD80 (16-10A1), CD86 (GL-1) and CD40 (3/23), FITC-labeled CD4, streptavidin PE, isotype control mAbs, biotinylated anti-transforming growth factor (TGF)-β (A75-3) and purified anti-CD16/CD32R (2.4G2) as Fc receptor block were purchased from BD PharMingen (San Diego, CA, USA). APC anti-Foxp3 (FJK-16S), PE anti-cytotoxic T-lymphocyte antigen 4 (UC10-4F10-11), PE anti-CD4 (GK1.5), PE anti-IL-10 (JES5-16E3) and neutralizing rat mAb to mouse IL-10 receptor (IL-10R) (1B1.3a) were obtained from eBioscience (San Diego, CA, USA). Carboxyfluorescein succinimidyl ester (CFSE) was purchased from Invitrogen and neutralizing antibody to TGF-β (1D11) was purchased from R&D Systems (Minneapolis, MN, USA).
T cells, DCs and mTg
Spleens and lymph nodes were isolated and CD4+25+ and CD4+CD25− T cells were purified by positive and negative selection, respectively, using antibodies bound to magnetic beads (Miltenyi Biotec, Auburn, CA, USA). CD11c+ DCs were first isolated from spleens by positive selection using CD11c-labeled magnetic beads, and CD8a+ and CD8a− DCs were further purified using the MoFlo cell sorter (Beckman Coulter Cyan ADP) after staining with fluorochrome-labeled CD11c and CD8a. Normal mouse thyroids were obtained from (Biochemed, Winchester, VA, USA) and the mTg was prepared as described earlier (29).
GM-CSF treatment and immunization with thyroglobulin
Mice were given intra-peritoneal injections of GM-CSF (2 μg per mouse per day) for five consecutive days from days 1 to 5 and 15 to 19. Control mice received PBS. Mice were immunized subcutaneously with mTg (100 μg per mouse) emulsified in CFA on days 6 and 20. Mice from these groups were sacrificed at different time points for different experiments as indicated in the figure legends.
Effects of GM-CSF treatment on DC maturation
GM-CSF-treated and control mice were sacrificed 48 h after mTg immunization. Purified CD8a+ and CD8a− DCs obtained from spleens were stained with fluorochrome-labeled anti-mouse CD11c in combination with anti-mouse MHC class II, CD80, CD86 or CD40 antibodies and analyzed using a flow cytometer (Cyan; DakoCytomation). RNA for reverse transcription (RT)–PCR was isolated from these purified DC populations using Trizol (Invitrogen). Cytokine transcript levels for IL-10, IL-6, tumor necrosis factor (TNF)-α, IL-1β, and IL-12 were determined by RT–PCR using a commercial cytokine kit following manufacturer's guidelines (Maxim Biotech, Rockville, MD, USA).
Antigen presentation assay
Purified CD8a+ (GM-CD8a+) or CD8a− (GM-CD8a−) DCs from spleens of GM-CSF-treated and untreated control mice (i.e. C-CD8a+ and C-CD8a− DCs) (2 × 104 cells per well) were cultured with either total CD4+ T cells or sorted CD4+25+ or CD4+25− T cells, at a 1:1 ratio. After 6 days in culture, cells were analyzed by flow cytometry. For some experiments, IL-1β and/or IL-12 were added to the DC–T cell co-cultures at a concentration of 10 ng and 100 pg ml−1, respectively. To study the effects of toll-like receptor (TLR) ligands on the DCs, DC cultures were first exposed to LPS (5 μg ml−1; Salmonella enteritidis; Sigma–Aldrich) or cytosine-phosphate-guanine (CpG)-oligodeoxynucleotide (3 μg ml−1; ODN 1826: TCCATGACGTTCCTGACGTT; InvivoGen) for 24 h. The DCs were then washed and co-cultured with CD4+ T cells as described above. In some experiments, these cells were assessed for their ability to suppress antigen-specific T cell proliferation, as measured by CFSE dilution in CD4+CD25− responder T cells from mTg-immunized mice, in the presence of irradiated splenocytes (Cs-137, 2000 rads) which served as APCs. Cell-free supernatants were collected for cytokine analysis by ELISA.
Measurement of cytokine production
Cell-free supernatants were assayed for Th1/Th2 or pro-inflammatory cytokines, IL-1β and IL-12 by ELISA following the manufacturer's instructions (eBioscience). The optical density values were determined at 450 nm using a microplate reader (Bio-Rad). Cytokine levels were measured using corresponding cytokine standards. Suggested lowest detection levels using this kit are 15 pg ml−1 for IL-12, IL-1β, IFN-γ and IL-10, 2 pg ml−1 for IL-2 and 4 pg ml−1 for IL-4. TGF-β levels were determined using the DuoSet ELISA Kit (R&D Systems).
Adoptive transfer of CD4+ T cells into SCID mice
CB17-Prkdcscid/J SCID mice were treated with GM-CSF (2 μg per mouse per day) or PBS for five consecutive days from days 1 to 5 and 15 to 19. Fourteen days later (i.e. Day 33), 2 × 106 purified CD4+ T cells or CD3+ T cells were transferred intravenously (i.v.) into these mice. Two and 16 days after receiving the cells, the recipient mice were immunized with mTg emulsified in CFA. Mice were sacrificed 24 days after the second immunization and draining lymph nodes, spleens and thyroids were collected and used for analyzing mTg-specific immune responses. Splenic CD4+ T cells were pulsed with mTg in vitro and the percentage of cytokine-secreting cells was determined by intracellular staining using fluorochrome-labeled anti-IL-10 or anti-TGF-β antibodies followed by FACS analysis. Culture supernatants were analyzed for cytokines by ELISA as described above.
Adoptive transfer of CD11C+8a− DCs from SCID mice into wild-type mice
CB17-Prkdcscid/J SCID mice treated with GM-CSF or PBS, as described above, were sacrificed within 48 h after the last treatment and CD8a− DCs isolated. 2 × 106 purified CD8a− DCs from either control or GM-CSF-treated mice were adoptively transferred i.v. into wild-type CBA/J mice. The recipient mice were immunized twice with mTg emulsified in CFA on days 2 and 16 after adoptive transfer. Mice were sacrificed 24 days after the second immunization, and draining lymph nodes and spleens were collected for analyzing mTg-specific immune responses.
Evaluation of EAT
Thyroids were fixed in formalin, embedded in paraffin, sectioned across both lobes and stained with hematoxylin and eosin. Thyroid pathology was evaluated and the extent of thyroid lymphocytic infiltration, as a marker of disease severity, was scored using a scale of 1+ to 5+. An infiltrate of at least 125 cells in one or several foci was scored 1+. Ten to twenty foci of cellular infiltration involving up to 25% of the gland was scored 2+. An infiltration involving up to 25–50% of the gland was scored 3+. Destruction of >50% of the gland was scored 4+, and near-complete destruction of the gland with very few or no remaining follicles was scored 5+. Thyroids were evaluated and scored in a blinded fashion.
Statistical analysis
Mean, standard deviation, and statistical significance were calculated using the SPSS application software. Statistical significance was determined using the non-parametric Wilcoxon signed test. In most cases, values of individual-treated and immunized groups were compared with that of untreated but immunized group unless mentioned otherwise. In studies comparing more than two groups, one-way analysis of variance was used to determine P values and assess significance. A P value of ≤0.05 was considered significant.
Results
GM-CSF treatment induces Foxp3+ and IL-10+ Tregs
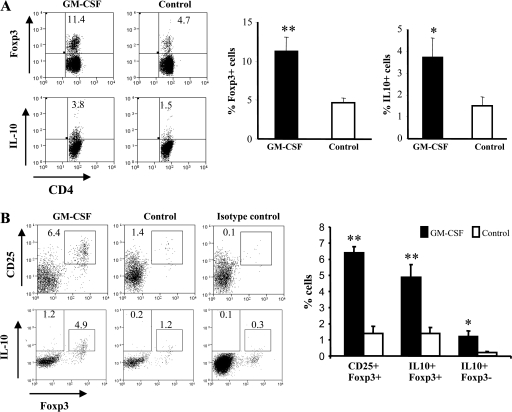
Our earlier studies revealed that GM-CSF treatment can increase the frequencies of CD8a− DCs and CD4+CD25+ T cells and suppress mTg-induced EAT through an IL-10-dependent mechanism (26, 27). Since CD25 is expressed on most activated T cells, we investigated to see what proportion of CD4+ T cells were Tregs by measuring the expression of a Treg-specific marker, Foxp3 and suppressor cytokine, IL-10. As shown in Fig. 1(A), significantly higher percentages of CD4+Foxp3+ cells (11.4 versus 4.7%; P = 0.007) and CD4+IL-10+ T cells (3.8 versus 1.5%; P = 0.03) were seen in GM-CSF-treated mice that were immunized with mTg relative to the controls.
Fig. 1.
GM-CSF treatment expands Foxp3+ and IL-10+ Tregs in vivo: (A) CBA/J mice were treated with GM-CSF or PBS for five consecutive days starting on days 1 and 15. Mice were immunized twice subcutaneously with mTg (100 μg 100 μl−1) emulsified in CFA on days 6 and 20. Two days after the second immunization, mice were sacrificed and spleens collected. Splenocytes were stained for CD4 and Foxp3 and IL-10 using the corresponding fluorochrome-labeled antibodies and analyzed by FACS. The plots shown were gated on CD4+ cells. Percentages of double-positive cells are indicated on the plots. Specific regions were marked, and the gates and quadrants were set while analyzing the data based on isotype control antibody background staining. Representative scatterplots (left panels) and mean ± SD of three independent experiments carried out using five mice per group (right panels) are shown. (B) Female C3Smn.CD17-Prkdcscid/J SCID mice were treated with either GM-CSF or PBS starting on days 0 and 15 for five consecutive days. Fourteen days after the last GM-CSF treatment, these mice received CD4+ T cells (2 × 106), isolated from pooled splenocytes of three naive wild-type CBA/J mice, through the tail vein. Recipient mice were immunized with mTg emulsified in CFA (100 μg 100 μl−1), 2 days and 16 days after the adoptive transfer of T cells, and sacrificed 24 days after the second immunization. Splenocytes were cultured in the presence of 50 μg ml−1 of mTg and stained with fluorochrome-labeled antibodies for Foxp3 along with either CD25 or IL-10 and analyzed by flow cytometry. Representative scatterplots (left panels) and mean ± SD of three independent experiments carried out using three to five mice mice per group (right panels) are shown. Extreme right dot plots represent a sample stained using fluorochrome-labeled CD25-specific and isotype control antibodies. Results shown are representative of two independent experiments carried out in triplicates. T-test was carried out to compare GM-CSF groups with control groups. * P < 0.05, ** P < 0.01.
To further confirm this observation and to understand the role of GM-CSF-exposed DCs in inducing an increase in Treg numbers, we adoptively transferred enriched CD3+ or CD4+ T cells from naive CBA/J mice into syngeneic SCID mice previously treated with GM-CSF or PBS. To minimize exposure of transferred cells to exogenous GM-CSF, the cells were transferred 14 days after the last treatment with GM-CSF. On days 2 and 16 after the cell transfer, recipient mice were challenged with mTg emulsified in CFA. Twenty-four days after the second mTg challenge, animals were sacrificed and their splenocytes examined for Tregs. GM-CSF-treated CD4+ T cell-recipient SCID mice showed significant numbers of CD25+Foxp3+ cells compared with controls (P = 0.007) (Fig. 1B). Importantly, in these mice, a higher percentage of Foxp3+ cells were also IL-10+ (P = 0.008). In addition, a considerable number of CD4+Foxp3− T cells were also IL-10+ in GM-CSF-treated mice (1.2 versus 0.2%). A similar trend in Foxp3+IL-10+ T cell frequency was seen in GM-CSF-treated mice adoptively transferred with CD3+ T cells (data not shown). Our results suggested that Tregs are induced by GM-CSF-exposed DCs in vivo.
GM-CSF treatment primarily modulates CD8a− DCs in vivo
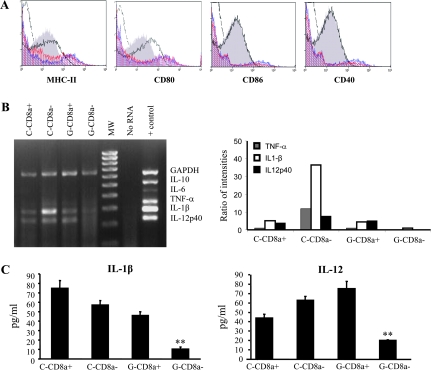
To understand the potential mechanism of action of GM-CSF on DCs, we characterized purified CD8a+ and CD8a− DCs from GM-CSF-treated (GM-CD8a+ and GM-CD8a−, respectively) and PBS-treated control CBA/J mice (C-CD8a+ and C-CD8a− DCs, respectively). While surface expression levels of MHC class II and CD80 were lower in GM-CD8a− DCs compared with C-CD8a− DCs or CD8a+ DCs from either group, DCs from both groups of mice had comparable levels of CD86 and CD40 (Fig. 2A). Importantly, the levels of transcripts for pro-inflammatory cytokines, IL-12, and IL-1β (Fig. 2B) were considerably lower only in GM-CD8a− DCs relative to the other three groups. Moreover, amounts of IL-12 and IL-1β secreted by GM-CD8a− DCs were significantly lower compared with C-CD8a− DCs (P < 0.008) (Fig. 2C). These observations suggested that GM-CD8a− DCs may have tolerogenic ability and led us to speculate that antigen presentation by these DCs could lead to the induction/expansion of Tregs.
Fig. 2.
DCs: GM-CSF suppresses pro-inflammatory cytokine transcript levels in CD8a- dendritic cells. CBA/J mice were treated with either GM-CSF or PBS for five consecutive days starting on days 1 and 15. Mice were immunized twice subcutaneously with mTg (100 μg 100 μl−1) emulsified in CFA on days 6 and 20. Two days after the second immunization, mice were sacrificed and spleens collected. CD11c+ DCs were isolated from pooled splenocytes using CD11c-labeled magnetic beads. (A) Purified CD11c+ DCs were stained with fluorochrome-conjugated anti-CD11c and anti-CD8a antibodies and sorted into CD8a+ and CD8a− sub-populations using a cell sorter. The sorted populations were further stained with anti-MHC II, anti-CD80, anti-CD86 or anti-CD40 and analyzed by flow cytometry. Broken lines indicate isotype control, red and blue histograms represent GM-CSF-treated and untreated CD8a+ DCs, respectively; gray filled and empty histograms (dark lines) represent GM-CSF-treated and untreated CD8a− DCs, respectively. (B) RNA was isolated from CD8a+ and CD8a− DCs isolated from either GM-CSF-treated or untreated mice and used in a multiplex RT–PCR assay to detect cytokine transcripts (C = control PBS-treated mice, G = GM-CSF-treated mice, + control = positive control provided with the multiplex PCR kit from Maxim Biotech). RT–PCR products were resolved on a 2% agarose gel (left panel). Densitometry analysis was performed and band densities represented as ratios of densities relative to the reduced form of guanosine adenine dinucleotide phosphate bands from the corresponding cells (right panel). (C) Purified CD8a+ or CD8a− DCs were cultured (1 × 106 cells per ml) for 48 h and culture supernatants were tested for secreted IL-1β and IL-12 concentrations by ELISA. Representative panels or mean ± SD values from three independent assays carried out in triplicate are shown. One-way analysis of variance was used to determine statistical significance. ** P < 0.01.
Antigen presentation by GM-CD8a− DCs causes expansion of Foxp3+ and IL-10+ cells
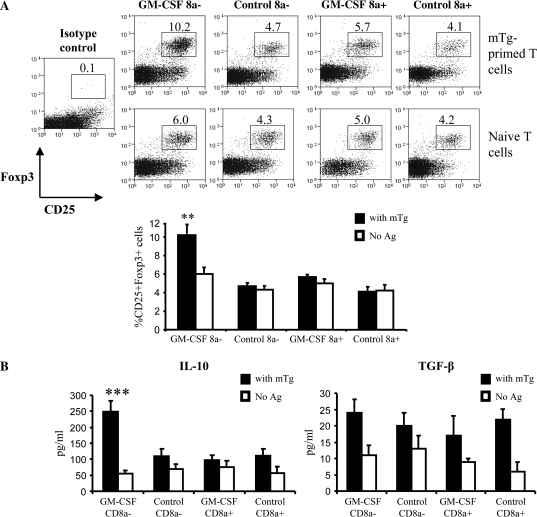
To assess the role of DC sub-populations from GM-CSF-treated mice in the generation of Tregs, enriched CD8a+ or CD8a− DCs from GM-CSF-treated and untreated mice along with CD4+ T cells from mTg-primed and naive mice were cultured in the presence of mTg. After 6 days, T cells from these cultures were examined for their phenotype by flow cytometry. We noted a significant increase in the percentage of CD25+Foxp3+ cells in cultures containing mTg-primed T cells and GM-CD8a− DCs (10.2%) relative to 4.7% in cultures containing C-CD8a− DCs (Fig. 3A, upper panel; P < 0.005). In contrast, only a modest increase in CD25+Foxp3+ cells was observed in cultures containing GM-CD8a+ DCs (5.7%) relative to that noted in the presence of C-CD8a+ DCs (4.1%). Notably, cultures containing naive T cells failed to show a significant increase in Foxp3+ T cells (Fig. 3A, lower panel). Also, as seen in Fig. 3(B), antigen presentation by GM-CD8a− DCs, but not GM-CD8a+ DCs or control DCs, induced IL-10 secretion by mTg-primed T cells (all P values were <0.01). In contrast, there were no significant differences in the levels of TGF-β. These observations suggested that GM-CSF can render CD8a−, but not CD8a+ DCs tolerogenic.
Fig. 3.
DCs from GM-CSF-treated mice cause expansion of Foxp3+ IL-10-secreting T cells in vitro: CBA/J mice (5 mice per group) were treated with GM-CSF or PBS for five consecutive days starting on days 1 and 15. CD11c+ DCs isolated on day 21 from pooled splenocytes of GM-CSF-treated or control mice were further sorted into CD8a+ and CD8a− populations as described above. CD8a+ or CD8a− DCs (2 × 104 cells) were co-cultured with mTg-primed or naive CD4+ splenic T cells (purified by using CD4-labeled magnetic beads) at a 1:1 ratio in 96-well round-bottom plates. DC–T cell cultures were pulsed with 50 μg ml−1 of mTg. (A) After 6 days in culture, CD4+T cells were analyzed for Foxp3 expression along with CD25 by flow cytometry. Representative scatterplots (upper panels) and mean ± SD values of CD25+Foxp3+ cells from three independent experiments carried in triplicate (lower panel) are shown. (B) IL-10 and TGF-β levels in supernatants from 48-h culture were quantified by ELISA. Results shown are mean ± SD values from three independent experiments carried out in triplicates. One-way analysis of variance was used to determine statistical significance. ** P < 0.01.
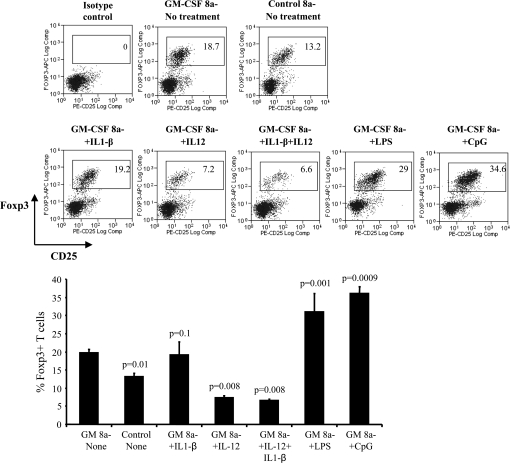
IL-12 abrogates induction of Foxp3+ T cells by GM-CD8a− DCs
To understand the functional consequence of suppressed IL-1β and IL-12 production by GM-CD8a− DCs compared with control DCs in inducing Foxp3+ T cells, we supplemented CD4+ T cell–GM-CD8a− DC cultures with recombinant IL-12 and/or IL-1β. As seen in Fig. 4, while exogenous IL-1β had no significant effect on the ability of GM-CD8a− DCs to induce Foxp3+ T cells, addition of IL-12 alone, or along with IL-1β, dramatically reduced the number of Foxp3+ T cells in the cultures (P < 0.005 for all comparisons). This suggested that suppressed IL-12 secretion by GM-CSF-exposed DCs could be a critical factor contributing to their ability to induce Foxp3+ T cells.
Fig. 4.
IL-12 inhibits expansion of Foxp3+ T cells by GM-CSF-exposed CD8a− DCs in vitro: CD8a-CD11c+ DCs were isolated on day 21 from pooled splenocytes of GM-CSF-treated or control mice as described above. CD8a− DCs (2 × 104 cells) were co-cultured with purified mTg-primed splenic T cells at a 1:1 ratio in 96-well round-bottom plates. DC–T cell cultures received 50 μg ml−1 of mTg along with IL-1β or IL-12, or both. For studies using TLR ligands, 2 × 104 CD8a− DCs were cultured in the presence of LPS or CpG for 24 h. Cells were then washed and cultured in the presence of mTg (50 μg ml−1) and CD4+ T cells isolated from mTg-primed mice. After 6 days in culture, CD4+ T cells were analyzed for Foxp3 expression along with CD25 by flow cytometry. Representative scatterplots and mean ± SD values (bar graphs) of Foxp3+ cells from three independent experiments are shown. Foxp3 expression in CD4+ T cells from untreated GM-CSF-exposed and control DCs and GM-CSF-exposed CD8a− DCs that were treated with IL-12, IL-1β, both IL-12 and IL-1β and LPS or CpG were compared and t-test was used to determine statistical differences. Corresponding P values are indicated above the bar graphs. P < 0.05 was considered significant.
To examine whether TLR signaling can abrogate the tolerogenic ability of GM-CD8a− DCs, CD4+ T cell–GM-CD8a− DC cultures were supplemented with bacterial LPS (TLR4 ligand) or CpG-ODN (TLR9 ligand). Interestingly, both LPS and CpG-ODN not only failed to over ride the tolerogenic effect of GM-CSF on CD8a− DCs but also profoundly enhanced the ability of these DCs to induce Foxp3 expression in CD4+ T cells.
CD8a− DCs induce Foxp3+ and IL-10+ T cells
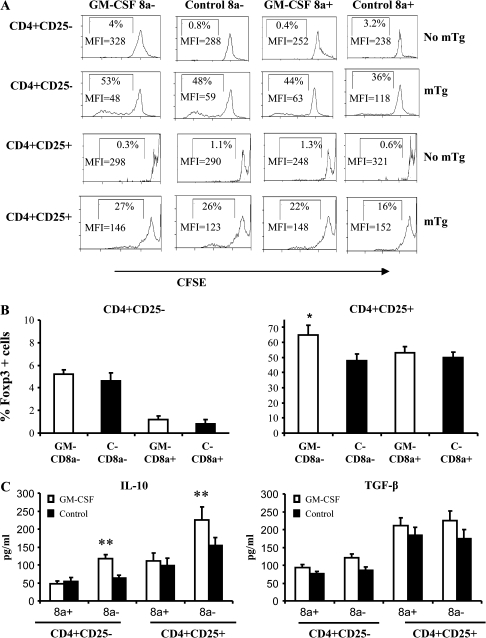
Next, we examined the effects of GM-CSF-exposed DC sub-populations on the proliferation of CD4+CD25+ and CD4+CD25− cells. Cells were enriched for CD25+ and CD25− T cells from spleens of mTg-primed mice, labeled with CFSE and co-cultured with different preparations of DCs. Relative to unstimulated cultures (4%), both CD8a+ and CD8a− DCs from GM-CSF-treated as well as control mice induced comparable levels of proliferation of T cells from mTg-primed mice (Fig. 5A). Approximately 5% of proliferated CD4+CD25− T cells were Foxp3+ when antigen was presented by CD8a− DCs from either group (Fig. 5B). In contrast, there was a significant increase in Foxp3-expressing cells among the CD4+CD25+ population when cultured with GM-CD8a− DCs. However, measuring secreted cytokines revealed that both CD25− and CD25+ subsets could produce higher amounts of IL-10 (P < 0.003, P < 0.001, respectively) in the presence of GM-CD8a− DCs compared with control DCs (Fig. 5C). There were no significant differences in the secreted TGF-β levels between the CD4+CD25− or the CD4+CD25+ cells cultured in the presence of CD8a− or CD8a+ DCs from either group. These observations suggest that CD25+ T cells that expanded upon mTg presentation are most likely activated effector T cells and not naturally existing Tregs.
Fig. 5.
GM-CSF-exposed CD8a− DCs expand CD4+25+ T cells that show increased IL-10 secretion: CD8a+ or CD8a− DCs from either control or GM-CSF-treated mice were cultured with either CFSE-stained CD4+25− or CD4+CD25+ T cells in the presence of mTg as described under Fig. 3. CD4+25+ and CD4+CD25− T cells were purified from spleens of mTg-primed or naive mice by positive and negative selection, respectively, using antibodies bound to magnetic beads. (A) Six days later, CFSE dilution was analyzed by flow cytometry after gating on CD4+ cells. Percentage and mean fluorescence intensity values of proliferated (CFSElow) cells are displayed in the histograms. (B) Foxp3 expression in CD4+ T cells from the above assay was examined by FACS. Mean ± SD of percentage values of cells expressing Foxp3 are plotted as bar diagrams. Each bar represents the mean ± SD values of three independent experiments carried out in triplicate. (C) IL-10 and TGF-β levels in 48-h culture supernatants from the above assay were quantified by ELISA. Each bar represents the mean ± SD values of three independent experiments carried out in triplicate. * P < 0.05.
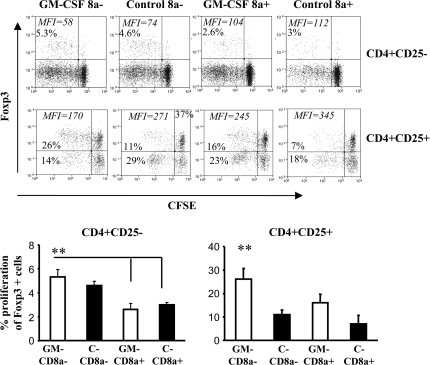
GM-CD8a− DCs induce Foxp3+ expression in effector T cells
To study the differences in the potential of various DC preparations to induce T cell proliferation, CFSE-labeled cells from antigen presentation assay similar to that described for Fig. 5 were examined for Foxp3 expression. As observed in Fig. 6, while both GM-CD8a− DCs and C-CD8a− DCs could induce Foxp3 expression in a considerable number of proliferating CD4+CD25− cells, however, GM-CD8a− DCs showed superior ability to induce and/or expand Foxp3 in CD4+CD25+ T cells compared with C-CD8a− DCs (P < 0.01). On the other hand, a majority of the C-CD8a− DC-expanded CD4+CD25+ T cells remained Foxp3 negative further supporting the notion that these proliferating CD25+ T cells are activated effector T cells. Interestingly, GM-CD8a+ DCs also demonstrated the ability to induce Foxp3 expression in proliferating CD4+CD25+ T cells from mTg-primed mice compared with C-CD8a+ DCs. These results, along with results in Fig. 5, suggested that GM-CD8a− DCs are highly effective in inducing Foxp3+ and IL-10-secreting Tregs from T cells that have been exposed to the antigen.
Fig. 6.
GM-CSF-exposed CD8a− DCs induce and expand CD4+25+Foxp3+ T cells: CBA/J mice (5 mice per group) were treated with GM-CSF or PBS for five consecutive days starting on days 1 and 15. CD11c+ DCs isolated on day 21 from pooled splenocytes of GM-CSF-treated or control mice were further sorted into CD8a+ and CD8a− populations as described above. CD8a+ or CD8a− DCs from either control or GM-CSF-treated mice were cultured with either CFSE-stained CD4+25− or CD4+CD25+ T cells in the presence of mTg as described under Fig. 5. CFSE-labeled cells were examined for Foxp3 expression by flow cytometry. Dot plots show expansion of Foxp3+ cells as indicated by CFSE dilution, Percentage of Foxp+ cells with CFSE dilution and their mean fluorescence intensity values are shown in each scatterplot (upper panel). Bars in the lower panel represent mean ± SD values for three independent experiments carried out in triplicate. One-way analysis of variance was used to compare values between all four groups. ** P < 0.01.
GM-CD8a− DCs induced Tregs function through IL-10
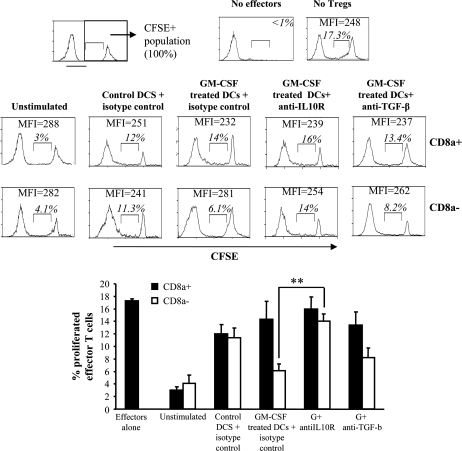
To examine whether Foxp3+ and IL-10+ T cells generated upon antigen presentation by GM-CD8a− DCs have the ability to suppress effector T cells, co-culture assays were carried out. T cells from antigen presentation assay using CD8a+ and CD8a− DCs described above were co-cultured with CD4+CD25− effector T cells from mTg-primed mice in the presence of irradiated spleen cells and mTg. T cells obtained from antigen presentation assays using GM-CD8a− DCs, but not GM-CD8a+ DCs (Fig. 7), suppressed mTg-specific proliferation of CD25− effector T cells (14 versus 6.1%) (P < 0.01). This suppression was abrogated by the addition of anti-IL-10R (anti-IL-10R) antibody (6.1 versus 14%; P < 0.01), but not to the same extent by the anti-TGF-β antibody (6.1 versus 8.2%; P < 0.08). These results show that GM-CSF-exposed CD8a− DCs induce Tregs that suppress effector T cells primarily through IL-10.
Fig. 7.
GM-CD8a− DCs cause expansion of Tregs that suppress CD4+CD25− T cell proliferation in an IL-10-dependent manner: GM-CSF-treated (as described under Fig. 3) and control mice were sacrificed on day 21 to obtain CD11c+ DCs from pooled splenocytes using CD11c-labeled magnetic beads. These cells were further separated into CD8a+ and CD8a− DCs by cell sorting. CD4+ T cells from mTg-primed donors were purified using antibody-labeled magnetic beads and cultured in the presence of mTg-pulsed CD8a+ or CD8a− DCs to generate Tregs. This mixture of cells was added to CFSE-labeled CD4+CD25− effector T cells obtained from spleens of mTg-primed mice (2:1 effector T cell:Treg ratio). Cultures were further stimulated with mTg in the presence of saturating concentrations of anti-IL-10R, anti-TGF-β or isotype control antibodies. Irradiated splenocytes from naive mice were used as APCs. After 5 days of culture, cell proliferation was assessed by CFSE dilution using flow cytometry. The CFSE+ population was considered 100%. The percentage and mean fluorescence intensity values of CFSElow cells are shown in each scatterplot (upper panel). Bar diagram (lower panel) shows mean ± SD of the percentage of CFSElow effector cells in the presence of neutralizing antibodies to IL-10 and TGF-β and corresponding isotype control antibodies. T-test was used to determine P values and assess significance. ** P < 0.01.
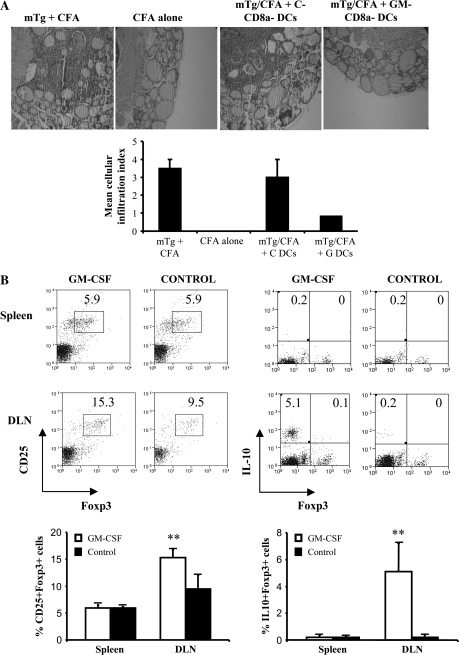
GM-CD8a− DCs are sufficient to induce Foxp3+ and IL-10+ Tregs in vivo and protect against EAT
To substantiate that GM-CSF induces Tregs primarily through its effects on CD8a− DCs, we adoptively transferred purified CD8a− DCs from GM-CSF-treated and untreated SCID mice into naive CBA/J mice that were then immunized with mTg to induce EAT. Mice that received GM-CD8a− DCs developed less severe thyroiditis as compared with recipients of C-CD8a− DCs (Fig. 8A). Surprisingly, there was no difference in the percentages of splenic CD25+Foxp3+ cells or Foxp3+IL-10+ cells (Fig. 8B, upper histograms and lower panel) between recipients of DCs from either donor. However, mice that received GM-CD8a− DCs showed a highly significant increase in CD25+Foxp3+ T cells in the draining lymph nodes compared with C-CD8a− DC recipients (Fig. 8B, lower histograms and lower panel, P = 0.002). Further, the frequency of Foxp3–IL-10+ cells was also significantly increased (Fig. 8B, lower histograms and lower panel, P = 0.001). These results showed that GM-CSF-induced tolerogenic CD8a− DCs are sufficient to induce CD25+Foxp3+ and CD4+IL-10+ T cells in vivo and protect recipient mice from developing thyroiditis.
Fig. 8.
Adoptive transfer of CD8a− DCs from GM-CSF-treated SCID mice expands Foxp3+ Tregs and suppresses thyroiditis: C3Smn.CD17-Prkdcscid/J mice (5 mice per group) were treated with GM-CSF or PBS for five consecutive days starting on days 1 and 15. Purified CD8a− DCs (2 × 106) were transferred via tail vein into 7-week-old naive wild-type CBA/J mice (5 mice per group). These mice were immunized with mTg emulsified in CFA 3 and 17 days after the adoptive transfer. (A) Forty days after transfer, mice were sacrificed, thyroids removed and fixed in formalin. Paraffin-embedded sections were prepared and stained with hematoxylin and eosin. Representative photomicrographs of hematoxylin and eosin-stained thyroid sections from different treatment groups are shown. Severity of thyroid lymphocytic infiltration, as described under Materials and methods was used as a marker of disease severity. Mean cellular infiltration index for different groups is shown in the bar graph (C = Control, G = GM-CSF treated). (B) CD4+ cells from spleen and thyroid-draining lymph nodes (DLN) of these mice were examined for Foxp3 and IL-10 expression. Representative scatterplots (upper panels) and mean ± SD of percentage values of Foxp3+CD25+ and Foxp3+ IL-10+ CD4+ T cells from 5 mice per group (lower panel) are shown. T-test was used to determine P values and assess significance. ** P < 0.01.
Discussion
In this study, we investigated the underlying mechanism of GM-CSF treatment-induced expansion of self-antigen-specific Tregs. Our results showed that GM-CSF could enhance not only the number of CD8a− DCs but also help maintain them in a semi-mature tolerogenic status. Antigen presentation by these ‘tolerogenic’ CD8a− DCs, but not CD8a+ DCs, leads to the induction of Tregs from effector T cells that can suppress mTg-specific immune response primarily in an IL-10-dependent manner.
Cytokine modulators such as GM-CSF and Flt3-L can profoundly affect expansion, maturation and function of certain subsets of DCs (25–27,30–34). Earlier we showed that GM-CSF-induced suppression of ongoing EAT was associated with an increase in CD8a− DCs and CD4+CD25+Foxp3+ T cell frequencies and was mediated through IL-10 produced by CD4+CD25+ T cells (26, 27). Similarly, a recent study in NOD mice demonstrated that GM-CSF treatment can suppress T1D through CD4+CD25+Foxp3+ Treg expansion (28). This report also showed that GM-CSF-influenced DCs have an immature phenotype and decreased capacity to activate CD8+ T cells. These findings were extended and further substantiated by our recent findings of prolonged suppression of T1D in NOD mice even when the GM-CSF treatment was initiated at later stages of insulitis. CD4+CD25+ T cells from GM-CSF-treated mice could suppress effector T cell response and T1D through enhanced production of IL-10 and TGF-β1. Moreover, transfer of GM-CSF-exposed DCs to naive NOD mice resulted in an expansion of Foxp3+ T cells leading to a significant delay in the onset of T1D and indicated that GM-CSF primarily acts on DCs. (35). These and our earlier observations (26, 27) prompted us to further study the underlying mechanism by which GM-CSF-exposed DC sub-populations induce/expand Tregs with the ability to suppress EAT.
CD4+ T cells from mice that were immunized with mTg to induce EAT and then treated with GM-CSF showed an increase in Foxp3+ and IL-10+ populations indicating that GM-CSF can induce heterogeneous adaptive Treg response. Importantly, in vivo adoptive transfer of CD4+ T cells into GM-CSF-treated SCID mice followed by mTg challenge resulted in a significant increase in the Foxp3+ and IL-10+ populations indicating that GM-CSF acts primarily on APCs to induce Tregs in vivo. However, it was not apparent whether Tregs’ induction is functionally linked to GM-CSF-exposed CD8a− DCs and if GM-CSF can affect the other major sub-population of CD8a+ DCs.
Reduced expression of MHC class II and CD80 combined with suppressed secretion of IL-1β and IL-12 cytokines by GM-CD8a− DCs, but not GM-CD8a+ DC, indicated that GM-CSF selectively affected CD8a− DCs and can render them tolerogenic. The notion that GM-CSF treatment induces tolerogenic CD8a− DCs is supported by our observation that antigen (mTg) presentation by GM-CD8a− DCs, and not GM-CD8a+ DCs or control DCs, could induce significant numbers of CD25+FoxP3+ and IL-10-producing T cells. These observations are consistent with earlier reports demonstrating the ability of immature and semi-mature DCs to induce adaptive Tregs (36, 37).
Treg-inducing ability of GM-CSF-exposed CD8a− DCs may be linked to suppressed expression of MHC II and pro-inflammatory cytokines such as IL-1β and IL-12. Class II genes have been shown to be important in the development of autoimmunity, both by their ability to present auto-antigens and to induce CD4+CD25+ Tregs (38). In addition, pro-inflammatory cytokines are known to influence tolerance induction. While one study has shown that infusion of thyroglobulin-pulsed, TNF-α-treated, semi-mature DCs can lead to the generation of Tregs and suppression of EAT in recipient mice (39), others have shown that mAbs to TNF receptors enable autoreactive T cells to circumvent suppression by Tregs (40, 41). Recent studies have demonstrated contrasting effects of IL-1 cytokines on APCs. While one study showed that IL-1β can break tolerance by directly expanding the effector T cell population in NOD mice (42), another study demonstrated that Foxp3+ T cells can be expanded in the presence of splenic DCs and IL-1β (43). It has also been shown that IL-1β can render DCs resistant to pro-inflammatory maturation-inducing conditions and thus allow them to retain their tolerogenic potential (44). Consistent with these studies, our observations show that IL-1β does not abrogate the tolerogenic properties of GM-CSF-exposed CD8a− DCs. However, IL-12, a Th1-promoting cytokine, could eliminate the ability of GM-CSF-exposed DCs to induce Foxp3+ T cells suggesting that down-regulation of pro-inflammatory cytokines like IL-12 could be the major tolerogenic effect of GM-CSF on CD8a− DCs.
It has been widely believed that while immature or semi-mature DCs induce T cell tolerance upon antigen presentation by enhancing Treg number and function, mature DCs promote effector T cell response against both self and foreign antigens. In addition to pro-inflammatory cytokines, TLR ligands are known to cause the activation and maturation of DCs and contribute to the development of autoimmune diseases (45–48). In contrast, recent studies have demonstrated that signaling through TLR ligands can enhance the ability of DCs to induce and/or expand Foxp3-expressing T cells (49–52). Interestingly, our results show that exposure of GM-CD8a− DCs to TLR4 and TLR9 ligands enhanced their ability to induce and expand Foxp3+ T cells. While additional studies are necessary to understand how TLR signaling enhances the tolerogenic function of GM-CD8a− DCs, our observations strongly suggest that inflammatory immune response, if any, during GM-CSF treatment may produce an enhanced antigen-specific tolerogenic effect, but not an adverse effector T cell response.
Our earlier studies have shown that GM-CSF treatment can induce an increase in the frequency of Tregs in vivo in an EAT model (26, 27). Therefore, we examined whether this increase is associated with an expansion of naturally existing Tregs. Both GM-CSF-exposed and control DCs maintained their ability to facilitate mTg-induced proliferation of CD4+CD25+ T cells from mTg-primed mice, but not naive T cells, suggesting that proliferating CD4+CD25+ T cells could be mTg-specific activated effector T cells. Supporting this notion, we found that a significant number of CD4+CD25+ T cells from mTg-primed mice to be Foxp3−. Further, it has been well established that naturally existing CD4+CD25+ Tregs are anergic in that they do not proliferate upon either CD3 (53, 54) or antigenic stimulation (55–58). Our observations show that while the magnitude of CD4+CD25+ T cell proliferation induced by both GM-CD8a+ and GM-CD8a− DCs was comparable, a significant increase in the total number of proliferated cells that express Foxp3+ was seen only with GM-CD8a− DCs. This shows that GM-CSF-exposed CD8a+ and CD8a− DCs differ in their abilities to induce the differentiation and/or expansion of antigen-specific T cells.
Studies have shown that Tregs that originated from antigen-specific effector T cells (adaptive Tregs) are more likely to produce significant amounts of IL-10 than natural Tregs (59–61). Our observation that GM-CD8a− DC-expanded T cells produce significant amounts of IL-10 further support the notion that these are adaptive Tregs that originated from mTg-specific effector/memory T cell pool. While our observations strongly indicate that these adaptive Tregs might be originating from effector T cells, CD8a− DC's ability to selectively expand a small pool of existing antigen-specific Tregs cannot be ruled out. Collectively, our observations show that GM-CD8a− DCs are more efficient in inducing and/or expanding Foxp3+ and IL-10+ T cells upon cognate antigen presentation compared with GM-CD8a+ DCs and control DCs.
GM-CD8a− DC-induced Foxp3+ T cells secrete significant amounts of IL-10, suggesting their ability to suppress effector T cells through this cytokine. This notion was substantiated by the observation that addition of anti-IL-10R antibody was sufficient to release mTg-specific effector T cells from the suppressive effect of GM-CD8a− DC-induced Tregs. These results combined with our earlier reports on the reversal of GM-CSF-induced suppression of mTg-specific proliferation by anti-IL-10 antibodies in vitro (27) or EAT by in vivo administration of anti-IL-10R antibodies (26) clearly demonstrate that GM-CD8a− DC-induced Treg-mediated suppression of effector T cell function is IL-10 dependent.
This study, in conjunction with our earlier reports on the prevention and/or treatment of autoimmune conditions with GM-CSF (25–27), suggested that GM-CSF can have a profound modulatory effect primarily on CD8a− DCs and this sub-population alone is sufficient to achieve the therapeutic effect upon adoptive transfer. This protective effect was accompanied by a significant increase in Foxp3+ and IL-10+ T cells in the draining lymph nodes of the recipient mice. It is likely that the adoptively transferred DCs migrated specifically to the draining lymph nodes and induced Tregs upon presentation of antigen to effector T cells. These results further demonstrate that the effects of GM-CSF in vivo are primarily mediated through CD8a− DCs. Although the ability of GM-CSF-exposed CD8a+ DCs in inducing T cell tolerance cannot be completely ruled out, our observation that adoptive transfer of purified GM-CD8a− DCs into CBA/J mice was sufficient to protect recipients from developing EAT upon immunization with mTg validates the notion that tolerance is induced primarily by GM-CSF-exposed CD8a− DCs. Our observations are consistent with a recent study which showed that GM-CSF-influenced CD8a− DCs, but not CD8a+ DCs, exert their tolerogenic function on diabetogenic T cells (28, 35).
In addition to myeloid DCs, CD8a− DC preparations may contain a small percentage of CD11clow plasmacytoid dendritic cells (pDCs). pDCs are known to have the ability to induce antigen-specific Tregs (28). A recent study has shown that GM-CSF-exposed pDCs can reduce expression of pro-inflammatory genes and ameliorate disease in a mouse model of colitis (51). While additional systematic analyses are necessary to understand the effect of GM-CSF on pDCs and the contribution of pDCs in the generation of Tregs by GM-CD8a− DCs, our current study clearly demonstrates the ability of GM-CSF to selectively modulate CD8a− to render them tolerogenic. Our ongoing studies would help define the relative contributions of CD8a− DCs of both myeloid and plasmacytoid origin, and the role of expression of reduced levels of co-stimulatory molecules and pro-inflammatory cytokines in GM-CSF treatment induced immune tolerance and suppression of autoimmunity.
In summary, our results show that GM-CSF acts primarily on CD8a− DCs and renders them tolerogenic most likely by maintaining them in a semi-mature state. These tolerogenic CD8a− DCs induce/expand antigen-specific Foxp3+ and IL-10+ Tregs, which suppress autoreactive T cells through an IL-10-dependent mechanism. These findings provide additional insights into the mode of tolerance induction by GM-CSF and further advocate its potential as a therapeutic molecule in the treatment of autoimmune conditions.
Funding
The National Institutes of Health (grant RO1 AI058190 to B.S.P., NIH R21 AI069848 to C.V.); Myasthenia Gravis Foundation of America post-doctoral fellowship award to J.R.S.
Glossary
Abbreviations
- APC
antigen-presenting cell
- CFSE
carboxyfluorescein succinimidyl ester
- CpG
cytosine-phosphate-guanine
- DC
dendritic cell
- EAT
experimental autoimmune thyroiditis
- Foxp3
forkhead box P3
- GM-CD8a− and GM-CD8a+
CD8a− and CD8a+ DCs from GM-CSF-treated mice
- C-CD8a− and C-CD8a+
CD8a− and CD8a+ DCs from control mice
- i.v.
intravenously
- IL-10R
IL-10 receptor
- mTg
mouse thyroglobulin
- NOD
non-obese diabetic
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid dendritic cells
- RT
reverse transcription
- T1D
type 1 diabetes
- TGF
transforming growth factor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- Treg
regulatory T cell
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Schnorrer P, Behrens GM, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl Acad. Sci. USA. 2006;103:10729. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(-) dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 2001;167:4345. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- 5.Lahoud MH, Proietto AI, Gartlan KH, et al. Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J. Immunol. 2006;177:372. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 6.Grohmann U, Fallarino F, Bianchi R, et al. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J. Immunol. 2001;167:708. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 7.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J. Exp. Med. 1996;183:1789. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazekas de St Groth B. The evolution of self-tolerance: a new cell arises to meet the challenge of self-reactivity. Immunol. Today. 1998;19:448. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 9.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 13.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Shea JJ, Paul WE. Regulation of T(H)1 differentiation—controlling the controllers. Nat. Immunol. 2002;3:506. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 16.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001;166:5448. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 17.Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv. Immunol. 2001;78:233. doi: 10.1016/s0065-2776(01)78005-4. [DOI] [PubMed] [Google Scholar]
- 18.Parajuli P, Mosley RL, Pisarev V, et al. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp. Hematol. 2001;29:1185. doi: 10.1016/s0301-472x(01)00722-6. [DOI] [PubMed] [Google Scholar]
- 19.O'Keeffe M, Hochrein H, Vremec D, et al. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 20.Curti A, Fogli M, Ratta M, Tura S, Lemoli RM. Stem cell factor and FLT3-ligand are strictly required to sustain the long-term expansion of primitive CD34+DR- dendritic cell precursors. J. Immunol. 2001;166:848. doi: 10.4049/jimmunol.166.2.848. [DOI] [PubMed] [Google Scholar]
- 21.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes. 2005;54:78. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 22.Dogan RN, Vasu C, Holterman MJ, Prabhakar BS. Absence of IL-4, and not suppression of the Th2 response, prevents development of experimental autoimmune Graves’ disease. J. Immunol. 2003;170:2195. doi: 10.4049/jimmunol.170.4.2195. [DOI] [PubMed] [Google Scholar]
- 23.Buchsel PC, Forgey A, Grape FB, Hamann SS. Granulocyte macrophage colony-stimulating factor: current practice and novel approaches. Clin. J. Oncol. Nurs. 2002;6:198. doi: 10.1188/02.CJON.198-205. [DOI] [PubMed] [Google Scholar]
- 24.Balducci L, Carreca I. The role of myelopoietic growth factors in managing cancer in the elderly. Drugs. 2002;62(Suppl. 1):47. doi: 10.2165/00003495-200262001-00004. [DOI] [PubMed] [Google Scholar]
- 25.Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J. Immunol. 2006;177:5296. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 26.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 2005;174:7006. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 27.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 2003;170:5511. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 28.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 2007;179:3638. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 29.Esquivel PS, Rose NR, Kong YC. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J. Exp. Med. 1977;145:1250. doi: 10.1084/jem.145.5.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulendran B, Banchereau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165:566. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 32.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484. [PubMed] [Google Scholar]
- 33.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 34.Ganesh BB, Cheatem DM, Vasu C, Prabhakar BS. Induction of peripheral tolerance to treat autoimmune thyroiditis. In: Wiersinga WM, Drexhage HA, Weetman AP, Butz S, editors. The Thyroid and Autoimmunity. Stuttgart, Germany: Georg Thieme Verlag; 2007. 17. [Google Scholar]
- 35.Cheatem DM, Ganesh BB, Gangi E, Vasu C, Prabhakar BS. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin. Immunol. 2008 doi: 10.1016/j.clim.2008.12.001. doi:10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado-Lopez R, De Smedt T, Pajak B, et al. Role of CD8alpha+ and CD8alpha- dendritic cells in the induction of primary immune responses in vivo. J. Leukoc. Biol. 1999;66:242. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 37.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat. Rev. Immunol. 2005;5:251. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 38.Morris GP, Yan Y, David CS, Kong YC. H2A- and H2E-derived CD4+CD25+ regulatory T cells: a potential role in reciprocal inhibition by class II genes in autoimmune thyroiditis. J. Immunol. 2005;174:3111. doi: 10.4049/jimmunol.174.5.3111. [DOI] [PubMed] [Google Scholar]
- 39.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J. Immunol. 2005;174:7433. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 40.Morris GP, Kong YC. Interference with CD4+CD25+ T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J. Autoimmun. 2006;26:24. doi: 10.1016/j.jaut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Morris GP, Chen L, Kong YC. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell. Immunol. 2003;226:20. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 42.O'Sullivan BJ, Thomas HE, Pai S, et al. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J. Immunol. 2006;176:7278. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- 43.Brinster C, Shevach EM. Costimulatory effects of IL-1 on the expansion/differentiation of CD4+CD25+Foxp3+ and CD4+CD25+Foxp3- T cells. J. Leukoc. Biol. 2008;84:480. doi: 10.1189/jlb.0208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnquist HR, Sumpter TL, Tsung A, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 2008;181:62. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 46.Hurst J, von Landenberg P. Toll-like receptors and autoimmunity. Autoimmun. Rev. 2008;7:204. doi: 10.1016/j.autrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J. Immunol. 2005;174:131. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- 48.Zaccone P, Fehervari Z, Blanchard L, Nicoletti F, Edwards CK, 3rd, Cooke A. Autoimmune thyroid disease induced by thyroglobulin and lipopolysaccharide is inhibited by soluble TNF receptor type I. Eur. J. Immunol. 2002;32:1021. doi: 10.1002/1521-4141(200204)32:4<1021::AID-IMMU1021>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Bleich A, Janus LM, Smoczek A, et al. CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction. Gastroenterology. 2009;136:278. doi: 10.1053/j.gastro.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Kwan WH, Boix C, Gougelet N, Fridman WH, Mueller CG. LPS induces rapid IL-10 release by M-CSF-conditioned tolerogenic dendritic cell precursors. J. Leukoc. Biol. 2007;82:133. doi: 10.1189/jlb.0406267. [DOI] [PubMed] [Google Scholar]
- 51.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173:4433. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 52.Lau AW, Biester S, Cornall RJ, Forrester JV. Lipopolysaccharide-activated IL-10-secreting dendritic cells suppress experimental autoimmune uveoretinitis by MHCII-dependent activation of CD62L-expressing regulatory T cells. J. Immunol. 2008;180:3889. doi: 10.4049/jimmunol.180.6.3889. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 54.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 2002;3:33. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 56.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25 + 4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl Acad. Sci. USA. 2002;99:8213. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 58.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 2002;3:756. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 59.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 60.Wilczynski JR, Radwan M, Kalinka J. The characterization and role of regulatory T cells in immune reactions. Front. Biosci. 2008;13:2266. doi: 10.2741/2840. [DOI] [PubMed] [Google Scholar]
- 61.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]