Abstract
Objective
Because depressive symptoms are prevalent among patients with peripheral arterial disease (PAD), our goal was to study the effect of depressive symptoms over time on functional decline among patients with PAD.
Methods
We conducted a prospective cohort study of 417 patients with PAD followed annually for 2 years. A Geriatric Depression Scale Short Form (GDS-S) score >5 was considered positive for depressive symptoms. Depressive symptom categories based on annual GDS-S measures included persistent, new, resolved, and no depressive symptoms. Outcome variables were change in 6-minute walk distance, 4-meter fast walking velocity, and short physical performance battery (0–12 scale, 12 = best). Results are adjusted for age, sex, race, body mass index, marital status, exercise level, smoking, ankle brachial index, leg symptoms, comorbidities, beta-blocker medication use, anti-depressant medications, and interim medical events.
Results
In adjusted analyses, patients with new depressive symptoms had greater annual decline in fast walking velocity compared with that of patients with no depressive symptoms (−0.08 versus −0.01 meters/second per year, p = .02). Patients with persistent depressive symptoms had greater annual decline in 6-minute walk distance (−86.4 versus −41.5 feet/yr, p = .04), fast walking velocity (−0.08 versus −0.01 meters/second per year, p = .004), and short physical performance battery (−0.73 versus −0.18 per year, p = .005) compared with that of patients with no depressive symptoms.
Conclusions
Among patients with PAD, persistent and new depressive symptoms are associated with greater annual decline in functional performance. Further study is needed to determine the mechanisms of these associations and whether treatment of depressive symptoms prevents functional decline in persons with PAD.
Keywords: depression, functional status, peripheral arterial disease, intermittent claudication
Introduction
Compared with patients without lower extremity peripheral arterial disease (PAD), patients with PAD have more functional impairment and a faster rate of decline in lower extremity functioning leading to more disability (1–4). Risk factors contributing to PAD-related decline are still not fully understood and there are few effective treatments for PAD to abate this decline. Coexisting conditions such as depression might affect the evolution of functional decline in PAD. The identification of mechanisms of functional decline in patients with PAD could lead to the development of new therapies for preventing functional decline in these patients. Specifically, if greater depressive symptoms are associated with an increased rate of functional decline in PAD, then identifying and treating depressive symptoms might improve the outcome of PAD patients.
With up to 26% of patients with PAD having a significant number of depressive symptoms (5), depression is a prevalent comorbidity among these patients. Our previous work shows that depressive symptoms are associated with functional impairment among patients with PAD in cross-sectional data (5). However, the association between depressive symptoms and objective measures of functional decline in persons with PAD has not been studied previously. Although prior studies have shown that depression is associated with increased rates of functional decline in non-PAD patients (6–8), it is unclear whether this association occurs in PAD patients. Because patients with PAD have greater functional impairment than non-PAD patients and a higher prevalence of significant depressive symptoms (1–3), it is important to study the association between depressive symptoms and functional decline among patients with PAD.
In this study, our main objective was to examine the longitudinal effects of depressive symptoms and change in depressive symptoms on functional decline. We hypothesized that patients with persistent or new depressive symptoms would have more functional decline than would those with resolved or no depressive symptoms.
Methods
We performed a prospective cohort study of men and women with PAD enrolled in the Walking and Leg Circulation Study (WALCS) (3), a prospective observational study of adults age ≥55. The Institutional Review Board at Northwestern University approved the study. Cohort participants were enrolled from October 1998 through January 2000. All PAD participants had a confirmed ankle brachial index (ABI) of <0.90 at their baseline visit. Outcome measures were performed at baseline and at two annual follow-up visits.
Exclusion Criteria
Exclusion criteria have been previously reported (4). Patients who were nursing home residents, wheelchair-bound, or had dementia, recent major surgery, or foot or leg amputations were excluded. Non-English speaking patients were excluded because investigators were not fluent in non-English languages. Participants who underwent interim lower extremity revascularization or hip replacements were excluded from analyses because these procedures are likely to substantially influence the natural history of lower extremity functioning independent of depressive symptoms. There were no exclusion criteria based on depressive symptom score.
Depressive Symptom Categories
Depressive symptoms were measured annually using the Geriatric Depression Scale Short Form (GDS-S), a 15-item questionnaire with scores ranging from 0 to 15 assessing the number of depressive symptoms (9). The objective of our analysis is to evaluate the effect of a longitudinal pattern of depression on functional performance. Treating the GDS-S score as a continuous variable would not allow for a simple summary measure of the presence or absence of depression for this objective. For these reasons, we used the cutpoint of GDS-S score >5 for the creation of the depression pattern categories because a score of >5 has been shown in a prior study to have a sensitivity of 92% and a specificity of 81% for clinical depression, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-IIIR) (9).
To capture the dynamic nature of depressive symptoms, we coded depressive symptoms in each follow-up year as a four-state ordinal variable based on the results of each follow-up year and the prior year GDS-S scores. At each follow-up visit, depressive symptom status was determined relative to the prior year. At the first follow-up visit (FV-1), four categories were defined: persistent depressive symptoms (a score >5 at baseline and FV-1), new depressive symptoms (a score ≤5 at baseline but a score >5 at FV-1), resolved depressive symptoms (a score >5 at baseline but a score ≤5 at FV-1), and no depressive symptoms (the reference group, a score ≤5 at baseline and FV-1). These same categories were defined at the second follow-up visit (FV-2) based on the depressive symptom status at FV-2 relative to FV-1. The four-state depressive symptom variable may be interpreted as a reflection of depressive symptoms for each one-year follow-up period. Each participant could contribute up to two person-years of follow-up.
Functional Performance Measures
Six-minute Walk Distance
In older adults, the 6-minute walk test is a better measure of community walking ability than is treadmill performance (10–13). In accordance with a standardized protocol (14), participants were instructed to walk up and down a 100-foot hallway for 6 minutes, covering as much distance as possible during that time.
Rapid Pace 4-meter Walking Velocity
Walking velocity was measured with a 4-meter walk performed at “fastest” pace. The walk was performed twice and the faster walk velocity was used in the analyses (15,16).
Short Physical Performance Battery
This global measure of lower-extremity function combines data from three tests: usual paced 4-meter walking velocity, standing balance, and time to rise five times consecutively from a seated position (15,16). The short physical performance battery predicts mobility loss, nursing home placement, and mortality among community-dwelling elders (15,16). To calculate the short physical performance battery score, a score of 0 to 4 was assigned for performance on each test, based on cut-points derived from normative data of community samples (15,16). These scores were combined to obtain the short physical performance battery score (range 0 to 12, 12 = best).
Education Level
Education level was categorized by the highest level of education completed: less than high school, high school or college graduate, and graduate or professional school.
Marital Status
Marital status was assessed by patient self-report. Response choices included a) married or living with a partner as if married; b) divorced, separated, or widowed; and c) single.
Current Smoking
Current cigarette smoking was assessed by patient report.
Ankle Brachial Index (ABI)
Using established methods, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries (17,18). Each pressure was measured twice. The ABI was calculated in each leg by dividing the mean of all four dorsalis pedis and posterior tibial pressures by the mean of the four brachial pressures (17). Average brachial pressures in the arm with the highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg, because, in such cases, subclavian stenosis was possible (17,18). The lowest leg ABI was used in analyses.
Body Mass Index
Body Mass Index (BMI) was calculated as weight in kilograms divided by height squared in meters.
Exercise Level
At each visit, participants were asked how often they walked for exercise during the prior two weeks. Exercise frequency was entered as a categorical variable (none, ≤3 times/wk, >3 times/wk) because of the extremely left-skewed distribution.
Antidepressant Use or Beta-Blocker Use
Participants listed their medications at each visit. The study principal investigator (M.M.M.) reviewed all medications and classified them according to whether each was an antidepressant medication, beta-blocker medication, or other medication class. The study principal investigator was blinded to other patient characteristics during this classification.
Comorbidities
Comorbidities were identified and adjudicated annually based on algorithms developed for the Women's Health and Aging Study (19). These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and physician questionnaire. We used the American College of Rheumatology criteria to diagnose knee and hip arthritis (20,21). Comorbidities assessed included diabetes mellitus, pulmonary disease, musculoskeletal conditions, cardiac disease, stroke, coronary or carotid revascularization, and cancer. Musculoskeletal conditions included lower-extremity arthritis, sciatica, herniated disk, and spinal stenosis. We defined cardiac disease as the presence of angina, myocardial infarction, or heart failure. Coronary or carotid revascularization included carotid endarterectomy, percutaneous coronary angiography, and coronary artery bypass surgery. Cancer was defined as history of any cancer other than nonmelanoma skin cancer.
Leg Symptoms
Leg symptoms were categorized into five groups, using the San Diego claudication questionnaire (2). Four groups had exertional leg symptoms based on an affirmative response to the question, “Do you get pain in either leg or buttock on walking?” and were further classified as a) intermittent claudication; b) leg pain on exertion and rest; c) atypical exertional leg pain/carry on (exertional leg symptoms that do not stop the individual from walking); d) atypical exertional leg pain/stop (exertional leg symptoms that stop the individual from walking, but are otherwise not consistent with classic intermittent claudication). A fifth group was defined as asymptomatic (no exertional leg pain).
Statistical Methods
Baseline characteristics stratified by depressive symptom category were compared using general linear models for continuous variables and chi-square tests for categorical variables.
We used mixed effects linear regression models (22) with annual changes in functional performance measures as the response variable to evaluate the dynamic association between depressive symptoms and changes in functional performance measures. A subject-specific random-effect model was used to account for the potential correlations among successive annual differences in each functional measure of the same participant.
In our multivariable model, we included baseline values for age, education, smoking status, diabetes mellitus, pulmonary disease, and musculoskeletal conditions. For each follow-up visit, we also included time-dependent variables using prior year values for functional performance, exercise level, and ABI. We included time dependent variables, using values from the same follow-up visit, for BMI, marital status, antidepressant use, beta-blocker use, and leg symptoms. For cardiac disease, coronary or carotid revascularization, stroke, and cancer, we hypothesized that participants would be affected differently by recent events compared with more distant events. Thus, for these comorbidities, we included separate variables for events occurring a) since the previous year's visit and b) before the previous year's visit.
We chose to include the above covariables because we considered them as potential confounders in the relationship between depression and functional performance. For example, significant comorbidities may be expected to be associated with decline in functioning (23). Smoking has been shown to be associated with progression of PAD (24,25). Exercise, BMI, ABI, and leg symptoms have been shown to be associated with functional impairment or decline, or both (3,4,23,26,27). Education is also a reasonable determinant of functional decline (28,29). The additional covariables, marital status and beta-blocker use, were added to the multivariable model in response to comments from peer review.
The missing data were handled using a pattern-mixture model in the mixed effects linear regression analysis. Specifically, the different patterns of missing data were included in the analysis as binary indicator covariates. By including patterns of missing data in the analyses and averaging over these patterns using adjusted least squares means, one can obtain an unbiased estimate of the marginal means when one can not ignore the missing data (30).
As requested by peer review, we also performed sensitivity analyses using current and prior years' GDS scores as continuous variables in the mixed effects linear regression to confirm that the categorization of GDS scores did not lead to artificial findings. The results from these analyses were similar to those reported in this article. We chose to present the multivariable model using the depressive symptom categories created using dichotomized GDS scores because a) our primary research question was to examine the longitudinal effects of specific predefined categories of depression status on functional performance; b) the cutpoint of a GDS score >5 has been commonly used clinically as a depression screen; c) for ease of interpretation; and d) statistical testing of our data suggest that the relationship between the change in GDS scores and functional performance measures is not linear for the 6-minute walk.
Analyses were performed using SAS software, version 9.1 (SAS Institute, Inc., Cary, NC). All p values were 2 tailed, and p < .05 was considered statistically significant.
Results
At baseline, the WALCS study included 460 patients with PAD (3). Four hundred ten PAD patients returned for FV-1 (26 had died or dropped out, 17 had interim lower-extremity revascularization, and 7 had hip or knee replacement surgery). Three hundred fifty-two patients were evaluated at FV-2 (35 had died or dropped out, 16 had lower-extremity revascularization, and 7 had hip or knee replacement surgery) (Figure 1). At FV-1, 352 had complete data on depression assessment for depression categorization. After FV-2, 313 had complete data for depression categorization.
Figure 1.
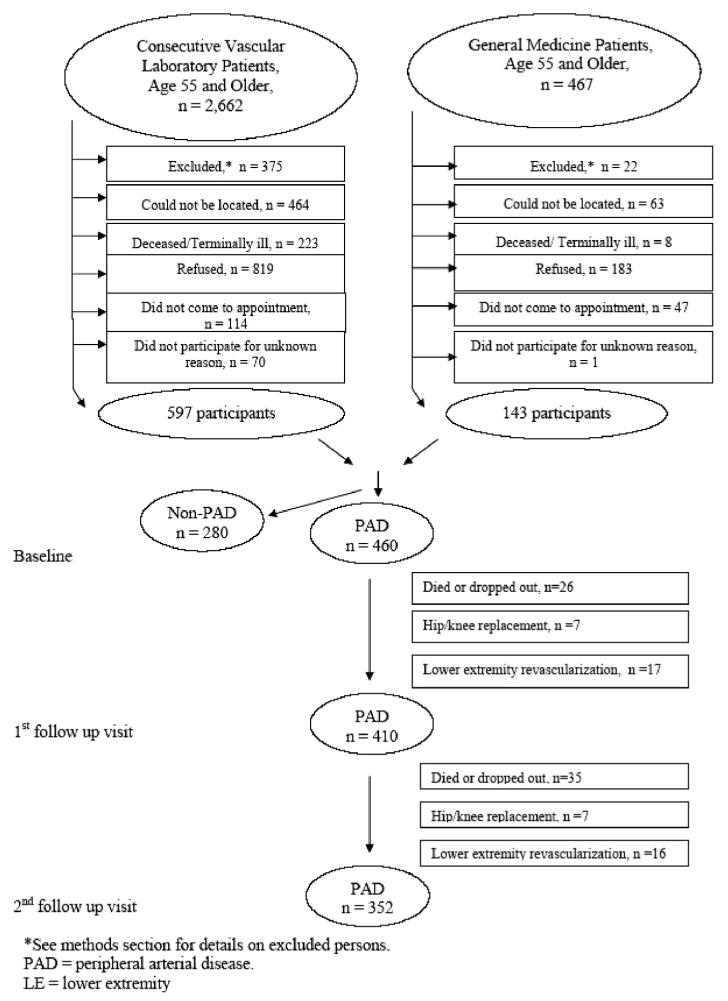
Participant recruitment and follow-up flowchart.
At baseline, the prevalence of more than five depressive symptoms was 24.5% among this cohort. At FV-1, 70.3% of these individuals had persistent depressive symptoms, whereas 29.7% had resolution of their depressive symptoms. Of those without depressive symptoms at baseline, 14.2% developed new depressive symptoms at FV-1. At FV-2, 66.3% of those with more than five depressive symptoms at the prior year's visit had persistent depressive symptoms, whereas 33.7% had resolution of their depressive symptoms. Of those without depressive symptoms at FV-1, 8.9% developed new depressive symptoms. Of the 665 person-years of follow-up, 117 (17.6%) person-years were from individuals with persistent depressive symptoms, 58 (8.7%) from individuals with new depressive symptoms, and 54 (8.1%) from individuals with resolved depressive symptoms.
Table 1 shows cohort characteristics stratified by depressive symptom category. PAD participants with persistent depression were less educated, were more likely to be female, and had an increased prevalence of stroke, pulmonary disease, musculoskeletal disease, and antidepressant use compared with the group without depressive symptoms. Individuals with persistent depressive symptoms also had more leg symptoms and a lower prevalence of walking for exercise at least three times per week. Individuals with persistent depressive symptoms had significantly lower 6-minute walk distances, 4-meter fast walking velocities, and short physical performance battery scores at baseline than did individuals with no depressive symptoms. Participants with resolved depression were younger and had an increased prevalence of diabetes mellitus and cardiac disease compared with those without depressive symptoms (Table 1).
TABLE 1. Characteristics of Men and Women With Peripheral Arterial Disease Stratified by Depressive Symptom Category (N = 665 person-years).
| Persistent Depressive Symptoms (N = 117 person-years from 78 individuals) | New Depressive Symptoms (N = 58 person-years from 58 individuals) | Resolved Depressive Symptoms (N = 54 person-years from 54 individuals) | No Depressive Symptoms (N = 436 person-years from 258 individuals) | p Value for Trend | |
|---|---|---|---|---|---|
| Baseline1 | |||||
| Age, mean (SD) | 71.7 (8.7) | 73.5 (8.7) | 68.8 (7.9)* | 71.8 (8.5) | .03 |
| Female, % | 49.6* | 48.3 | 38.9 | 36.5 | .04 |
| African-American, % | 18.8 | 6.9 | 13.0 | 15.6 | .21 |
| BMI ≥ 25, % | 62.6 | 65.5 | 79.3 | 70.4 | .14 |
| Current smokers, % | 29.9* | 19.0 | 29.6 | 20.0 | .06 |
| Beta-blocker use, % | 35.9 | 37.9 | 33.3 | 32.3 | .78 |
| Marital status | * | .05 | |||
| Married/living with partner, % | 43.6 | 56.9 | 51.9 | 55.7 | |
| Divorced/separated/widowed, % | 40.2 | 32.8 | 33.3 | 34.9 | |
| Single, % | 16.2 | 10.3 | 14.8 | 9.4 | |
| Education level | * | .002 | |||
| <High school, % | 14.5 | 13.8 | 11.1 | 7.6 | |
| High school or college, % | 72.7 | 74.1 | 77.8 | 69.2 | |
| Professional or graduate school, % | 12.8 | 12.1 | 11.1 | 23.2 | |
| At beginning of person-year2 | |||||
| cardiac disease, % | 56.4 | 65.5 | 74.1* | 61.5 | .16 |
| stroke, % | 20.5* | 12.1 | 13.0 | 11.0 | .06 |
| cancer, % | 18.0 | 17.2 | 16.7 | 14.7 | .82 |
| diabetes mellitus, % | 33.3 | 29.3 | 44.4* | 31.4 | .26 |
| pulmonary disease, % | 43.4* | 39.7 | 48.2 | 34.2 | .09 |
| musculoskeletal disease, % | 72.7* | 48.3 | 50.0 | 44.0 | <.001 |
| Walking for exercise, frequency | * | .003 | |||
| ≥3 times/week, % | 20.5 | 29.3 | 28.3 | 38.3 | |
| <3 times/week, % | 22.2 | 27.6 | 20.8 | 20.0 | |
| none, % | 57.3 | 43.1 | 50.9 | 41.7 | |
| Leg symptoms | * | ||||
| No exertional pain, % | 12.0 | 17.2 | 18.5 | 23.9 | .91 |
| Pain on exertion and rest, % | 27.4 | 24.1 | 16.7 | 11.9 | |
| Leg pain/stop, % | 17.1 | 22.4 | 22.2 | 20.2 | |
| Intermittent claudication, % | 36.8 | 29.3 | 37.0 | 31.2 | |
| Leg pain/carry-on, % | 6.8 | 6.9 | 5.6 | 12.8 | |
| Anti-depressant use, % | 21.4* | 10.3 | 14.8 | 3.9 | <.001 |
| At end of person-year3 | |||||
| GDS-S score, mean (SD) | 8.6 (2.8)* | 7.0 (2.4)* | 2.6 (1.4)* | 1.5 (1.3) | <.001 |
| Baseline1 | |||||
| 6-min walk distance, mean feet (SD) | 963 (392)* | 1114 (390)* | 1134 (361) | 1216 (354) | <.001 |
| 4-m fast walking velocity (m/s), mean (SD) | 1.13 (0.30)* | 1.19 (0.29) | 1.18 (0.31) | 1.26 (0.26) | <.001 |
| Short physical performance battery (0 to 12 scale, 12 = best), mean (SD) | 8.9 (2.8)* | 11.5 (4.2) | 9.8 (2.5)* | 10.0 (3.5) | <.001 |
p < .05 for pairwise comparison between the group and the no depressive symptoms group.
Baseline = at time of study enrollment.
At the beginning of person-year = the value at baseline for person-year ending at the first annual follow-up visit or the value at the first annual follow-up visit for person-year ending at the second annual follow-up visit.
At the end of person-year = the value at the first annual follow-up visit for the person year ending at the first annual follow-up visit or the value at the second annual follow-up visit for the person year ending at the second annual follow-up visit.
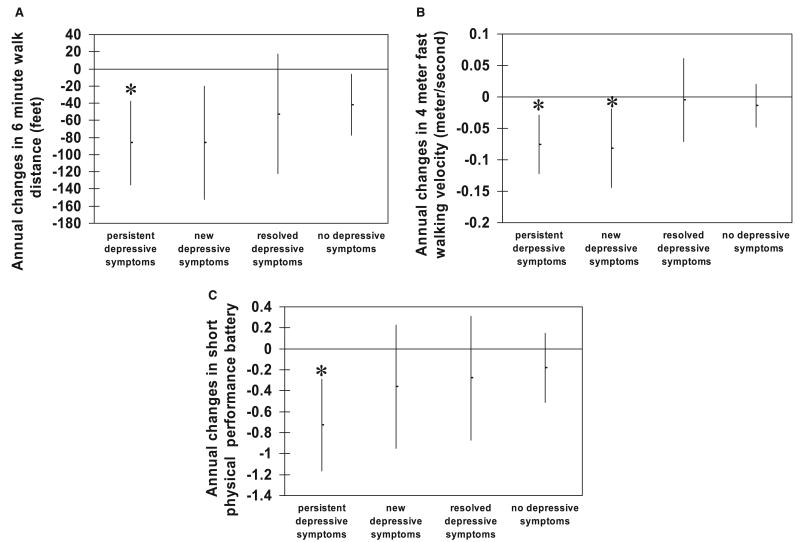
Compared with their baseline performance, the referent group, PAD patients with no depressive symptoms, had a statistically significant average annual decline in 6-minute walk distance (−41.5 feet, 95% confidence interval (CI) −76.9 to −6.2), a statistical trend toward an average annual decline in fast 4-meter walking velocity (−0.01 meters/second per year, 95% CI −0.05 to 0.02), and an average annual decline in the short physical performance battery (−0.18 per year, 95% CI −0.51 to 0.15).
In analyses adjusted for age, gender, race, marital status, BMI, ABI, education, comorbidities, exercise level, smoking status, antidepressant and beta-blocker medications, leg symptoms, and baseline performance scores, PAD patients with persistent depressive symptoms had a significantly greater average annual decline in 6-minute walk distance (−86.4 feet/yr versus −41.5 feet/yr, p = .04), fast 4-meter walking velocity (−0.08 meters/second per year versus −0.01 meters/second per year, p < .01), and short physical performance battery (−0.73 per year versus −0.18 per year, p < .01) than did those with no depressive symptoms (Figure 2). PAD patients with new depressive symptoms had a significantly greater average annual decline in fast 4-meter walking velocity compared with the rate of those with no depressive symptoms (−0.08 meters/second per year versus −0.01 meters/second per year, p = .02). PAD patients with resolved depressive symptoms did not differ significantly in annual rate of decline in any of the performance measures compared with those with no depressive symptoms (Figure 2).
Figure 2.
Adjusted annual change in (A) 6-minute walk distance, (B) fast 4-meter walking velocity, and (C) short physical performance battery, by depressive symptom category among persons with peripheral arterial disease. *p < .05 compared with no depressive symptoms.
To better understand the magnitude of the association of persistent or new depressive symptoms and annual decline in functional performance measures, we compared the effect sizes with those of other clinical comorbidities or events. For example, the association between persistent depressive symptoms and average annual decline in 6-minute walk distance is of the same order of magnitude as having diabetes mellitus or having had a coronary or carotid revascularization procedure more than a year ago (Figure 3). Although recently diagnosed stroke or cancer were not statistically significantly associated with decline in 6-minute walk distance, the magnitudes of their associated effect sizes are similar to that of persistent depressive symptoms (Figure 3).
Figure 3.
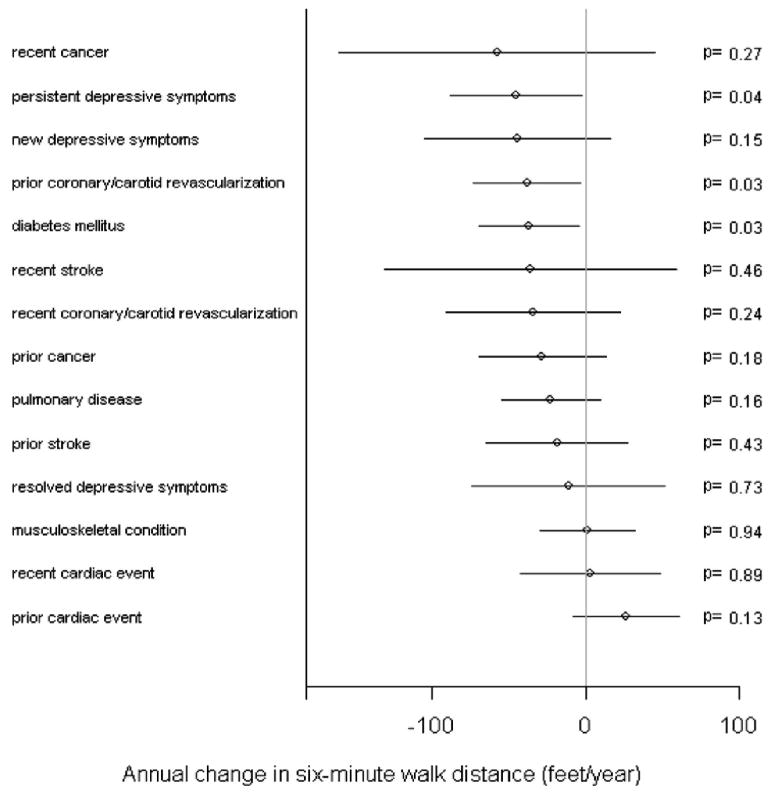
Adjusted magnitudes of annual change in 6-minute walk distance for various clinical factors among persons with peripheral arterial disease. The lines around the point estimates represent 95% confidence intervals (CIs).
With regard to annual change in fast 4-meter walking velocity, a history of pulmonary disease had an association with an effect size similar to that of persistent depressive symptoms (Figure 4). Although the effect size for stroke within the past year on fast 4-meter walking velocity was not statistically significant, the effect size was similar to that of persistent or new depressive symptoms (Figure 4). Persistent depressive symptoms were associated with a magnitude of annual decline in short physical performance battery score comparable with that of a cardiac event occurring within the past year (Figure 5).
Figure 4.
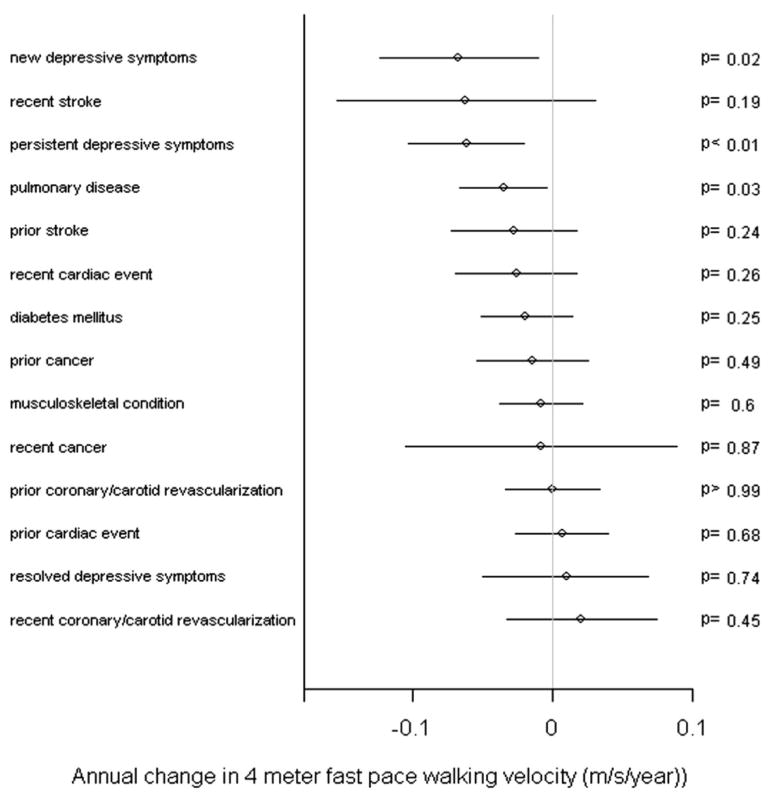
Adjusted magnitudes of annual change in fast 4-meter walking velocity for various clinical factors among persons with peripheral arterial disease. The lines around the point estimates represent 95% CIs.
Figure 5.
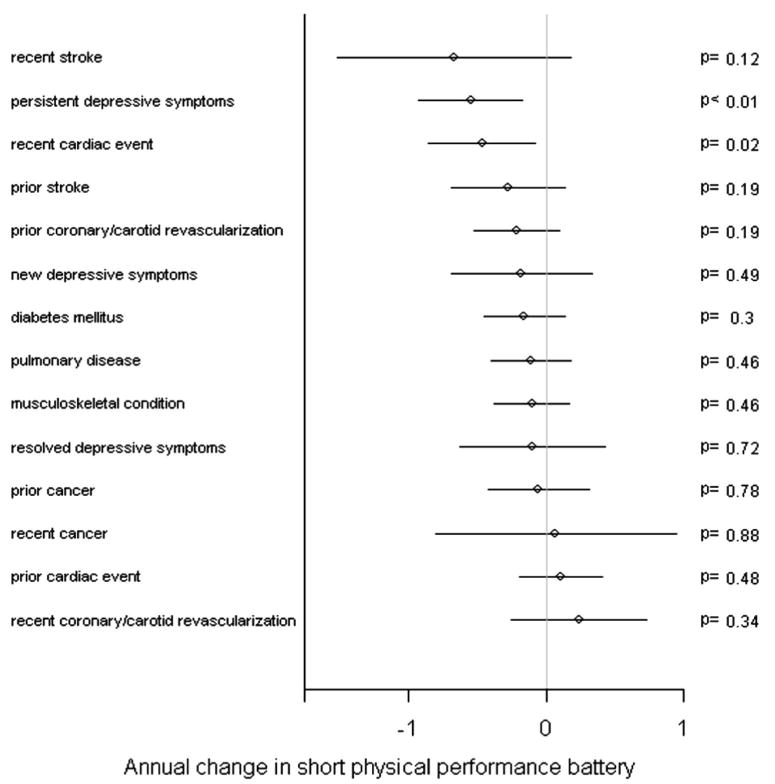
Adjusted magnitudes of annual change in short physical performance battery for various clinical factors among persons with peripheral arterial disease. The lines around the point estimates represent 95% CIs.
Discussion
PAD patients who were persistently depressed had a greater average annual decline in objective measures of functioning than did PAD patients without any depression at either the beginning or end of the year of follow-up. Among patients with PAD, the magnitude of the association between persistent depressive symptoms and functional decline is comparable with that of recent cardiovascular events. These associations persisted after adjustment for known and potential confounders.
These findings underscore the importance of identifying depressive symptoms among PAD patients. A previous study showed that persons with PAD have an increased rate of functional decline compared with that of persons without PAD (4). Results reported here show that PAD patients with persistent depressive symptoms are at particularly increased risk of functional decline. Given the important impact of functional performance on use of medical services and quality of life (8), treatment of depressive symptoms should be considered as a potentially cost-effective intervention among patients with PAD. However, further study is needed.
Previous studies have found that the chronicity of depression increases its impact on functional status in the elderly without PAD (31,32). One study showed that treatment of depression improves self-reported physical functioning in elders (33). To our knowledge, no prior studies have prospectively assessed associations between depressive symptoms or the chronicity of depressive symptoms and functional decline in persons with PAD.
Several limitations must be considered when interpreting our results. First, there were relatively small numbers of person-years of follow-up representing new or resolved depressive symptoms. Second, because only those who completed the questionnaires were included, there is the potential for survivor bias. The nonsurvivors or those who dropped out of the study had a higher prevalence of depressive symptoms. For example, the 26 participants who died or were lost to follow-up before FV-1 had a greater mean GDS-S score than that of the 434 participants who had a first year follow-up visit (5.25 versus 3.18, p = .002). However, this bias would lead to an underestimation of the association between depressive symptoms and functional decline. Third, the assessment of functional performance depends on participant motivation, and potential lack of effort among patients with persistent depression could lead to underestimation of functional performance. This limitation is less important when looking at intra-individual change in performance. Fourth, in the multivariable analyses, the time-dependent variables of ABI or walking exercise frequency can be entered for the same year's values in order to account for effects as confounders rather than using the prior year's values in order to examine their effects as predictor variables. However, even with exercise frequency or ABI modeled using the same year's values in alternative models, the findings were consistent with those reported above. Fifth, as in all measures of association, there is the possibility of unmeasured confounders such as the impact of comorbidity severity or medication adherence on our findings.
One potential mechanism for the association between depressive symptoms and functional decline is a lower activity level among depressed participants. Greater depressive symptoms have been previously shown to be associated with reduced frequency of walking for exercise (34,35). In our cohort, the association between depressive symptoms and functional decline was present even after accounting for frequency of walking for exercise in multivariable models. Supervised and self-directed exercise has been shown to be associated with improvement or less decline in lower extremity performance among patients with PAD (27,36,37). However, other studies have not demonstrated consistent benefits of unsupervised exercise on walking performance (37,38).
Other potential mechanisms for the association between depressive symptoms and greater functional decline include the possibility that depressed patients have higher levels of inflammation than do nondepressed patients. Compared with nondepressed adults, depressed but otherwise healthy adults have higher circulating levels of interleukin-6 (39–41). Interleukin-6 is associated with increased rates of functional decline in the elderly (42,43). Depression could also trigger dysregulation of the neurohormonal systems responsible for cortisol and catecholamine secretion (44).
In summary, we found that among patients with PAD, persistent depressive symptoms are associated with a greater rate of annual decline in functional performance compared with that of patients with resolved or no depressive symptoms. The magnitude of this association was comparable with that of recent cardiovascular events on functional decline. Given the high prevalence of depressive symptoms among patients with PAD and the relative lack of interventions to prevent declining functional status among patients with PAD, further studies are needed to determine the mechanism of this association and whether screening for and treating depressive symptoms could decrease the rate of decline in lower extremity functioning among persons with PAD.
Acknowledgments
Supported by grants #R01-HL58099, #R01-HL64739, and #R01-HL071223 from the National Heart Lung and Blood Institute and by grant #RR-00048 from the National Center for Research Resources, National Institutes of Health.
- ABI
Ankle Brachial Index
- BMI
Body Mass Index
- FV-1
first follow-up visit
- FV-2
second follow-up visit
- GDS-S
Geriatric Depression Scale Short Form
- PAD
Peripheral Arterial Disease
- WALCS
Walking and Leg Circulation Study
References
- 1.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101:1007–12. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, Chan C, Schneider J, Sharma L, Taylor LM, Arseven A, Quann M, Celic L. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18:461–7. doi: 10.1046/j.1525-1497.2003.20527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. Depression without sadness: functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 1997;45:570–8. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 7.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–6. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 8.Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–9. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–54. [PubMed] [Google Scholar]
- 10.Swerts PM, Mostert R, Wouters EF. Comparison of corridor and treadmill walking in patients with severe chronic obstructive pulmonary disease. Phys Ther. 1990;70:439–42. doi: 10.1093/ptj/70.7.439. [DOI] [PubMed] [Google Scholar]
- 11.Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging (Milano) 2000;12:274–80. doi: 10.1007/BF03339847. [DOI] [PubMed] [Google Scholar]
- 12.Peeters P, Mets T. The 6-minute walk as an appropriate exercise test in elderly patients with chronic heart failure. J Gerontol A Biol Sci Med Sci. 1996;51:M147–51. doi: 10.1093/gerona/51a.4.m147. [DOI] [PubMed] [Google Scholar]
- 13.Greig C, Butler F, Skelton D, Mahmud S, Young A. Treadmill walking in old age may not reproduce the real life situation. J Am Geriatr Soc. 1993;41:15–8. doi: 10.1111/j.1532-5415.1993.tb05941.x. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–71. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 18.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–9. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Fried L, Simonsick E. The Women's Health and Aging Study: health and social characteristics of older women with disability. Bethesda: National Institute on Aging; 1995. Appendix E. [Google Scholar]
- 20.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Feldman D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 22.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 23.McDermott MM, Guralnik JM, Ferrucci L, Criqui MH, Greenland P, Tian L, Liu K, Tan J. Functional decline in lower-extremity peripheral arterial disease: associations with comorbidity, gender, and race. J Vasc Surg. 2005;42:1131–7. doi: 10.1016/j.jvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Juergens JL, Barker NW, Hines EA., Jr Arteriosclerosis obliterans: review of 520 cases with special reference to pathogenic and prognostic factors. Circulation. 1960;21:188–95. doi: 10.1161/01.cir.21.2.188. [DOI] [PubMed] [Google Scholar]
- 25.Liedberg E, Persson BM. Age, diabetes and smoking in lower limb amputation for arterial occlusive disease. Acta Orthop Scand. 1983;54:383–8. doi: 10.3109/17453678308996589. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Criqui MH, Ferrucci L, Guralnik JM, Tian L, Liu K, Greenland P, Tan J, Schneider JR, Clark E, Pearce WH. Obesity, weight change, and functional decline in peripheral arterial disease. J Vasc Surg. 2006;43:1198–204. doi: 10.1016/j.jvs.2006.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, Ferrucci L, Criqui MH, Greenland P, Guralnik JM, Tian L, Schneider JR, Pearce WH, Tan J, Martin GJ. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med. 2006;144:10–20. doi: 10.7326/0003-4819-144-1-200601030-00005. [DOI] [PubMed] [Google Scholar]
- 28.Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–50. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 29.Zunzunegui MV, Nunez O, Durban M, Garcia de Yebenes MJ, Otero A. Decreasing prevalence of disability in activities of daily living, functional limitations and poor self-rated health: a 6-year follow-up study in Spain. Aging Clin Exp Res. 2006;18:352–8. doi: 10.1007/BF03324830. [DOI] [PubMed] [Google Scholar]
- 30.Fitzmaurice GM, Laird NM, Shneyer L. An alternative parameterization of the general linear mixture model for longitudinal data with non-ignorable drop-outs. Stat Med. 2001;20:1009–21. doi: 10.1002/sim.718. [DOI] [PubMed] [Google Scholar]
- 31.Penninx BW, Deeg DJ, van Eijk JT, Beekman AT, Guralnik JM. Changes in depression and physical decline in older adults: a longitudinal perspective. J Affect Disord. 2000;61:1–12. doi: 10.1016/s0165-0327(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 32.Penninx BW, Guralnik JM, Mendes de Leon CF, Pahor M, Visser M, Corti MC, Wallace RB. Cardiovascular events and mortality in newly and chronically depressed persons > 70 years of age. Am J Cardiol. 1998;81:988–94. doi: 10.1016/s0002-9149(98)00077-0. [DOI] [PubMed] [Google Scholar]
- 33.Callahan CM, Kroenke K, Counsell SR, Hendrie HC, Perkins AJ, Katon W, Noel PH, Harpole L, Hunkeler EM, Unutzer J. Treatment of depression improves physical functioning in older adults. J Am Geriatr Soc. 2005;53:367–73. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet F, Irving K, Terra JL, Nony P, Berthezene F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–44. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 35.van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32:81–7. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 36.McDermott MM, Tiukinhoy S, Greenland P, Liu K, Pearce WH, Guralnik JM, Unterreiner S, Gluckman TJ, Criqui MH, Ferrucci L. A pilot exercise intervention to improve lower extremity functioning in peripheral arterial disease unaccompanied by intermittent claudication. J Cardiopulm Rehabil. 2004;24:187–96. doi: 10.1097/00008483-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–8. doi: 10.1016/s0741-5214(97)70352-5. discussion 318–9. [DOI] [PubMed] [Google Scholar]
- 38.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 39.Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14:103–7. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 40.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–8. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 41.Dentino AN, Pieper CF, Rao MK, Currie MS, Harris T, Blazer DG, Cohen HJ. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 44.Plotsky P, Owens MJ, Nemeroff CB. Neuropeptide alterations in mood disorders. In: Bloom F, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 971–81. [Google Scholar]