Abstract
Context
Maintaining independence of older persons is a public health priority, and identifying the factors that contribute to decline in physical function is needed to prevent or postpone the disablement process. The potential deleterious effect of poor nutrition on decline in physical function in older persons is unclear.
Objective
To determine whether a low serum concentration of micronutrients is associated with subsequent decline in physical function among older men and women living in the community.
Design, Setting, and Participants
Longitudinal study of 698 community-living persons 65 years or older who were randomly selected from a population registry in Tuscany, Italy. Participants completed the baseline examination from November 1, 1998, through May 28, 2000, and the 3-year follow-up assessments from November 1, 2001, through March 30, 2003.
Main Outcome Measure
Decline in physical function was defined as a loss of at least 1 point in the Short Physical Performance Battery during the 3-year follow-up. Odds ratios (ORs) were calculated for the lowest quartile of each nutrient using the other 3 quartiles combined as the reference group. Two additional and complementary analytical approaches were used to confirm the validity of the results.
Results
The mean decline in the Short Physical Performance Battery score was 1.1 point. In a logistic regression analysis that was adjusted for potential confounders, only a low concentration of vitamin E (<1.1 μg/mL [<24.9 μmol/L]) was significantly associated with subsequent decline in physical function (OR, 1.62; 95% confidence interval, 1.11-2.36; P=.01 for association of lowest α-tocopherol quartile with at least a 1-point decline in physical function). In a general linear model, the concentration of vitamin E at baseline, when analyzed as a continuous measure, was significantly associated with the Short Physical Performance Battery score at follow-up after adjustment for potential confounders and Short Physical Performance Battery score at baseline (β=.023; P=.01). In a classification and regression tree analysis, age older than 81 years and vitamin E (in participants aged 70-80 years) were identified as the strongest determinants of decline in physical function (physical decline in 84% and 60%, respectively; misclassification error rate, 0.33).
Conclusions
These results provide empirical evidence that a low serum concentration of vitamin E is associated with subsequent decline in physical function among community-living older adults. Clinical trials may be warranted to determine whether an optimal concentration of vitamin E reduces functional decline and the onset of disability in older persons.
The decline in physical function that occurs with aging often represents the early stage of a continuum leading to disability and other important adverse outcomes such as institutionalization. For example, although the prevalence of disability in the United States declined from 1982 to 2004,1 the absolute number of disabled older US adults is projected to increase as the population ages over the next 2 decades,2 with a detrimental effect on the health-related costs and long-term care.3 Thus, disentangling the mechanisms underlying the disablement process has been identified as a high-research priority,4 and the assessment of physical function has become an essential feature of the comprehensive clinical evaluation of older persons.5 Standardized measures such as the Short Physical Performance Battery6 have been developed to study the etiology and progression of functional decline and disability.
Poor nutrition may play a role in the disabling process through different mechanisms, for example, by increasing the levels of markers of inflammation and oxidative stress,7 with subsequent muscle or neuronal cell damage8,9 and decline in physical and cognitive function.10,11 Despite this strong theoretical basis, relatively little empirical evidence links poor nutrition to decline in physical function. Previous studies12 and our recent findings13-16 have shown that poor nutrition is associated with reduced physical function, frailty, and disability in older persons. However, these studies have been limited by their cross-sectional design12-15 or nonrepresentative samples, which have included, for example, only older women with some level of difficulty in physical function.16
The purpose of this study was to determine whether a low concentration of specific micronutrients is associated with subsequent decline in physical function. We used data from a population-based longitudinal study of community-living older adults, which included objective measures of both nutritional status and physical function.
Methods
Invecchiare in Chianti (InCHIANTI) is a population-based study of risk factors contributing to decline in physical function in older persons living in 2 municipalities located in Tuscany, adjacent to the city of Florence, Italy. The design and methods on data collection have been described in detail else-where.17 In brief, potential participants were randomly selected from the population registry, and 1155 persons 65 years or older agreed to participate in the study. The response rate was 91.6%.
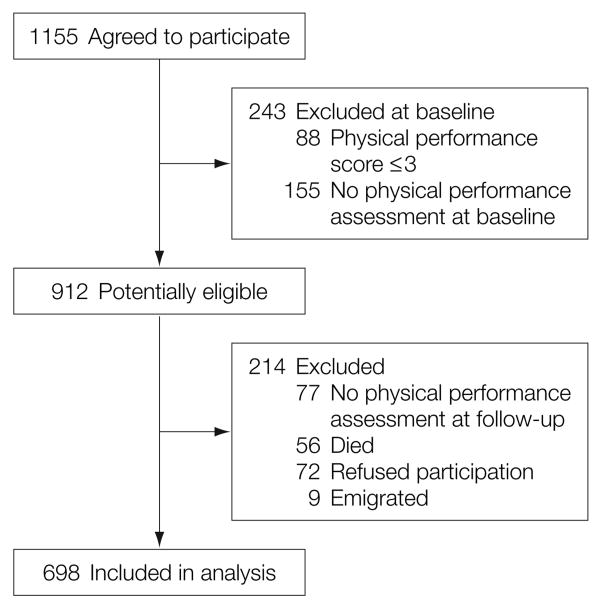
Data collected at baseline and during a 3-year follow-up assessment were used for the current study. As shown in Figure 1, participants were excluded if they had either missing Short Physical Performance Battery data at baseline or during follow-up or had a score of 3 or less at baseline (to exclude participants with very poor functional status who had little opportunity to decline further).
Figure 1. Profile of the Study Population.
Physical performance scores were based on the Short Physical Performance Battery. See “Methods” section for physical performance assessment (range, 0-12) with higher scores representing better performance.
Trained interviewers administered a structured assessment in the participant's home, including questions on education, socioeconomic status, household composition, and health and functional status. Cognitive function was assessed by the Mini-Mental State Examination,18 and depressive symptoms were assessed using the Center for Epidemiological Studies-Depression Scale (CES-D).19 Participants were asked to specify their level of physical activity, which was subsequently classified as (1) sedentary: completely inactive or light physical activity (ie, walking) for less than 1 h/wk; (2) light: light physical activity for 2 to 4 h/wk; or (3) moderate to intense: light physical activity for more than 4 h/wk or moderate physical activity (ie, swimming etc) 1 to 2 h/wk or more. A validated food frequency questionnaire was administered to estimate intake of energy and nutrients.20
A medical examination and an assessment of micronutrient concentrations and physical function were subsequently performed in the study clinic by physicians and therapists, respectively. Weight and height were measured according to standard protocols, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The presence of major chronic conditions was established by trained geriatricians according to algorithms based on information from the medical history, drug treatments, signs and symptoms, and hospital discharge records.21 The number of chronic conditions (including diabetes, arthritis, stroke, angina pectoris, congestive heart failure, chronic obstructive pulmonary disease, myocardial infarction, and cancer) served as the indicator of comorbidity. The Italian National Research Council of Aging Ethical Committee approved the study protocol. Participants provided written informed consent to participate in the study.
Assessment of Micronutrient Concentrations
Fasting blood samples were obtained by venipuncture between 7 am and 10:30 am.
Serum folate and vitamin B6 and B12 concentrations were obtained by centrifuging blood, which was collected in evacuated tubes without anticoagulant and stored at −80°C. Vitamin B6 was measured by high-pressure liquid chromatography (Immundiagnostik, Bensheim, Germany) and vitamin B12 and folate by radioimmunoassay (ICN Pharmaceuticals, New York, New York). The minimum detectable concentrations were 0.6 ng/mL (1.5 nmol/L) for folate, 0.2 ng/mL (0.8 nmol/L) for vitamin B6, and 75 pg/mL (55.3 pmol/L) for vitamin B12; the intra-assay coefficients of variation were 4.1% for folate, 2.8% for vitamin B6, and 11.2% for vitamin B12; and the interassay coefficients of variation were 7.1% for folate, 4.1% for vitamin B6, and 12.3% for vitamin B12. Plasma vitamin E (α-tocopherol) concentrations were measured by reverse-phase high-pressure liquid chromatography. Triplicate analysis of the reference samples provided by the American Association for Laboratory Accreditation (Washington, DC) showed intrabatch and interbatch coefficients of variation of 3% and 4.2% respectively. 25-Hydroxyvitamin D was measured by radioimmunoassay (DiaSorin Inc, Stillwater, Minnesota), after extraction of samples with acetonitrile. Intra-assay and interassay coefficients of variation were each 8.1%. Intra-assay coefficient of variance was less than 3.0% and interassay, less than 5.5%. Iron was assessed using a colorimetric assay (Roche Diagnostics, Mannheim, Germany).
Assessment of Physical Function
The physical performance score was derived from 3 objective tests of physical function: 4-meter walking speed, repeated chair rises, and standing balance in progressively more challenging positions.6 Walking speed was defined as the best performance (time) of 2 walks at usual pace over a 4-meter course. For the chair-stand test, participants were asked to rise 5 times from a seated position as quickly as possible with their hands folded across the chest; and performance was expressed as total time to complete the test. For the standing-balance test, participants were asked to stand in 3 progressively more difficult positions for 10 seconds each: feet in side-by-side, semitandem, and full-tandem positions.
For each of these 3 physical performance tests, participants received a score from 0 to 4, with a value of 0 indicating the inability to complete the test and 4 the highest level of performance. The values were summed to create a total score ranging from 0 to 12 with higher scores representing better performance. Previous studies have demonstrated that older, nondisabled persons with a low score are at high risk of developing disability.6 The Short Physical Performance Battery score has excellent reliability and is highly sensitive to clinically important change.22,23
Statistical Analysis
Descriptive analyses were performed to provide information on general characteristics of the study population. The physical performance score at baseline was subtracted from the score at the 3-year follow-up to identify participants who declined in physical function. Because the loss of 1 point in the physical performance score is considered a clinically meaningful23 and a potentially modifiable24 change, a dichotomized variable (1 indicates a loss of ≥1 point; 0, no loss) was created and used as the primary outcome in the current study. An analysis that included micronutrients and physical performance scores as continuous variables (with 99% power to detect an interaction) confirmed that the effect of nutrients on change in the physical performance score did not differ over the range of physical performance scores at baseline, thereby justifying our decision to include all participants who lost at least 1 point in a single group.
As in a previous study,16 low concentration for each nutrient was defined as the lowest quartile of the baseline distribution. The cutoff values were 1.1 μg/mL (24.9 μmol/L) for α-tocopherol, 275 pg/mL (202.9 pmol/L) for vitamin B12, 4.35 ng/mL (17.6 pmol/L) for vitamin B6, 1.9 ng/mL (4.31 nmol/L) for folate, 305 ng/dL (761.28 nmol/L) for 25-hydroxyvitamin D, and 55 μg/dL (9.8 μmol/L) for iron. This analytical approach was used because nutrient requirements for older persons are inadequately documented and cutoff points for this segment of the population have not been defined.25
Logistic regression models were used to evaluate the association between low concentration of the specific micronutrients and subsequent decline in physical function. The odds ratios (ORs) were calculated for the lowest quartile of each nutrient, using the other 3 quartiles combined as the reference group. Four separate logistic models were used for each nutrient: (1) unadjusted; and adjusted for (2) age; (3) age and sex; and (4) age, sex, educational achievement, marital status, household composition, smoking, physical activity level, chronic conditions, BMI, and CES-D and Mini-Mental State Examination scores. As previously suggested,26 we adjusted the analyses on vitamin E for total cholesterol. Because cholesterol-adjusted and cholesterol-unadjusted models yielded similar results, cholesterol-unadjusted models are presented.
Two additional and complementary analytical approaches, namely multiple general linear models and classification and regression tree (CART) analysis, were used to evaluate more completely the association between low concentration of micronutrients and subsequent decline in physical function and to confirm the validity of our primary results. Separate general linear models were used to evaluate whether serum concentrations of micronutrients at baseline (continuous) were associated with the physical performance score at follow-up (continuous) after adjustment for the physical performance score at baseline (continuous) and potential confounders, as in the fully adjusted model. Finally, CART analysis was performed to identify a hierarchical order of and potentially complex interactions between the concentration of different micronutrients (continuous) and the other variables included in the fully adjusted logistic model (model 4) with the outcome of decline in physical function (dichotomous). CART analysis uses recursive partitioning to define the optimum cutoff point for continuous predictors and identifies homogeneous groups having the largest difference in the outcome variable (minimum misclassification error rate).27 Interactions between independent variables are evaluated recursively instead of simultaneously as in linear regression. This process results in a classification rule with the optimum cut point for continuous variables and is represented as a tree. Cross validation was applied and the tree with the smallest deviance (sum of squares for residuals) was considered to have the optimal size.27
Finally, based on the results of the preceding analyses, we plotted the relationship between vitamin E concentration and decline in the physical performance score to evaluate graphically whether these values had a dose-response relationship. Because the graph suggested a change in the slope above specific concentrations of vitamin E, we created dummy variables of quartiles of vitamin E and tested the difference in the change of the physical performance score between each of the lowest 3 quartiles vs the upper quartile, using logistic-regression analysis adjusted for age, sex, educational achievement, marital status, household composition, smoking, physical activity level, chronic conditions, BMI, CES-D and Mini-Mental State Examination scores, and physical performance score at baseline.
The CART analysis was performed using S-PLUS statistical software.28 All other analyses were performed using SAS version 8.1.29 Two-tailed P<.05 was considered statistically significant.
Results
Table 1 provides the baseline characteristics of participants according to decline in physical function. Participants who declined were older and more likely to be women, had lower educational achievement and physical activity level, poorer cognitive function, and more depressive symptoms. The final analytical sample included 698 participants 65 years or older. Only 1 individual was taking a vitamin E supplement. The mean decline in physical performance score over the 3-year follow-up period was 1.1 point, with 50.4% declining by at least 1 point. Participants excluded from the study (n=457) were older (P<.001), had a lower level of physical activity (P<.001), poorer cognitive (P<.001) and physical function (P<.001), and a lower concentration of vitamin D (P=.01).
Table 1.
Baseline Characteristics of the Study Patients 65 Years or Older, According to Subsequent Decline in Physical Function
| Overall (N = 698) |
Decline in Physical Functiona (n = 352) |
No Decline in Physical Functiona (n = 346) |
P Valueb | |
|---|---|---|---|---|
| Age, mean (SD), y | 73.7 (6.2) | 75.6 (6.8) | 71.8 (5.0) | <.001 |
| Women, No. (%) | 380 (54.4) | 209 (59.4) | 171 (49.4) | .01 |
| Living alone, No. (%) | 128 (18.3) | 72 (20.5) | 56 (16.2) | .15 |
| ≥Elementary school education No. (%)c | 501 (72) | 269 (76) | 232 (67) | .01 |
| BMI, mean (SD) | 27.5 (3.9) | 27.4 (4.2) | 27.6 (3.7) | .64 |
| Physical activity, No. (%) | ||||
| Sedentary | 92 (13.3) | 63 (17.9) | 29 (8.4) | |
| Light | 322 (46.3) | 166 (47.2) | 156 (45.1) | ] .001 |
| Moderate to intense | 284 (40.4) | 123 (34.9) | 161 (46.5) | |
| Current smokers, No. (%) | 100 (14.3) | 46 (13.1) | 54 (15.6) | .34 |
| Chronic conditions, mean (SD)d | 0.41 (0.70) | 0.45 (0.69) | 0.37 (0.72) | .38 |
| Mental health, mean (SD), score | ||||
| MMSE25 | 25.6 (3.1) | 25.1 (3.5) | 26.2 (2.5) | .02 |
| CES-D26 | 11.1 (3.8) | 11.6 (4.0) | 10.6 (3.6) | .03 |
| Daily dietary intake, mean (SD)e | ||||
| Energy, kcal | 1966 (562) | 1922 (563) | 2010 (558) | .80 |
| Vitamin E, mg | 6.40 (1.97) | 6.32 (2.04) | 6.51 (1.90) | .61 |
| Vitamin B6, mg | 1.70 (0.47) | 1.66 (0.47) | 1.75 (0.45) | .59 |
| Folic acid, μg | 264 (77) | 258 (79) | 269 (75) | .96 |
| Vitamin D, μg | 1.85 (0.86) | 1.79 (0.92) | 1.91 (0.79) | .57 |
| Iron, mg | 12.9 (3.70) | 12.5 (3.6) | 13.2 (3.8) | .64 |
Abbreviations: BMI, body mass index, which is calculated as weight in kilograms divided by height in meters squared; CES-D, Center for Epidemiological Studies-Depression Scale; MMSE, Mini-Mental State Examination.
Decline in physical function is defined by a loss of at least 1 point in the Short Physical Performance Battery score.
Adjusted for age (except age comparison).
Completed elementary school through the fifth grade.
Number of chronic conditions.
Dietary intake of vitamin B12 has not been assessed.
In the unadjusted analyses (Table 2), low concentrations of both vitamin E and vitamin D were significantly associated with subsequent decline in physical function, with ORs of 1.65 (95% confidence interval [CI], 1.17-2.34, P=.01) and 1.45 (CI, 1.03-2.05, P=.03), respectively. In the fully adjusted model, the association between vitamin E and physical function remained statistically significant (OR, 1.62; CI, 1.11-2.36; P=.01). Even after adjustment for energy intake, the results did not change appreciably (OR, 1.63; CI, 1.12-2.38; P=.01). The association of vitamin E with change in the physical performance score over 3 years did not depend on the initial physical performance score (interaction term, β=−.005; P=.33).
Table 2.
Logistic Regression Models Evaluating the Effect of Low Concentrations of Micronutrients on Subsequent Decline of Physical Functiona
| Nutrient and Cutoff for Lowest Quartile | Model 1b |
Model 2b |
Model 3b |
Model 4b |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Vitamin E, 1.1 μg/mL | 1.65 (1.17-2.34) | .01 | 1.54 (1.07-2.22) | .02 | 1.62 (1.12-2.35) | .01 | 1.62 (1.11-2.36) | .01 |
| Vitamin B12, 275 pg/mL | 1.17 (0.83-1.65) | .36 | 0.99 (0.69-1.43) | .98 | 1.03 (0.72-1.49) | .87 | 1.03 (0.71-1.50) | .86 |
| Vitamin B6, 4.35 ng/mL | 1.33 (0.94-1.88) | .11 | 1.02 (0.71-1.48) | .91 | 1.06 (0.73-1.54) | .76 | 1.04 (0.71-1.53) | .83 |
| Folic acid, 1.9 ng/mL | 0.81 (0.57-1.13) | .21 | 0.69 (0.48-0.99) | .05 | 0.71 (0.49-1.02) | .06 | 0.72 (0.49-1.03) | .08 |
| Vitamin D, 305 ng/mL | 1.45 (1.03-2.05) | .03 | 1.08 (0.74-1.56) | .69 | 0.98 (0.67-1.43) | .91 | 0.92 (0.63-1.36) | .69 |
| Iron, 55 μg/dL | 1.15 (0.82-1.61) | .42 | 1.11 (0.78-1.58) | .57 | 1.11 (0.77-1.58) | .58 | 1.10 (0.77-1.59) | .60 |
Abbreviations: CI, confidence interval; OR, odds ratio.
SI conversion factors: to convert vitamin E from μg/mL to μmol/L, multiply by 23.22; vitamin B12 from pg/mL to pmol/L, multiply by 0.7378; vitamin B6 from ng/mL to nmol/L, multiply by 4.046; vitamin D from ng/mL to nmol/L, multiply by 2.496; and folic acid from ng/mL to nmol/L, multiply by 2.266.
Nutrients were entered in separate models (1st quartile vs upper 3 quartiles). Outcome represents dichotomized variables (1 indicates a decline of at least 1 point in the Short Physical Performance Battery score; 0, no decline).
Model 1 is not adjusted; model 2 adjusts for age; model 3 adjusts for age and sex; and model 4 adjusts for age, sex, educational achievement, marital status, household composition, smoking, physical activity level, number of diseases, body mass index, Center for Epidemiological Studies-Depression Scale, and Mini-Mental State Examination.
In the multivariate general linear regression analyses, only a lower concentration of vitamin E (continuous) was significantly associated with a lower physical performance score at follow-up after adjustment for potential confounders and the physical performance score at baseline (β=.02; P=.01). Further adjustment for energy intake had little effect on the results (β=.022; P=.01). When the logistic and general linear models were adjusted for each of the chronic conditions, rather than the number of chronic conditions, the results did not change appreciably (logistic model: OR, 1.58; 95% CI, 1.08-2.33; general linear model: β=.023, P=.01). Because lipid intake, in particular vegetable lipids, could potentially confound the relationship between vitamin E and physical function, we completed a series of additional analyses that adjusted individually for dietary intake of total lipids, total vegetable lipids, and monosaturated and polyunsaturated fatty acids, and found that the results were essentially unchanged for each model (β=.023; P=.01).
Using a logistic regression analysis, we found that the slope of the relationship between vitamin E concentration and decline in physical function changes above specific values of Vitamin E. In fact, we found that the average decline in the physical performance score among participants with vitamin E concentrations in the first and second lowest quartiles were 0.66 (P=.006) and 0.43 (P=.06) points, respectively, compared with that among participants in the upper quartile. Although the quadratic term included in the logistic model was not significant (β=−.004; P=.41), we found almost no difference (0.08 points; P=.74) in the average decline in the physical performance score when participants in the third quartile of vitamin E were compared with those in the upper quartile. Higher vitamin E concentrations were associated with younger age, female sex, and higher BMI (Table 3).
Table 3.
Baseline Characteristics of the Study Participants, According to Quartiles of Vitamin Ea
| Quartile of Vitamin E (μg/mL) |
P Value for Trend | ||||
|---|---|---|---|---|---|
| 1st (<1.1) | 2nd (1.1-1.3) | 3rd (1.3-1.5) | 4th (≥1.5) | ||
| No. (%) of patients | 174 (24.93) | 174 (24.93) | 175 (25.0) | 175 (25.07) | |
| Age, mean (SD), y | 74.6 (6.8) | 74.1 (6.7) | 72.8 (5.5) | 73.3 (5.8) | .03 |
| Women, No. (%) | 80 (46.0) | 87 (50) | 79 (54.9) | 58 (66.9) | .001 |
| Living alone, No. (%) | 29 (16.7) | 40 (23) | 32 (18.3) | 27 (15.4) | .28 |
| ≥Elementary school education No. (%)b | 123 (71) | 129 (74) | 119 (68) | 130 (74) | .50 |
| BMI | 26.5 (3.9) | 27.5 (4.1) | 27.5 (3.7) | 28.4 (3.8) | <.001 |
| Physical activity, No. (%)c | |||||
| Sedentary | 32 (18.4) | 26 (14.9) | 17 (9.7) | 17 (9.7) | |
| Light | 71 (40.8) | 78 (44.8) | 81 (46.3) | 92 (52.6) | ] .10 |
| Moderate to intense | 71 (40.8) | 70 (40.2) | 77 (44) | 66 (37.7) | |
| Current smokers, No. (%) | 25 (14.4) | 27 (15.5) | 24 (13.7) | 24 (13.7) | .96 |
| Evaluation scores, mean (SD) | |||||
| MMSE | 25.5 (3.3) | 25.6 (3.4) | 25.6 (2.9) | 25.9 (2.7) | .53 |
| CES-D | 11 (3.7) | 11.1 (3.7) | 11.2 (4.1) | 11.1 (3.9) | .99 |
| SPPB | 10.6 (1.9) | 10.6 (2.1) | 10.9 (1.7) | 10.7 (1.8) | .43 |
| Chronic conditions, mean (SD)d | 0.42 (0.65) | 0.4 (0.64) | 0.35 (0.74) | 0.47 (0.78) | .33 |
Abbreviations: BMI, body mass index, which is calculated as weight in kilograms divided by height in meters squared; CES-D, Center for Epidemiological Studies-Depression Scale; MMSE, Mini-Mental State Examination; SPPB, Short Physical Performance Battery.
SI conversion factor: To convert vitamin E from μg/mL to μmol/L, multiply by 23.22.
The corresponding vitamin E values were mean, 1.3 μg/mL (30.6 μmol/L); median, 1.3 μg/mL (29.8 μmol/L); and interquartile range, 0.4 μg/mL (10.4 μmol/L).
Completed elementary school through fifth grade.
For a definition of levels of physical activity, see the “Methods” section.
Number of chronic conditions.
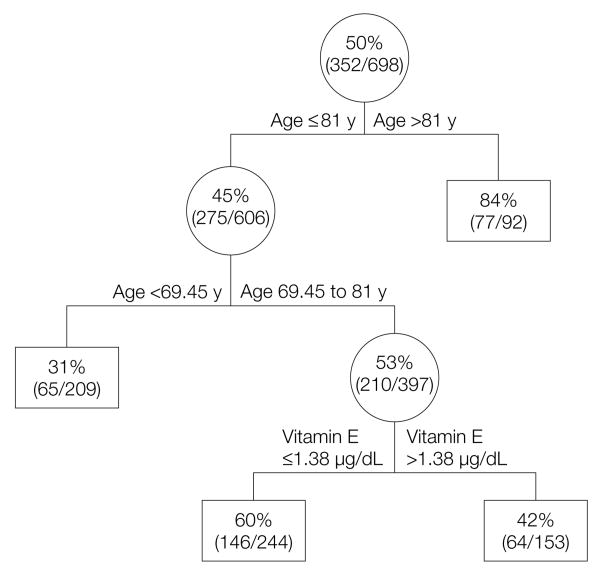
As shown in Figure 2, among the 17 factors evaluated in the CART analysis (the 6 micronutrients and the 11 covariates used in the fully adjusted models), age and vitamin E were identified as the strongest determinants of decline in physical function. Participants older than 81 years had the highest risk of physical function declining (84%), while those 70 years or younger had the lowest risk. Among persons aged 70 to 80 years, the strongest predictor of decline in physical function was a concentration of vitamin E of 1.4 μg/mL (32 μmol/L) or less. The misclassification error rate for the CART analysis was 0.33.
Figure 2. Classification Tree for Decline in Physical Performance Score.
Tree selected among the following factors: α-tocopherol, vitamin B12, vitamin B6, folate, 25-hydroxyvitamin D, iron, age, sex, educational achievement, marital status, household composition, smoking, physical activity level, number of chronic conditions, body mass index, Center for Epidemiological Studies-Depression Scale, and Mini-Mental State Examination. To convert vitamin E from μg/mL to μmol/L, multiply by 23.22. Includes selectively those participants who declined in physical function (352 [50.4%]) and the specified percentage represents the proportion of participants who declined in physical function for each specific node.
Comment
In a population-based sample of community-living older men and women, we evaluated whether a low concentration of micronutrients was associated with subsequent decline in physical function. Using 3 analytical approaches, we consistently found that a low concentration of vitamin E was associated with subsequent decline in physical function.
As the major lipid-soluble antioxidant, vitamin E plays a critical role in the defense from oxidative stress by donating electrons and neutralizing free radicals. Low concentrations of vitamin E may affect this neutralization by creating an imbalance between oxidants and antioxidants and, consequently, a highly reactive milieu. Because molecular oxygen promptly accepts unpaired electrons to form reactive oxygen species,30 this imbalance may lead to excessive formation of reactive oxygen species and, consequently, to oxidative stress that may cause lipid peroxidation31 and DNA,8 muscle,8 and neuronal9 damage.
This chain of events may explain, at least in part, our findings on the association between low concentrations of vitamin E and subsequent decline in physical function. The hypothesis that antioxidants play a role in the etiology of decline in physical function and disability is supported by our previous findings13-16 and other studies suggesting that oxidative stress is involved in muscle fatigue32 and that antioxidants play a preventive role in muscle damage by reducing oxidative injury.33 Interestingly, vitamin E plays a differential role in oxidative metabolism of different muscle fibers (type I and type II). Type I fibers are plentiful in myoglobin and mitochondrial enzymes and replenish phosphocreatine more efficiently via oxidative phosphorylation than do type II fibers,34 which theoretically generate more free radicals. Thus, it has been suggested that type I (slow) fibers require more vitamin E than type II (fast) fibers.34 Furthermore, high concentration of vitamin E has been associated with higher levels of creatine kinase activity, suggesting the possibility of increased skeletal muscle repair.35 In addition, vitamin E deficiency has been associated with increased lipid peroxidation and risk of cardiovascular diseases,31 as well as with neurodegenerative disorders.9
Thus, at least 3 different mechanisms may explain the effect of low concentration of vitamin E on subsequent decline in physical function: (1) increased oxidative stress leading to muscle or DNA damage,8 (2) exacerbation of atherosclerosis31 or other pathologic conditions,7 and (3) development of neurodegenerative disorders.9 Although a low concentration of other micronutrients could potentially play a role in decline of physical function through alternative mechanisms, we could not establish associations in the current study between vitamin B12, vitamin B6, folic acid, vitamin D, or iron and subsequent decline in physical function.
To our knowledge, this is the first longitudinal study to have evaluated the effect of low concentrations of different micronutrients on subsequent decline in physical function using a population-based sample of older men and women living in the community. We used objective measures for the evaluation of both the exposure (concentration of micronutrients) and the outcome (decline in physical function). Hence, our results are not biased by self-report. Furthermore, we used an indicator of physical function derived from the assessment of 3 performance tests, which increases its reliability and accuracy.22 Finally, the validity of our findings is strengthened by the use of 3 analytical approaches, each of which demonstrated the same result: low vitamin E concentration was associated with subsequent decline in physical function. Although observed over the entire range of vitamin E concentrations, decline in physical function was particularly pronounced for vitamin E concentrations in the lowest 2 quartiles, corresponding to values 1.29 μg/mL (≤29.8 μmol/L). Of note, the cutoff of vitamin E selected by the CART analysis was 1.38 μg/mL (32 μmol/L) in participants aged 70 to 80 years old, and greater than 1.29 μg/mL (>30 μmol/L) is the cutoff used to define optimal status of vitamin E.36 Hence, our results have face validity.
Potential limitations, however, warrant comment. First, our results may have been biased by losses to follow-up. Participants in the InCHIANTI study who were not included in the current study were older, more sedentary, and had lower cognitive function and physical performance scores compared with those who were included. In longitudinal studies of older persons, age-related problems—such as progressive cognitive impairment, morbidity and mortality—are inevitable causes of attrition,36,38 leading to loss of power and underestimation of decline in physical function over time.38 Second, the InCHIANTI study is an Italian population-based sample, raising potential concerns about the generalizability of our findings. It is unlikely, however, that the basic biological mechanisms underlying decline in physical function with age differ substantially from one country to another. The low percentage of participants in this study who used nutritional supplements (4%), in contrast to that in the United States (>50%)39 provided us with a unique opportunity to evaluate the “pure effect” of poor nutrition on decline in physical function.
Third, although vitamin D was associated with decline in physical function in bivariate analysis, this association was not observed in the adjusted analyses. Nevertheless, because persons who were not included in the study had a significantly lower concentration of vitamin D and worse physical function, it is possible that we missed a true association between these 2 variables. Fourth, because 6 micronutrients were evaluated, multiple testing may have increased the possibility of a false-positive result. This concern, however, is attenuated by the consistency of the results across the 3 analytical approaches. Finally, it is possible that vitamin E may simply be a sensitive marker of differences in health status and that the adjustments used in the current study were not adequate. This possibility is diminished, however, by the specificity of the relationship between vitamin E and decline in physical function and the stability of the effect size despite sequential adjustment for multiple potential confounders. In a prior study13 using baseline data from the InCHIANTI study, we found that the concentration of α-tocopherol, a common indicator of vitamin E status, was significantly correlated with dietary intake of vitamin E (r=0.126, P=<.001). Because dietary intake of vitamin E includes not only α-tocopherol but also tocotrienols and all other tocopherols, we would not expect such a summary measure to be highly correlated with plasma α-tocopherol concentration. In the current study, only 1 participant reported taking a vitamin E supplement; hence, our findings do not suggest that vitamin E supplementation would prevent decline in physical function. Approximately 15 to 30 mg/d of dietary α-tocopherol is needed to achieve a plasma α-tocopherol concentration of 1.3 μg/mL (30 μmol/L),37 and this amount can be easily reached through diet, from sources such as almonds, tomato sauce, and sunflower seeds among others (http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w323.pdf).
In conclusion, the current study provides empirical evidence that a low concentration of vitamin E is associated with subsequent decline in physical function in a population-based sample of older persons living in the community. Although the findings from this epidemiological study cannot establish causality, they provide a solid base that low concentration of vitamin E contributes to decline in physical function. Clinical trials may be warranted to determine whether optimal concentration of vitamin E reduces functional decline and the onset of disability in older persons with a low concentration of vitamin E.
Acknowledgments
Funding/Support: The InCHIANTI study was supported by the Italian Ministry of Health and in part by contracts 263 MD 9164 13 and 263 MD 821336 from the Intramural Research Program of the National Institute on Aging, National Institutes of Health. Funding for the assessment of vitamin E was provided by Bracco Imaging SpA, Italy. Dr Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging. Dr Allore is funded in part by grant P30AG21342 from the Yale Claude D. Pepper Older Americans Independence Center. Dr Bartali is the recipient of a James Hudson Brown–Alexander B. Coxe Fellowship from Yale University School of Medicine.
Footnotes
Author Contributions: Dr Bartali had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bartali, Frongillo, Guralnik, Stipanuk, Ferrucci.
Acquisition of data: Bartali, Guralnik, Cherubini, Bandinelli, Ferrucci.
Analysis and interpretation of data: Bartali, Frongillo, Stipanuk, Allore, Cherubini, Gill.
Drafting of the manuscript: Bartali, Frongillo, Stipanuk, Allore.
Critical revision of the manuscript for important intellectual content: Bartali, Frongillo, Guralnik, Allore, Cherubini, Bandinelli, Ferrucci, Gill.
Statistical analysis: Bartali, Frongillo, Guralnik, Allore.
Study supervision: Bartali, Frongillo, Stipanuk, Gill.
Obtained funding: Bartali, Guralnik, Cherubini, Bandinelli, Ferrucci.
Administrative, technical, or material support: Cherubini, Bandinelli.
Financial Disclosures: None reported.
Role of the Sponsor: The Italian Ministry of Health had no role in the collection, analysis, or interpretation of the data or in the decision to submit the study for publication. The National Institute on Aging participated in data collection and data management and approved the manuscript. Bracco Imaging SpA provided funding to assess vitamin E but had no role in the collection, analysis, or interpretation of the data or in the decision to submit the study for publication.
References
- 1.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the US elderly population. Proc Natl Acad Sci U S A. 2006;103(48):18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washington, DC: US Census Bureau; 2000. Annual projections of the resident population by age sex, race, and Hispanic origin: lowest, middle, highest series and zero international migration series, 1999 to 2100 Table NP-D1-A. http://www.census.gov/population/www/projections/natdet.html. [Google Scholar]
- 3.Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care costs when older persons become more dependent. Am J Public Health. 2002;92(8):1244–1245. doi: 10.2105/ajph.92.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahim S. Disability in older people: a mass problem requiring mass solutions. Lancet. 1999;353(9169):1990–1992. doi: 10.1016/S0140-6736(99)00195-6. [DOI] [PubMed] [Google Scholar]
- 5.Applegate WB, Blass JP, Williams TF. Instruments for the functional assessment of older patients. N Engl J Med. 1990;322(17):1207–1214. doi: 10.1056/NEJM199004263221707. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 8.Mecocci P, Fano G, Fulle S, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26(34):303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 9.Coyle JT, Puttfarken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Kritchevsky SB, Nicklas BJ, et al. Lipoprotein peroxidation and mobility limitation: results from the Health, Aging, and Body Composition Study. Arch Intern Med. 2005;165(18):2148–2154. doi: 10.1001/archinte.165.18.2148. [DOI] [PubMed] [Google Scholar]
- 11.Lu T, Pan Y, Kao SY, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 12.Snowdon DA, Gross MD, Butler SM. Antioxidants and reduced functional capacity in the elderly: findings from the Nun Study. J Gerontol A Biol Sci Med Sci. 1996;51:10–16. doi: 10.1093/gerona/51a.1.m10. [DOI] [PubMed] [Google Scholar]
- 13.Cesari M, Pahor M, Bartali B, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79(2):289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 14.Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61(6):589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ble A, Cherubini A, Volpato S, et al. Lower plasma vitamin E levels are associated with the frailty syndrome: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61(3):278–283. doi: 10.1093/gerona/61.3.278. [DOI] [PubMed] [Google Scholar]
- 16.Bartali B, Semba RD, Frongillo EA, et al. Low micronutrient levels as a predictor of incident disability in older women. Arch Intern Med. 2006;166(21):2335–2340. doi: 10.1001/archinte.166.21.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159(15):1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 20.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. NIH publication no. 95-4009. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 22.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55(9):916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 25.Food and Nutrition Board. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academy of Sciences; 2006. [Google Scholar]
- 26.Traber MG, Jialal I. Measurement of lipid-soluble vitamins—further adjustment needed? Lancet. 2000;355(9220):2013–2014. doi: 10.1016/S0140-6736(00)02345-X. [DOI] [PubMed] [Google Scholar]
- 27.Allore H, Tinetti ME, Araujo KL, Hardy S, Peduzzi P. A case study found that a regression tree outperformed multiple linear regression in predicting the relationship between impairments and Social and Productive Activities scores. J Clin Epidemiol. 2005;58(2):154–161. doi: 10.1016/j.jclinepi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Data Analysis Products Division. S-PLUS 2000 Guide to Statistics. Vol. 1. Seattle, WA: MathSoft; 1999. [Google Scholar]
- 29.SAS Procedure Guide [computer program], Version 8.1. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 30.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(34):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 31.Diaz MN, Frei B, Vita JA, Keaney JFJ. Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 32.Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36(4):327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Fanò G, Mecocci P, Vecchiet J, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22(4):345–351. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 34.Pette D, Spamer C. Metabolic properties of muscle fibers. Fed Proc. 1986;45(13):2910–2914. [PubMed] [Google Scholar]
- 35.Evans WJ. Vitamin E, vitamin C, and exercise. Am J Clin Nutr. 2000;72(2 suppl):647S–652S. doi: 10.1093/ajcn/72.2.647S. [DOI] [PubMed] [Google Scholar]
- 36.Traber MG, Sies H. Vitamin E in humans: demand and delivery. Annu Rev Nutr. 1996;16:321–347. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 37.Liu IY, Anthony JC. Using the “Mini-Mental State” examination to predict elderly subjects' completion of a follow-up interview. Am J Epidemiol. 1989;130(2):416–422. doi: 10.1093/oxfordjournals.aje.a115350. [DOI] [PubMed] [Google Scholar]
- 38.Deeg DJ, van Tilburg T, Smit JH, de Leeuw ED. Attrition in the Longitudinal Aging Study Amsterdam: the effect of differential inclusion in side studies. J Clin Epidemiol. 2002;55(4):319–328. doi: 10.1016/s0895-4356(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 39.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]