Abstract
In many biological membranes, the major lipids are “non-bilayer lipids,” which in purified form cannot be arranged in a lamellar structure. The structural and functional roles of these lipids are poorly understood. This work demonstrates that the in vitro association of the two main components of a membrane, the non-bilayer lipid monogalactosyldiacylglycerol (MGDG) and the chlorophyll-a/b light-harvesting antenna protein of photosystem II (LHCII) of pea thylakoids, leads to the formation of large, ordered lamellar structures: (i) thin-section electron microscopy and circular dichroism spectroscopy reveal that the addition of MGDG induces the transformation of isolated, disordered macroaggregates of LHCII into stacked lamellar aggregates with a long-range chiral order of the complexes; (ii) small-angle x-ray scattering discloses that LHCII perturbs the structure of the pure lipid and destroys the inverted hexagonal phase; and (iii) an analysis of electron micrographs of negatively stained 2D crystals indicates that in MGDG-LHCII the complexes are found in an ordered macroarray. It is proposed that, by limiting the space available for MGDG in the macroaggregate, LHCII inhibits formation of the inverted hexagonal phase of lipids; in thylakoids, a spatial limitation is likely to be imposed by the high concentration of membrane-associated proteins.
Keywords: circular dichroism, chloroplast thylakoid membranes, electron microscopy, light-harvesting complex, lipid–protein interactions
The lamellar organization of biological membranes provides a structural matrix for various proteins and controls the permeability of organic molecules, water, and ions; it also prevents nonspecific protein–protein aggregation, whereas it allows protein diffusion and conformational changes in the membrane. However, biomembranes usually contain substantial amounts of non-bilayer lipids, which in purified form assume nonlamellar structures. In fact, in many membranes, e.g., thylakoid membranes of chloroplasts, membranes of Escherichia coli, rhodopsin, and mitochondria, non-bilayer lipids constitute about half or more of the total lipid content. It is well established that the physical and functional properties of these membranes depend to a large extent on protein–lipid interactions (1, 2). There are a few examples showing that lipid polymorphism can be modulated by proteins, and, in some cases, small unilamellar vesicles can be reconstituted from non-bilayer lipids and membrane proteins (e.g., refs. 3 and 4). However, the structural role of large amounts of non-bilayer lipids has remained enigmatic, and the assembly of extended bilayer lamellae from proteins and predominantly non-bilayer lipids is poorly understood (1, 2, 5). In this work, we use a simple system, the two main constituents of pea thylakoid membranes, purified non-bilayer lipids and isolated protein complexes, to demonstrate that the formation of a large, ordered lamellar structure is possible even in the presence of large amounts of lipids.
In chloroplast thylakoid membranes of green plants, monogalactosyldiacylglycerol (MGDG) accounts for about 50% of the total lipid content. The membranes also contain digalactosyldiacylglycerol (DGDG) (≈30%), sulfoquinovosyldiacylglycerol (SQDG) (≈5–12%), and phosphatidylglycerol (PG) (≈5–12%) (6). It is well known that, when dispersed in aqueous solution, MGDG forms an inverted hexagonal (HII) phase, and at physiological temperatures and a “realistic” pH and ionic strength of the medium no lamellar phase can be formed (7). The non-bilayer arrangement of lipids is likewise retained when purified thylakoid lipids are mixed, and the formation of lamellae can be observed only if the MGDG content is decreased substantially: in MGDG:DGDG mixtures, for example, it must be lower than 20% (8). As pointed out by Williams (9), “it is generally assumed, although it has never been directly demonstrated, that photosynthetic light harvesting proteins [suppress the HII formation… , and numerous data] indicate that lipid–protein interactions play a major part in imposing a bilayer configuration in the native membranes.” In the present work, by using electron microscopy, small-angle x-ray scattering, and circular dichroism (CD) spectroscopy, we provide direct experimental evidence that, when mixed together at lipid:protein ratios similar to or higher than those in thylakoids, the chlorophyll (Chl)-a/b light-harvesting antenna protein of photosystem II (LHCII) and MGDG spontaneously form large lamellar aggregates with long-range order of the complexes.
Materials and Methods
LHCII macroaggregates were isolated as described earlier from leaves of 2-wk-old pea (Pisum sativum, L.) grown in the greenhouse (10). Two types of preparations were used. For the reconstitution of lamellar aggregates by lipids, we applied disordered (type III) macroaggregates of LHCII, because the role of lipids in the formation of ordered macroarrays could be demonstrated most readily on these preparations. For detailed electron microscopic analysis, freshly prepared lamellar aggregates (type II) were used. These latter samples are capable of undergoing light-induced reversible structural changes (11), which can be enhanced significantly by freshly prepared thylakoid lipids (12). The ability of LHCII macrostructures to undergo light-induced structural changes is lost in the more delipidated preparations, i.e., in disordered macroaggregates and tightly stacked (type IV) lamellar aggregates of LHCII, and cannot be recovered by the addition of lipids.
MGDG was extracted by the procedure of Folch et al. (13) as modified by Vígh et al. (14), and the purified lipids were stored in hexane containing 0.5% bromohydroxytoluene at −20°C until use. Before the addition of lipids to LHCII, the hexane was evaporated under a nitrogen stream, and 20 mM Tricine buffer (pH 7.6) containing 0.08% Triton X-100 was then added to the dried lipids. The mixture was sonicated in a water bath sonicator for about 30 s, followed by vigorous vortexing until the lipids were dissolved. The lipid contents of the isolated and reconstituted complexes were determined as described earlier (10).
MGDG was incorporated into delipidated, disorganized (type III) macroaggregates of LHCII by adding saturating amounts of MGDG (about 6.5–7 mg lipid/mg Chl) to the LHCII preparation. The preparation was stored in the dark at 4°C until use within 1–2 h. For electron microscopy of ultrathin sections, samples were sedimented at 7,000 × g in an Eppendorf centrifuge, and the pellet was fixed and embedded in resin (Araldite) by means of published procedures (15); the micrographs were taken in a Zeiss/Opton 902 electron microscope. CD spectra of LHCII and MGDG-LHCII were recorded between 400 and 750 nm at room temperature in a Jobin-Yvon (Longjumeau, France) CD6 dichrograph. The pathlength of the optical cell was 1 cm; the Chl content of the samples was adjusted to 20 μg/ml. CD is expressed in units of absorbance.
Small-angle x-ray scattering experiments on preparations with different molar ratios MGDG:LHCII were carried out at the Austrian SAXS beamline (Station: 5.2L) of the 2 GeV electron storage ring of ELETTRA, Trieste (16). The samples were placed in a standard glass capillary with a diameter of 2 mm (Anton Paar, Graz). The initial concentration of MGDG was adjusted to approximately 2.5 mg lipid/ml, and the concentration of LHCII was varied by gradually adding the proteins to the sample. The sample to detector distance was set at 1.5 m to achieve a resolution between s = 0.04 and 0.40 1/nm with the 8 keV x-rays used. The diffraction pattern was measured by means of a one-dimensional delay line detector. The exposures used did not induce noticeable alterations in our samples.
For electron microscopy of negatively stained specimens, the ratio MGDG:LHCII was adjusted to approximately 1:5 (wt/wt): a droplet (5 μl) at 2 mg/ml lipid and 0.4 mg/ml LHCII (type II) was deposited onto a carbon-coated 400 mesh copper grid (Agar Scientific, Wetzlar, Germany), and adsorbed membranes were negatively stained with a 2% (wt/vol) solution of sodium phosphotungstate (pH 7.0) for 2 min. Electron micrographs were recorded in a Philips CM10 transmission electron microscope operated at 100 kV. The calibrated magnification for micrographs subjected to further processing was ×37,400. Films were digitized by using a Joyce–Loebl Scandig 3 rotating drum densitometer at 25 μm increments, corresponding to 0.67 nm/pixel at the specimen level. Fourier transforms were calculated, lattices were refined, and projection maps were calculated by using the PC-based crystallographic image processing software package crisp (17).
Results and Discussion
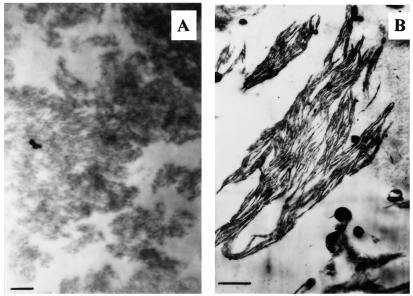
Fig. 1A shows that in delipidated LHCII the complexes are to be found in randomly distributed clusters (10). Upon the addition of any of the four purified thylakoid lipids, the disorganized macroaggregates were transformed into a lamellar structure that closely resembled the ultrastructure of freshly prepared LHCII (18). This is not surprising for DGDG, because of its well-known role in the formation of 2D crystals (19). For PG and SQDG, our finding is consistent with the notion that these lipids contribute to the lipid matrix that embeds integral proteins. However, reconstitution of the lamellar structure by MGDG (Fig. 1B) is somewhat unexpected, because this lipid is not capable alone of assembling into lamellae. Further, the uptake of MGDG was very high: the lipid content increased from 128 ± 25 in LHCII alone to 6200 ± 200 μg lipid/mg Chl in the lipid–protein complexes (mean values and SDs derived from five independent experiments). This corresponds to about 80 MGDG molecules per LHCII polypeptide, which is about twice the lipid:protein ratio (0.6–0.8 mg lipid/mg protein) in thylakoids (20). It is interesting that the amount of bound lipids per monomer was considerably higher for MGDG than for any of the other lipid species: for DGDG, SQDG, and PG, the lipid uptakes were 2200 ± 200, 1150 ± 50, and 950 ± 50 μg lipid/mg Chl, respectively. Thus, it appears that the maximum amount that can be bound by LHCII is proportional to the relative concentrations of these lipids in the thylakoids.
Figure 1.
Electron micrographs of ultrathin sections of delipidated disordered macroaggregates of LHCII before (A) and after (B) the admixing of 130 μg MGDG to 1 ml LHCII with a Chl content of 20 μg/ml. For further details, see Materials and Methods.
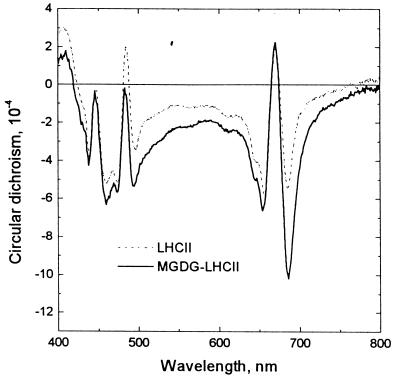
As shown in Fig. 2, the formation of lamellar aggregates of MGDG-LHCII is accompanied by the appearance of anomalous CD bands at around (−) 682 and (−) 495 nm. These nonconservative bands are superimposed on the excitonic bands originating from LHCII trimers, which predominate in the CD spectra of the disordered aggregates of the complexes (cf. ref. 10). These additional bands have been shown to be of psi type in origin (21), i.e., produced by long-range chiral order of the chromophores (22, 23) (psi, polymer or salt-induced). In thylakoid membranes, the macroaggregation of LHCII, which brings about the interconnection of photosystem II particles, has been shown to be responsible for the formation of psi-type macrodomains (24). Thus, it can be concluded that, in consequence of the association of MGDG with LHCII, the proteins are arranged in arrays with long-range chiral order.
Figure 2.
Circular dichroism spectra of delipidated disordered macroaggregates of LHCII before (A) and after (B) the addition of MGDG at a final concentration of 6.5 mg lipid/mg Chl. The Chl content of the sample was adjusted to 20 μg/ml; the optical pathlength was 1 cm. Typical structures of the samples are displayed in Fig. 1.
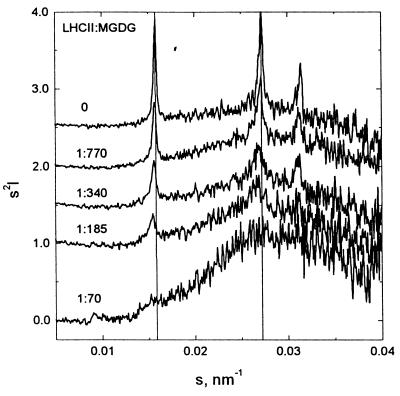
In accordance with expectations, when MGDG was dispersed in water it formed an HII phase, as seen from the x-ray diffraction peaks [1,0], [1,1], and [2,0] reflections (25) of an inverted hexagonal phase (Fig. 3). The gradual admixing of LHCII led to a pronounced decrease in intensity of the HII-signal and to a minor change in d-spacing. The decrease and final disappearance of the HII-signal clearly demonstrates that the interaction with the protein progressively “destroys” the HII-phase. At a lipid:protein ratio of about 100, a broad peak with a maximum around (4 nm)−1 became dominant, which agrees well with the lamellar 4.03-nm thickness in thylakoids (26), although a weak HII signal was retained. The origin of the minor change in the hexagonal d-spacing from 6.35 to 6.49 nm is not clear, but it can be speculated that the destruction of the hexagonal structure proceeds by means of domain fragmentation, with an increase in thermal disorder and a consequently larger, average separation of the hexagonal lattice positions.
Figure 3.
Small-angle x-ray scattering profiles after background subtraction, normalization to the primary intensity, and Lorentz correction for purified MGDG and MGDG-LHCII at different lipid:protein ratios, as indicated in the figure. The concentration of lipids was initially adjusted to about 2.5 mg/ml; LHCII was added stepwise. The dilution of the lipids was taken into account by multiplying the scattering intensities by different dilution factors.
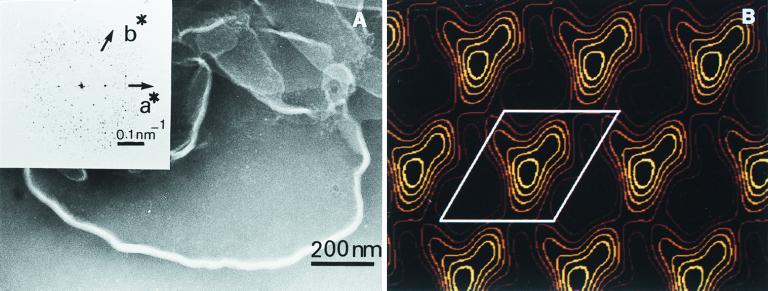
Negative staining electron microscopy on samples obtained following the addition of saturating amounts of MGDG to LHCII clearly revealed the presence of lamellae (Fig. 4A). Earlier, we have shown by ultrathin section electron microscopy that the incorporation of MGDG into lamellar aggregates of LHCII induces the formation of very large (≈10 μm in diameter), concentrically arranged membranes (12).
Figure 4.
(A) Electron micrograph of negatively stained MGDG-LHCII membranes. (Inset) The Fourier transform of an area of 256 × 256 pixels ≡ 171.52 nm × 171.52 nm. (B) Fourier projection map of LHCII reconstituted in MGDG. The first four (positive) contour lines were drawn equidistantly between the mean and the maximum density. One unit cell is indicated (a = 6.6 nm, b = 7.2 nm, γ = 121.2°).
In the Fourier transform of the 2D crystal of MGDG-LHCII (Fig. 4A, Inset), the first-order reflections clearly demonstrate the presence of a hexagonal type of long-range order within the artificial membrane. This indicates that MGDG provides a lipid matrix that is capable of accommodating the protein complexes while retaining long-range order in the macroarray, even when the concentration of this non-bilayer lipid is very high. The lipid content of the macroaggregates increased from 327 ± 43 to 15,000 ± 300 μg lipid/mg Chl, which at saturating concentration corresponds to about 200 lipid molecules per monomer. Although this latter value evidently includes significant amounts of nonincorporated MGDG molecules, which appear in the electron micrographs as osmiophilic droplets (12) or a coexisting HII phase (data not shown), there is no doubt that MGDG-LHCII contains large amounts of lipid molecules.
As revealed by the contour map, the unit cell dimensions are a = 6.6 nm and b = 7.2 nm with γ = 121.2°, and the map is characterized by well-defined densities covering a surface of approximately 4.8 × 3.1 nm, following a contour line 25% above the mean density (Fig. 4B). These values are in excellent agreement with data published for one LHCII monomer (27), suggesting that the densities correspond to single LHCII molecules. Although it is unclear whether or not the long-range order is facilitated by MGDG as compared with other lipids, these data are consistent with the proposal made by de Kruijff (1) on the role of the non-bilayer lipids in the packing of proteins in the membranes.
From the space available between the complexes, i.e., the estimated surface-exposed area of about 26–28 nm2 between the complexes (see Fig. 4B), and the area of 0.47 nm2 occupied by each MGDG molecule at the lipid-water interface (8), it may be estimated that in the macroarray each LHCII monomer can on average accommodate about 110–120 MGDG molecules. This is considerably higher than the MGDG:protein ratio in spinach thylakoids (20), and suggests that LHCII alone is in principle capable of “forcing” the non-bilayer lipids into a lamellar structure.
Although the space available between the complexes permits the incorporation of substantial amounts of lipids, it evidently does not allow formation of the HII phase. The repeat distance in the HII phase is around 6.4 nm (see above), whereas the diameter in the “hole” between the complexes evidently cannot be larger than 5 nm (Fig. 4B). Hence, the ordered macroarrays of complexes in 2D crystals of LHCII impose a severe restriction on the space available for lipids. Although it may not be straightforward to extrapolate these results, it can be assumed that in the stacked region of granal thylakoids the presence of LHCII-containing macrodomains with long-range order (21, 24) can impose a spatial restriction by essentially the same mechanism. In stroma membranes and other thylakoid membranes that do not contain an ordered macroarray, a high protein/lipid ratio (which brings about short average distances between the particles) and also the presence of external proteins may impose restrictions on the occurrence of the HII phase. It was earlier demonstrated that certain integral or extrinsic membrane proteins can inhibit the formation of non-bilayer structures (e.g., ref. 3) or stabilize the lamellar phase in the presence of high concentrations of non-bilayer lipids (28). In general, lipid polymorphism can be modulated by different membrane components and environmental factors (2, 29). Thus, it seems unlikely that spatial limitation is a unique mechanism of maintaining bilayer structures. Nonetheless, the fact that membranes with high protein contents, such as thylakoids, or mitochondrial and retinal rod membranes, tend to contain high amounts of non-bilayer lipids (cf. ref. 9) is consistent with the hypothesis that the bilayer configuration of lipids in thylakoid membranes can be preserved in consequence of spatial limitations. Indeed, in photosynthetic membranes, the dense population of proteins limits diffusion to the percolation threshold (30), which therefore indicates that these membranes do not contain large protein-free areas.
It is important to point out that this type of densely packed structure does not impede the structural dynamics of the membranes. Structural changes affecting the macroaggregation of LHCII in thylakoids have earlier been identified by CD spectroscopy (31) and probably play significant roles in the regulation of the dissipation of excess excitation energy in the antenna, and thereby in protecting plants against excessive radiation (32). Lamellar aggregates of LHCII and MDGD-LHCII are capable of undergoing light-induced reversible structural changes (12), which closely resemble the light-induced structural rearrangements in thylakoid membranes. Light-induced structural changes were recently identified in lipid-LHCII by means of scanning force microscopy and from surface-molecular area isotherms. These conformational transformations resulted in a change of about 10–20% in the surface area occupied by LHCII (33). Light-induced reversible structural changes in LHCII are also involved in the regulation of phosphorylation by light at the substrate level (34).
In summary, our results demonstrate that a single protein component can be considered to be responsible for transforming substantial amounts of non-bilayer lipids into lamellar structures, in which the complexes are to be found in structurally flexible, ordered macroarrays.
Acknowledgments
We thank Drs. I. Horváth and I. Dey for advice and help in the lipid analysis. This work was supported by Grants T019227 and T030324 from the Hungarian Research Fund. T.J. and A.H. gratefully acknowledge short-term fellowships from the Program “Biophysics of Photosynthesis,” European Science Foundation. G.G. received travel support from the Synchrotron Committee of Hungary.
Abbreviations
- CD
circular dichroism
- Chl
chlorophyll
- DGDG
digalactosyldiacylglycerol
- HII
inverted hexagonal phase
- LHCII
the chlorophyll-a/b light-harvesting antenna protein of photosystem II
- MGDG
monogalactosyldiacylglycerol
- PG
phosphatidylglycerol
- SQDG
sulfoquinovosyldiacylglycerol
References
- 1.de Kruijff B. Nature (London) 1997;386:129–130. doi: 10.1038/386129a0. [DOI] [PubMed] [Google Scholar]
- 2.Epand R M. Biochim Biophys Acta. 1998;1376:353–368. doi: 10.1016/s0304-4157(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 3.Taraschi T F, de Kruijff B, Verkleij A, Van Echteld C J. Biochim Biophys Acta. 1982;685:153–161. doi: 10.1016/0005-2736(82)90092-x. [DOI] [PubMed] [Google Scholar]
- 4.Rietveld A, van Kemenade T J, Hak T, Verkleij A J, de Kruijff B. Eur J Biochem. 1987;164:137–140. doi: 10.1111/j.1432-1033.1987.tb11004.x. [DOI] [PubMed] [Google Scholar]
- 5.Seddon J M, Templer R H, Warrender N A, Huang Z, Cevc G, Marsh D. Biochim Biophys Acta. 1997;1327:131–147. doi: 10.1016/s0005-2736(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 6.Murata N, Siegenthaler P A. In: Lipids in Photosynthesis: Structure, Function and Genetics. Siegenthaler P A, Murata N, editors. Dordrecht, the Netherlands: Kluwer; 1997. pp. 1–20. [Google Scholar]
- 7.Gounaris K, Barber J. Trends Biochem Sci. 1983;8:378–381. [Google Scholar]
- 8.Sen A, Williams W P, Quinn P J. Biochim Biophys Acta. 1981;663:380–389. doi: 10.1016/0005-2760(81)90167-3. [DOI] [PubMed] [Google Scholar]
- 9.Williams W P. In: Lipids in Photosynthesis: Structure, Function and Genetics. Murata N, Siegenthaler P A, editors. Dordrecht, the Netherlands: Kluwer; 1997. pp. 103–118. [Google Scholar]
- 10.Simidjiev I, Barzda V, Mustárdy L, Garab G. Anal Biochem. 1997;250:169–175. doi: 10.1006/abio.1997.2204. [DOI] [PubMed] [Google Scholar]
- 11.Barzda V, Istokovics A, Simidjiev I, Garab G. Biochemistry. 1996;35:8981–8985. doi: 10.1021/bi960114g. [DOI] [PubMed] [Google Scholar]
- 12.Simidjiev I, Barzda V, Mustárdy L, Garab G. Biochemistry. 1998;37:4169–4173. doi: 10.1021/bi972726m. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane-Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Vígh L, Horváth I, Thompson G A., Jr Biochim Biophys Acta. 1988;937:42–50. doi: 10.1016/0005-2736(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoppert M, Holzenburg A. Electron Microscopy in Microbiology. Oxford: BIOS Scientific; 1998. [Google Scholar]
- 16.Amenitsch H, Bernstorff S, Kriechbaum M, Lombardo D, Mio H, Rappolt M, Laggner P. J Appl Crystallogr. 1997;30:872–876. [Google Scholar]
- 17.Hovmöller S. Ultramicroscopy. 1992;40:121–135. [Google Scholar]
- 18.McDonnel A, Staehelin L A. J Cell Biol. 1980;84:40–56. doi: 10.1083/jcb.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussberger S, Dörr K, Wang D N, Kühlbrandt W. J Mol Biol. 1993;234:347–356. doi: 10.1006/jmbi.1993.1591. [DOI] [PubMed] [Google Scholar]
- 20.Douce R, Joyard J. In: Oxygenic Photosynthesis: The Light Reactions. Ort D R, Yocum C F, editors. Dordrecht, the Netherlands: Kluwer; 1996. pp. 69–101. [Google Scholar]
- 21.Barzda V, Mustárdy L, Garab G. Biochemistry. 1994;33:10837–10841. doi: 10.1021/bi00201a034. [DOI] [PubMed] [Google Scholar]
- 22.Keller D, Bustamante C. J Chem Phys. 1986;84:2961–2971. [Google Scholar]
- 23.Garab G. In: Biophysical Techniques in Photosynthesis. Amesz J, Hoff A J, editors. Dordrecht, the Netherlands: Kluwer; 1996. pp. 11–40. [Google Scholar]
- 24.Garab G, Faludi-Dániel Á, Sutherland J C, Hind G. Biochemistry. 1988;27:2425–2430. [Google Scholar]
- 25.Turner D C, Gruner S M. Biochemistry. 1992;31:1340–1355. doi: 10.1021/bi00120a009. [DOI] [PubMed] [Google Scholar]
- 26.Jávorfi T, Amenitsch H, Laggner P, Cseh Z, Mustárdy L, Borbély S, Rosta L, Garab G. In: Photosynthesis: Mechanisms and Effects. Garab G, editor. Dordrecht, the Netherlands: Kluwer; 1998. pp. 349–352. [Google Scholar]
- 27.Kühlbrandt W, Wang D N, Fujiyoshi Y. Nature (London) 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 28.Tampe R, Galla H J. Eur J Biochem. 1991;199:187–193. doi: 10.1111/j.1432-1033.1991.tb16108.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Quinn P J. Biophys Chem. 1999;80:93–101. doi: 10.1016/s0301-4622(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 30.Joliot P, Verméglio A, Joliot A. Biochim Biophys Acta. 1993;1141:151–174. [Google Scholar]
- 31.Garab G, Leegood R C, Walker D A, Sutherland J C, Hind G. Biochemistry. 1988;27:2430–2434. [Google Scholar]
- 32.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 33.Gruszecki W I, Grudzinski W, Matula M, Kernen P, Krupa Z. Photosynth Res. 1999;59:175–185. [Google Scholar]
- 34.Zer H, Vink M, Keren N, Dilly-Hartwig H G, Paulsen H, Herrmann R G, Andersson B, Ohad I. Proc Natl Acad Sci USA. 1999;96:8277–8282. doi: 10.1073/pnas.96.14.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]