Abstract
Stress-induced hypoalgesia (SIH) is an adaptive behavioral phenomenon mediated in part by the amygdala. Acute stress increases amygdalar noradrenaline levels and focal application of α2-adrenoceptor agonists in the central nucleus of the amygdala (CeA) is antinociceptive. We hypothesized that α2-adrenoceptor antagonist administration into the CeA may block SIH.
Bilateral microinjections of drug or saline via chronically implanted CeA cannulae were followed by either a period of restraint stress or rest. The nocifensive paw-withdrawal latency (PWL) to a focused beam of light was measured. PWLs were longer in restrained rats, constituting SIH. Microinjection of the α2-adrenoceptor antagonist idazoxan into the CeA prior to restraint blocked SIH. Idazoxan administration in unrestrained rats had no effect. Microinjection of the α2-adrenoceptor agonist clonidine in unrestrained rats caused dose dependent hypoalgesia, mimicking the effects of environmental stress. α2-Adrenoceptor function in the CeA is necessary for restraint-induced SIH.
Keywords: microinjection, α2-adrenoceptor, idazoxan, clonidine, restraint, nociception, stress-induced hypoalgesia
Hypoalgesia resulting from exposure to an acute stressor, or “stress-induced hypoalgesia” (SIH), is a well-described phenomenon in both humans and experimental animals (Hayes et al., 1978, Amit and Galina, 1986, Bodnar, 1986, van der Kolk et al., 1989, Rushen and Ladewig, 1991). SIH can be evoked by a variety of acute stressors including footshock and restraint, as well as more biologically relevant threat stimuli such as exposure to a predator or to odors from stressed conspecifics (Watkins et al., 1982b, Fanselow, 1985, Lester and Fanselow, 1985, Kavaliers, 1988, Rushen and Ladewig, 1991).
SIH may represent one component of a broader shift in attention towards salient environmental cues under stressful contingencies (Selden et al., 1990b, Berridge and Waterhouse, 2003, Morilak et al., 2005). This allows the organism to ignore distracting nociceptive stimuli, and attend to features of the environment important for escape or defense (Amit and Galina, 1988).
One neurotransmitter implicated in SIH is noradrenaline, and systemic treatment with the α2-adrenoceptor antagonists yohimbine or idazoxan blocks the expression of SIH in rodents (Coderre and Rollman, 1984, Oluyomi and Hart, 1990, Tokuyama et al., 1991). However, the site or sites of action of noradrenaline in evoking SIH are unknown.
SIH can be blocked by lesions of the central nucleus of the amygdala (CeA) (Helmstetter and Bellgowan, 1993). The CeA is densely populated by nociceptive responsive neurons, and electrical or chemical stimulation of the CeA results in altered nociceptive responsiveness (Mena et al., 1995, Manning et al., 2003). The CeA has reciprocal connections with the periaqueductal gray, an important link in brainstem nociceptive modulatory systems (Beitz, 1982, Bernard and Besson, 1990, Rizvi et al., 1991, Bernard et al., 1992, Neugebauer et al., 2003) and with the locus coeruleus (LC), the major source of forebrain noradrenaline. The LC may provide the noradrenergic link between stress and CeA activation to produce SIH. The LC is critical for the acute response to stressors such as electric shock, loud noise, tail-pinch, and immobilization (U'Prichard et al., 1980, Aston-Jones et al., 1991, Passerin et al., 2000, Pardon et al., 2002, Sved et al., 2002, Ma and Morilak, 2004). Lesions of the dorsal noradrenergic bundle attenuate stress-induced increases in noradrenaline levels in the CeA (Tanaka et al., 1991, Fendt et al., 1994, Quirarte et al., 1998, Khoshbouei et al., 2002). Moreover, focal application of an α2-agonist in the CeA mimics the effect of stress in inducing hypoalgesia (Ortiz et al., 2007).
These anatomical and functional data suggest that SIH may require stress-induced noradrenergic stimulation of α2-adrenoceptors in the CeA. To test this, we blocked α2-noradrenergic receptors in the CeA interfering with the expression of restraint-induced SIH in awake, behaving rats.
EXPERIMENTAL PROCEDURES
Animals and surgical preparation
Experimental protocols were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. All experiments conformed to the guidelines of the International Association for the Study of Pain (IASP, 1983). Efforts were made throughout experiments to minimize animal discomfort and to reduce the number of animals used. Male Sprague–Dawley rats (Taconic, Indianapolis, IN, USA; 250–300 g) were anesthetized with pentobarbital (60 mg/kg, i.p.), given a single dose of cefazolin (25 mg/kg, i.p.), and placed in a stereotactic head frame. Body temperature was maintained at approximately 37 °C by a circulating water pad. After incision of the scalp and retraction of the periosteum, craniotomies were drilled into the skull overlying the amygdala bilaterally. Two burr holes were also drilled for retaining screw placement. Bilateral guide cannulae (26 gauge, Plastics One, Roanoke, VA) were then placed under stereotactic guidance to overlie the CeA (relative to bregma: A/P − 2.8mm, M/L ± 4.2mm. relative to skull surface: D/V −6.3mm) and two retaining screws were placed. Polycarboxylate dental cement (Dentonics, Monroe, NC) was used to secure the cannulae to the skull. Once the dental cement was dry, dummy cannulae (Plastics One, Roanoke, VA) were placed into the guide cannulae to occlude the lumen. Animals were recovered and placed in fresh cages to be kept singly throughout the remainder of the experiment.
Nociceptive Testing Apparatus
A Hargreaves’ Box (IITC Life Science Inc., CA) was used for measurement of the PWL. This consists of a clear Plexiglas two-chamber ventilated box mounted atop a heated clear glass plate that can be maintained at constant temperature. The animal to be tested is placed in one of the two chambers and allowed to acclimate. A heat projector lamp below the glass floor plate is focused on the mid-plantar surface of the hind paw of the animal and a timer is started. When the animal withdraws its paw from the focused heat beam, the timer is stopped. The time from initial heat beam contact with the hind paw until the removal of the paw is the withdrawal latency, which provides a measure of nociceptive responsiveness. The projector beam was programmed to 75% maximal intensity, 15 sec cut-off time.
Protocols
Habituation Protocol for Post-surgical Animals
After one week of recovery from surgery, all animals were exposed to a habituation protocol designed to familiarize them with the nociceptive testing environment and procedures. Habituation was carried out between 1300 and 1600 hrs. Each animal was placed in the Hargreaves’ Box (H-box) for 15min, once per day, for six days. The projector lamp was switched on three times at random intervals during the habituation. The projector lamp was focused on a point away from the animal. Beginning on the fourth day of habituation, a sham injection procedure was performed for each animal prior to placement in the H-box. For this procedure, the animal was held firmly and a dummy cannula was removed from the guide cannula and then replaced. The animal was placed back in its home cage and allowed to move freely for 1 min. The procedure was then repeated for the contralateral dummy cannula.
Habituation Protocol for Surgically Naive nimals
Each animal was habituated to the H-box for 15 min, once per day, for three days. The projector lamp was switched on three times at random intervals during the habituation. The projector lamp was focused on a point away from the animal.
Microinjection Protocol
Each animal underwent microinjection of drug or vehicle prior to restraint and/or nociceptive testing. The animal was held firmly, the dummy cannula removed, and the microinjector placed into the guide cannula. The microinjection apparatus consisted of a microinjector (33 gauge, 8.1mm length, Plastics One, Roanoke, VA) connected by a length of polyethylene tubing (PE-50) to a glass microsyringe (1 µL, Hamilton, Reno, NV). The animal was then placed back in its home cage and allowed to move freely during the microinjection. Injections (0.2µL volumes were used in all experiments) were performed over 1 min, and the injector was left in place an additional 30 sec to allow for diffusion away from the tip. The animal was then held firmly while the injector was removed and the dummy cannula reinserted. The microinjection procedure was then repeated on the contralateral side.
Restraint-Stress Protocol
Animals were restrained by placing them in a tube formed from flexible plastic mesh (22.5 × 30 cm). The ends of the tube were clamped shut and this prevented the animals from moving but did not impede respiration. Based on the results of pilot experiments, surgically naive animals were restrained for 20 min and post-surgical animals were restrained for 1 hr.
Nociceptive Testing Protocol
This procedure was identical for surgically naive and post-surgical animals. The animal was placed in the nociceptive testing chamber and allowed to acclimate for 5 min. The projector lamp was then focused on the mid-plantar surface of the right hind paw and switched on. As the animal withdrew the hind paw, the projector lamp was switched off and the time interval was recorded. An automatic cutoff time of 15 sec was used to prevent tissue damage. This procedure was repeated at 5 min intervals, alternating the right and left hindpaws, for 6 consecutive trials. Each animal underwent nociceptive testing only once, after a single injection of vehicle, idazoxan, or clonidine (low or high dose).
Open-field Testing Protocol
At least three days following nociceptive testing animals were placed in an open-field arena to assess the sedative affects of clonidine by measuring locomotor activity. The animal was placed in the center of the walled chamber (0.9m × 0.9m) and allowed to explore. The number of squares (15cm × 15cm) the forepaws entered in 30 sec was counted. Three pre-infusion trials were performed at 5 min intervals to establish baseline activity. Each animal then underwent microinjection of drug (1.25mg or 5.0mg clonidine) or vehicle as described above. Following a 15 min interval, the animal was again placed in the center of the open-field arena and its activity recorded. Six consecutive 30 sec post-infusion trials were performed at 5 min intervals. The data for individual animals were summed across testing intervals. Animals underwent testing in the open-field on two separate occasions with at least two days between testing. Those receiving a microinjection of either dose of drug for the first test received microinjection of vehicle on the second, and vice versa.
Materials
Drugs
All drugs used were commercially available (Sigma/Aldrich, St. Louis, MO) and were dissolved in physiological saline. Drug solutions were prepared daily, and the pH adjusted to 7.4. The dose of idazoxan used was 9 µg per injection site. The dose of clonidine used was either 1.25 µg or 5 µg per injection site.
Histology
At the conclusion of the experiments, animals received an overdose of barbiturate. Infusion sites were marked by injection of Pontamine Sky Blue dye. Animals were then perfused intracardially with physiological saline followed by 10% formalin. Tissue was counterstained with Cresyl Violet, and infusion sites were verified and plotted on standardized sections (Paxinos and Watson, 1986).
Data Analysis
Student’s t-test for correlated means was used for comparing control and treatment group paw-withdrawal latencies. ANOVA was used for within group and between group comparisons. P < 0.05 was considered significant.
RESULTS
Restraint stress in surgically naive rats significantly increased the PWL (Fig. 1). Restraint stress also increased the PWL in operated rats following microinjection of saline into the CeA (Fig. 2). These findings constitute the expression of SIH in the current behavioral model. Microinjection of the α2-adrenoceptor antagonist idazoxan (9 µg) into the CeA prior to restraint completely blocked the expected increase in PWL. Intra-CeA microinjection of idazoxan (9 µg) without subsequent restraint had no effect.
Fig. 1.
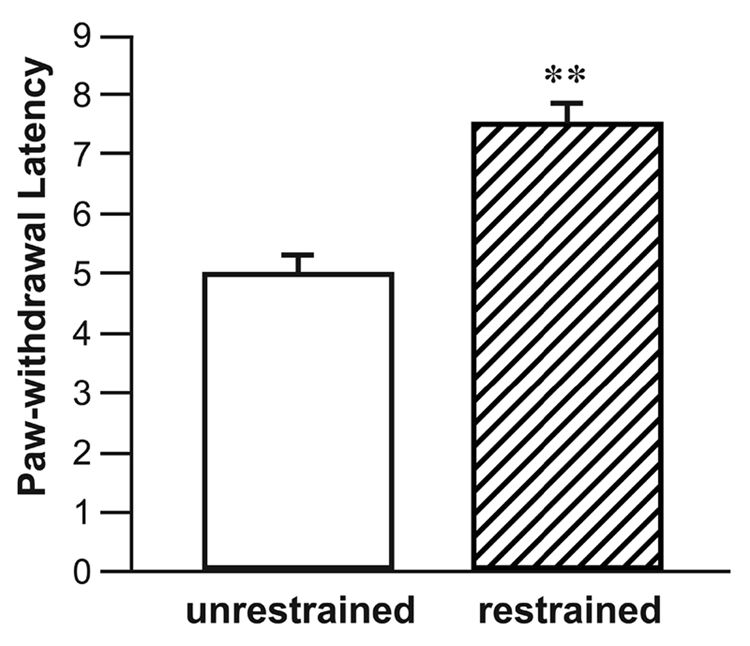
Surgically naive rats subjected to restraint had significantly elevated PWL’s (seconds) compared to unrestrained animals. **p<0.01, Student’s t-test. n = 13–16 per group.
Fig. 2.
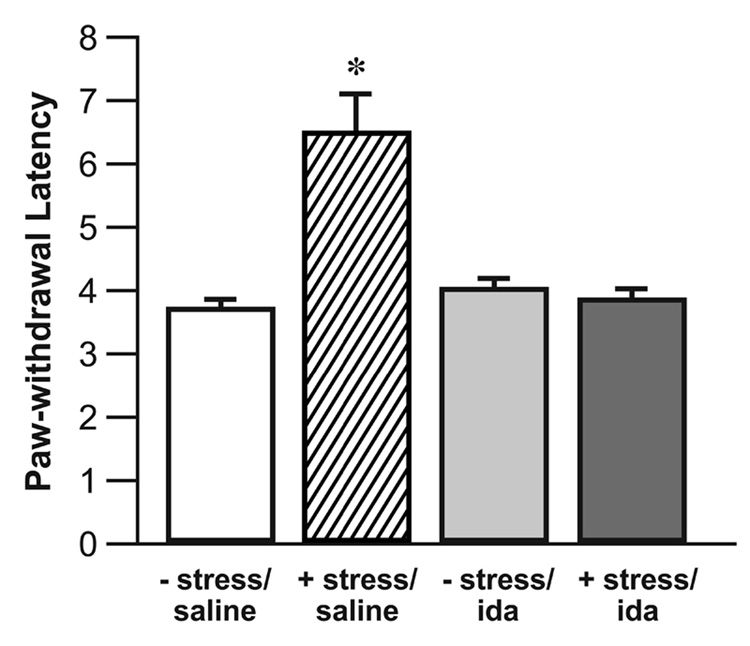
Microinjection of the α-2 adrenergic antagonist idazoxan into the CeA blocks SIH. Restraint significantly elevated PWL’s (seconds) in saline injected rats. Microinjection of α-2 adrenoceptor antagonist idazoxan (9 µg) in unrestrained animals had no effect. Microinjection of idazoxan (9 µg) prior to restraint blocked the expected elevation of the PWL. There was no significant difference among the − stress/saline, − stress/ida, and + stress/ida groups (ANOVA). n = 8 per group, (* P < 0.05, Student’s t-test, as compared to the − stress/saline group) − stress = no restraint, + stress = restrained, ida = idazoxan.
Microinjection of the α2-adrenoceptor agonist clonidine (1.25 µg or 5 µg) into the CeA without subsequent restraint resulted in a dose-dependent elevation of the PWL (Fig. 3). The 1.25 µg dose of clonidine produced an elevation in PWL similar to that produced by restraint stress alone.
Fig. 3.
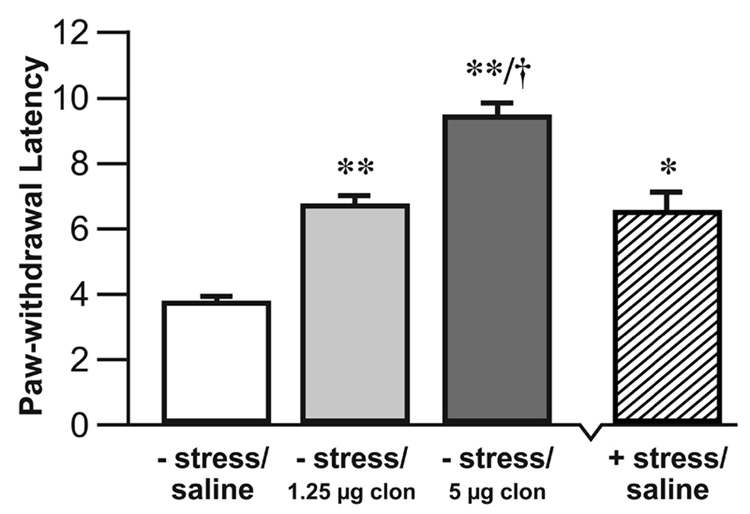
Microinjection of the α-2 agonist clonidine into the CeA resulted in a dose-dependent elevation of the PWL. Unrestrained rats microinjected with clonidine (1.25 µg) had significantly elevated PWL’s compared to saline injected unrestrained rats. Unrestrained rats microinjected with clonidine (5µg) had significantly elevated PWL’s compared to the control and 1.25 µg clonidine groups. The + stress/ saline group is shown to illustrate the comparable magnitude of the effects of restraint and intra-CeA microinjection of 1.25 µg clonidine on PWL. n = 8 – 10 per group, (* P < 0.05, ** P < 0.01, Student’s t-test, compared to the − stress/saline group. † P < 0.05, Student’s t-test, compared to the − stress/1.25 µg clon groups) − stress = no restraint, + stress = restrained, clon = clonidine.
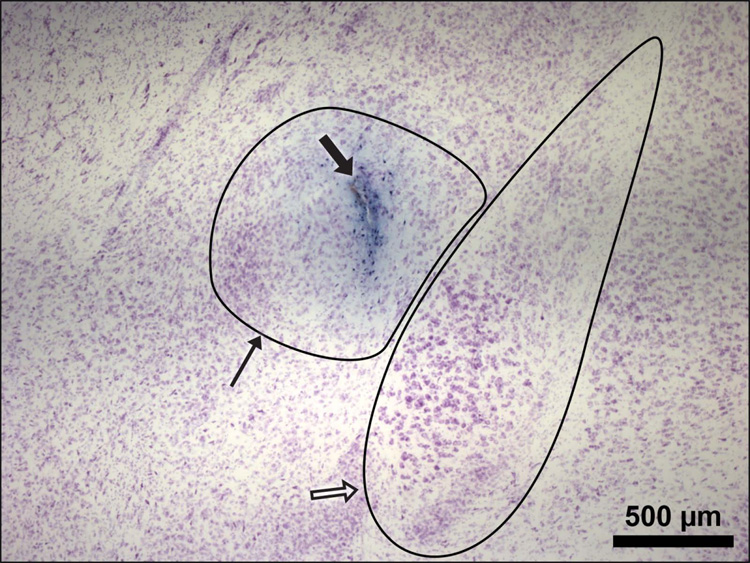
Only microinjections found on histological examination to have entered the target nucleus were included in the behavioral analysis (Fig. 4 and Fig. 5).
Fig. 4.
Representative section through the left amygdala showing the CeA (thin arrow) and BLA (open arrow), the microinjector tip placement is seen within the CeA (bold arrow). Magnification = 4×.
Fig. 5.
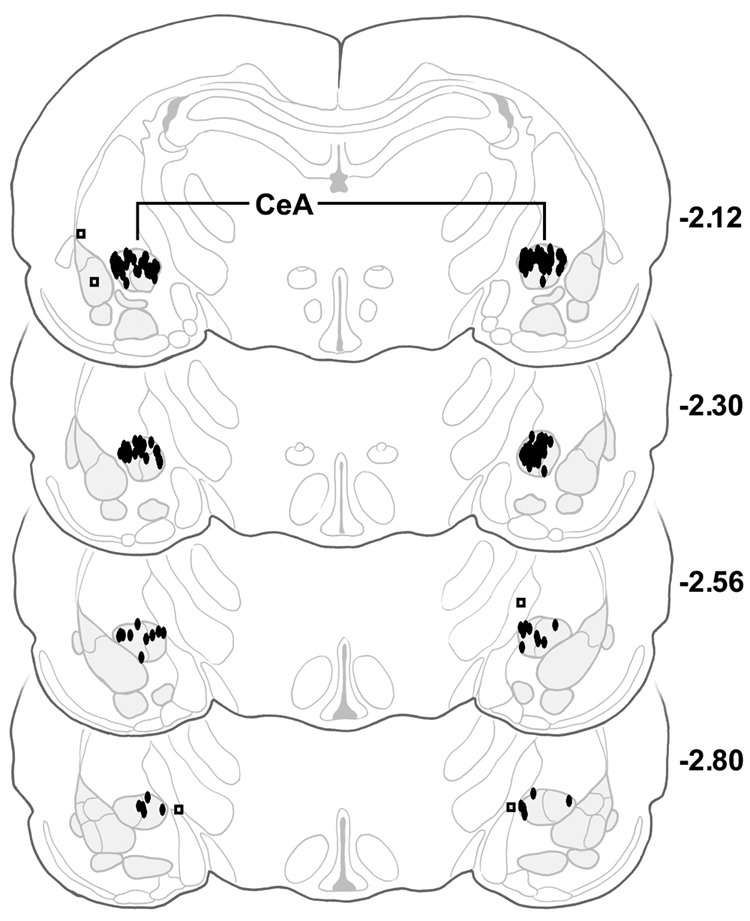
Serial coronal sections of the rat brain showing placement of microinjections (N = 50). Filled ovals = CeA injections, open squares = placement controls. CeA = central nucleus of the amygdala, numbers to the right of sections represent millimeters relative to bregma, adapted from the atlas of Paxinos and Watson (Paxinos and Watson, 1986).
Microinjection of 1.25 µg clonidine did not alter spontaneous locomotor activity in an open field test as compared with saline-injected controls (1.25 µg clonidine: 14.5 squares entered ± 3.26 s.e.m.; saline: 13.81 squares entered ± 1.49 s.e.m.; P = NS, Student’s t-test). Microinjection of 5 µg clonidine significantly reduced spontaneous motor activity during open-field testing (5 µg clonidine: 9.48 squares entered ± 0.88 s.e.m.; saline: 13.81 squares entered ± 1.49 s.e.m.; P < 0.05, Student’s t-test).
DISCUSSION
The CeA participates in the elaboration of SIH (Helmstetter and Bellgowan, 1993). We show here that the CeA requires α2-adrenergic transmission for SIH expression. Microinjection of the α2-adrenoceptor antagonist idazoxan into the CeA prior to restraint stress effectively blocked SIH. Intra-CeA idazoxan did not alter baseline nociceptive responsiveness in animals not exposed to restraint stress, arguing against a direct effect on nociceptive responsiveness or general activity, per se, as opposed to SIH.
Conversely, intra-CeA microinjection of the α2-adrenoceptor agonist clonidine produced hypoalgesia. A dose of clonidine with no effect on locomotor activity mimicked the hypoalgeric effects of restraint stress. Thus sedation is unlikely to account for the analgesic effects of clonidine under these conditions.
Alterations in baseline skin temperature may affect measurement of nociception using withdrawal latency from radiant heat (Hole and Tjolsen, 1993). However, restraint stress in rats also induces hypoalgesia measured by decreased licking and paw flexing after formalin injection (Aloisi et al., 1998). Furthermore, idazoxan injection into the CeA had no effect on baseline withdrawal latencies in unstressed animals in our study. Thus, the effects of local microinjection of adrenergic agents observed here appear most likely to result from an effect on nociceptive modulation.
We have previously demonstrated that the antinociceptive actions of clonidine microinjected into the CeA are blocked by α2- but not by α1-receptor antagonists and are seen only after injection into the CeA and not into the basolateral nucleus of the amygdala, suggesting the presence of both pharmacological and anatomical specificity (Ortiz et al., 2007).
The amygdala participates in the elaboration of SIH (Fox and Sorenson, 1994, Pavlovic et al., 1996). Lesions of the CeA block SIH but do not affect baseline nociception (Werka and Marek, 1990, Werka, 1994). Similarly, in the present experiments, blockade of α2-adrenoceptors in the CeA eliminated SIH but had no effect on nociception, per se. These results emphasize the modulatory nature of amygdalar influence on nociceptive responsiveness, particularly during challenging environmental conditions.
Recently, Delaney and colleagues have observed that noradrenaline acting at α2-presynaptic receptors potently inhibits single fiber inputs to the lateral division of the CeA from the nociceptive pontine parabrachial nucleus (Delaney et al., 2007). This circuit may allow for the interaction of nociceptive sensory information and noradrenergic, nociceptive modulatory influence within the CeA. Our results indicate that function of this circuit is also required for the behavioral expression of SIH.
Central Noradrenaline and Adaptive Responses to Environmental Stress
SIH is an important adaptive behavioral response that is conserved among diverse species, including humans. Stress may be defined as any stimulus that disturbs the physiological equilibrium of the organism or threatens to do so. By this definition, many stressors, including fear, pain, temperature extremes, novel environments, restraint, and the odor of stressed conspecifics, also trigger hypoalgesia (Watkins et al., 1982a, Fanselow, 1985, Lester and Fanselow, 1985, Kavaliers, 1988, Rushen and Ladewig, 1991). There is extensive anecdotal evidence regarding profound, albeit temporary, hypoalgesia resulting from battlefield and other severe stressors in humans (Wall, 1979).
SIH may facilitate adaptive responses to stressful environmental circumstances by allowing the organism to ignore distracting nociceptive input in order to attend to life or tissue preserving escape and defense behaviors (Amit and Galina, 1986). Noradrenergic transmission in other experimental contexts promotes various adaptive responses to stress, including focused attention to salient environmental stimuli and the systemic release of cortisol (Selden et al., 1990a, Selden et al., 1990b, Selden et al., 1991a, Selden et al., 1991b, Dalley et al., 2001). While brainstem noradrenergic fibers arise from a limited number of small nuclei, their diffuse forebrain projections allow them to coordinate numerous brain functions in various terminal areas, such as the frontal cortex, amygdala, hippocampus, and hypothalamus (Selden et al., 1991a, Selden et al., 1991b). Noradrenergic transmission via α2-receptors in the CeA may also participate in this coordinated response to stressful environmental contingencies, by promoting the expression of SIH.
CONCLUSION
Microinfusion of an α2-adrenoceptor antagonist into the CeA prior to restraint stress blocks SIH in awake behaving rats. Conversely, microinjection of an α2-adrenoceptor agonist into the CeA results in dose-dependent hypoalgesia. SIH may be part of a coordinated set of adaptive responses to stressful environmental contingencies that are mediated by noradrenergic transmission in various forebrain regions. α2-Adrenergic transmission in the CeA is required for the behavioral expression of SIH.
ACKNOWLEDGMENTS
Supported by grants from the National Institute of Neurological Disorders NS44255 (N.R.S.), the National Institute on Drug Abuse (DA05608, M.M.H.), and the Cameron Foundation (N.R.S.). We thank Shirley McCartney, Ph.D. for editorial assistance, Andy Rekito. M.S. for assistance with artwork and Kim J. Burchiel, M.D. for support of the Department of Neurological Surgery Laboratories at OHSU and personal encouragement.
LIST OF ABBREVIATIONS
- A/P
Anterior/posterior
- ANOVA
Analysis of variance
- CNS
Central nervous system
- CeA
Central nucleus of the amygdala
- D/V
Dorsal/ventral
- i.p.
Intra-peritoneal
- i.v.
Intravenous
- LC
Locus coeruleus
- M/L
Medial/lateral
- NS
Not significant
- PWL
Paw-withdrawal latency
- SIH
Stress-induced hypoalgesia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloisi AM, Ceccarelli I, Lupo C. Behavioural and hormonal effects of restraint stress and formalin test in male and female rats. Brain Res Bull. 1998;47:57–62. doi: 10.1016/s0361-9230(98)00063-x. [DOI] [PubMed] [Google Scholar]
- Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- Amit Z, Galina ZH. Stress induced analgesia plays an adaptive role in the organization of behavioral responding. Brain Res Bull. 1988;21:955–958. doi: 10.1016/0361-9230(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research - Brain Research Reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Neuropharmacological and neuroendocrine substrates of stress-induced analgesia. Ann N Y Acad Sci. 1986;467:345–360. doi: 10.1111/j.1749-6632.1986.tb14639.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Rollman GB. Stress analgesia: effects of PCPA, yohimbine, and naloxone. Pharmacol Biochem Behav. 1984;21:681–686. doi: 10.1016/s0091-3057(84)80002-7. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Odors released by stressed rats produce opioid analgesia in unstressed rats. Behav Neurosci. 1985;99:589–592. doi: 10.1037//0735-7044.99.3.589. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Amygdaloid noradrenaline is involved in the sensitization of the acoustic startle response in rats. Pharmacol Biochem Behav. 1994;48:307–314. doi: 10.1016/0091-3057(94)90532-0. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Sorenson CA. Bilateral lesions of the amygdala attenuate analgesia induced by diverse environmental challenges. Brain Res. 1994;648:215–221. doi: 10.1016/0006-8993(94)91120-7. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Bennett GJ, Newlon PG, Mayer DJ. Behavioral and physiological studies of non-narcotic analgesia in the rat elicited by certain environmental stimuli. Brain Res. 1978;155:69–90. doi: 10.1016/0006-8993(78)90306-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain Res. 1993;612:253–257. doi: 10.1016/0006-8993(93)91669-j. [DOI] [PubMed] [Google Scholar]
- Hole K, Tjolsen A. The tail-flick and formalin tests in rodents: changes in skin temperature as a confounding factor. Pain. 1993;53:247–254. doi: 10.1016/0304-3959(93)90220-J. [DOI] [PubMed] [Google Scholar]
- IASP. Ethical guidelines for investigations of experimental pain in conscious animals [Guest editorial] Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Kavaliers M. Brief exposure to a natural predator, the short-tailed weasel, induces benzodiazepine-sensitive analgesia in white-footed mice. Physiol Behav. 1988;43:187–193. doi: 10.1016/0031-9384(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Lester LS, Fanselow MS. Exposure to a cat produces opioid analgesia in rats. Behav Neurosci. 1985;99:756–759. doi: 10.1037//0735-7044.99.4.756. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Manning BH, Martin WJ, Meng ID. The rodent amygdala contributes to the production of cannabinoid-induced antinociception. Neuroscience. 2003;120:1157–1170. doi: 10.1016/s0306-4522(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Mena NB, Mathur R, Nayar U. Amygdalar involvement in pain. Indian J Physiol Pharmacol. 1995;39:339–346. [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RWt. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluyomi AO, Hart SL. Alpha-adrenoceptor involvement in swim stress-induced antinociception in the mouse. J Pharm Pharmacol. 1990;42:778–784. doi: 10.1111/j.2042-7158.1990.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Ortiz JP, Heinricher MM, Selden NR. Noradrenergic agonist administration into the central nucleus of the amygdala increases the tail-flick latency in lightly anesthetized rats. Neuroscience. 2007;148:737–743. doi: 10.1016/j.neuroscience.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF. Role of locus coeruleus in foot shock-evoked Fos expression in rat brain. Neuroscience. 2000;101:1071–1082. doi: 10.1016/s0306-4522(00)00372-9. [DOI] [PubMed] [Google Scholar]
- Pavlovic ZW, Cooper ML, Bodnar RJ. Enhancements in swim stress-induced hypothermia, but not analgesia, following amygdala lesions in rats. Physiol Behav. 1996;59:77–82. doi: 10.1016/0031-9384(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain and Stereotaxic Coordinates. 2nd Edition. New York: Academic Press; 1986. [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rushen J, Ladewig J. Stress-induced hypoalgesia and opioid inhibition of pigs' responses to restraint. Physiol Behav. 1991;50:1093–1096. doi: 10.1016/0031-9384(91)90566-7. [DOI] [PubMed] [Google Scholar]
- Selden NR, Cole BJ, Everitt BJ, Robbins TW. Damage to ceruleo-cortical noradrenergic projections impairs locally cued but enhances spatially cued water maze acquisition. Behav Brain Res. 1990a;39:29–51. doi: 10.1016/0166-4328(90)90119-y. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdale and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991a;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Robbins TW. Telencephalic but not diencephalic noradrenaline depletion enhances behavioural but not endocrine measures of fear conditioning to contextual stimuli. Behav Brain Res. 1991b;43:139–154. doi: 10.1016/s0166-4328(05)80064-6. [DOI] [PubMed] [Google Scholar]
- Selden NR, Robbins TW, Everitt BJ. Enhanced behavioral conditioning to context and impaired behavioral and neuroendocrine responses to conditioned stimuli following ceruleocortical noradrenergic lesions: support for an attentional hypothesis of central noradrenergic function. J Neurosci. 1990b;10:531–539. doi: 10.1523/JNEUROSCI.10-02-00531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington's nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yokoo H, Mizoguchi K, Yoshida M, Tsuda A, Tanaka M. Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain Res. 1991;544:174–176. doi: 10.1016/0006-8993(91)90902-8. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Takahashi M, Kaneto H. Participation of an alpha 2-mediated mechanism in the production of forced swimming-stress induced analgesia in mice. J Pharmacobiodyn. 1991;14:357–361. doi: 10.1248/bpb1978.14.357. [DOI] [PubMed] [Google Scholar]
- U'Prichard DC, Reisine TD, Mason ST, Fibiger HC, Yamamura HI. Modulation of rat brain alpha- and beta-adrenergic receptor populations by lesion of the dorsal noradrenergic bundle. Brain Res. 1980;187:143–154. doi: 10.1016/0006-8993(80)90500-4. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, Greenberg MS, Orr SP, Pitman RK. Endogenous opioids, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol Bull. 1989;25:417–421. [PubMed] [Google Scholar]
- Wall PD. On the relation of injury to pain. The John J. Bonica lecture. Pain. 1979;6:253–264. doi: 10.1016/0304-3959(79)90047-2. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Cobelli DA, Faris P, Aceto MD, Mayer DJ. Opiate vs non-opiate footshock-induced analgesia (FSIA): the body region shocked is a critical factor. Brain Res. 1982a;242:299–308. doi: 10.1016/0006-8993(82)90313-4. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Cobelli DA, Mayer DJ. Opiate vs non-opiate footshock induced analgesia (FSIA): descending and intraspinal components. Brain Res. 1982b;245:97–106. doi: 10.1016/0006-8993(82)90342-0. [DOI] [PubMed] [Google Scholar]
- Werka T. Post-stress analgesia in rats with partial amygdala lesions. Acta Neurobiol Exp (Warsz) 1994;54:127–132. [PubMed] [Google Scholar]
- Werka T, Marek P. Post-stress analgesia after lesions to the central nucleus of the amygdala in rats. Acta Neurobiol Exp (Warsz) 1990;50:13–22. [PubMed] [Google Scholar]