Abstract
Free radicals play a role in aging and age-related human diseases, including pulmonary emphysema. Cigarette smoke represents a source of oxidants and is considered an environmental hazard that causes pulmonary emphysema. Here, we show that caveolin-1 activates ataxia telangiectasia-mutated (ATM) after oxidative stress by sequestering the ATM inhibitor, the catalytic subunit of protein phosphatase 2A, into caveolar membranes. We demonstrate that cigarette smoke extracts promote stress-induced premature senescence in wild type but not caveolin-1 null lung fibroblasts and that caveolin-1 expression is required for activation of the ATM-p53-p21Waf1/Cip1 pathway following stimulation with cigarette smoke extracts in vitro. In vivo studies show that caveolin-1 expression is necessary for cigarette smoking-induced senescence of lung fibroblasts and pulmonary emphysema. These findings bring new insights into the molecular mechanism underlying free radical activation of the ATM-p53 pathway and indicate that caveolin-1 is a novel therapeutic target for the treatment and/or prevention of pulmonary emphysema.
Most cells cannot divide indefinitely due to a process termed cellular senescence. Growth arrest is associated with well defined biochemical alterations. These include irreversible cell cycle arrest, increased p53 activity, and increased p21Waf1/Cip1 protein expression (1, 2). Cellular stress can accelerate the onset of senescence. Oxidative stress, for example, is known to induce premature senescence in cells in culture (3, 4). Accumulation of senescent cells over time may contribute to aging and age-related diseases. In fact, their inability to proliferate contributes to reduced tissue function in aging organs. In addition, remaining metabolically active, senescent cells may play a role in tissue aging by influencing the neighboring tissue microenvironment (reviewed in Ref. 5).
Pulmonary emphysema is a fatal and age-related lung disease that occurs after a prolonged period of cigarette smoking. In the United States alone, there are about 4–5 million people with emphysema. Pulmonary emphysema is characterized by alveolar destruction, which promotes airspace enlargement with reduction of alveolar capillary exchange area. Because each puff of cigarette smoke contains ∼1015 free radicals in the gas phase, contains 1018 free radicals/gram of tar, and includes potent oxidants such as hydrogen peroxide, oxidative stress is believed to play an important role in the pathogenesis of emphysema (6, 7, 33). The classical concept of the pathogenesis of emphysema was based on lung inflammation caused by cigarette smoking and environmental pollutants, which leads to a protease/antiprotease imbalance, and ultimately, to the destruction of the alveolar wall (8).
However, emerging data indicate that cellular senescence may contribute to the cigarette smoke-induced emphysematous phenotype, which is consistent with the notion that oxidants promote cellular senescence (9–13). Because cellular senescence is characterized by cell cycle arrest, the presence of senescent cells may explain the lack of compensation for cell loss, which results in progressive destruction of alveolar architecture. The molecular mechanisms underlying free radical-induced senescence and emphysema remain to be elucidated.
Caveolae are vesicular invaginations of the plasma membrane. Caveolin-1 is the structural component of caveolae. It has been proposed that caveolin-1 participates in vesicular trafficking events and signal transduction processes (14) by acting as a scaffolding protein (15) to organize and concentrate specific lipids (cholesterol and glyco-sphingolipids) and lipid-modified signaling molecules (Src-like kinases, H-Ras, endothelial nitric oxide synthase, components of the p42/44 MAPK2 pathway, G-proteins, epidermal growth factor receptor, Neu, protein kinase A, and protein kinase C) within caveolar membranes (16–20). In addition to concentrating these signaling molecules within a specific region of the plasma membrane, caveolin-1 binding functionally regulates the activity of caveolae-associated molecules.
We have previously demonstrated that overexpression of caveolin-1 arrests cell cycle progression and induces a senescent phenotype (21, 22). We have also shown that oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 MAPK/Sp1-mediated activation of two GC-rich promoter elements (23). The signaling events, downstream of caveolin-1, activated by oxidative stress and whether caveolin-1 expression is required for SIPS remain to be determined. In addition, the role of caveolin-1 in the pathogenesis of emphysema is totally unknown. Here, we identify caveolin-1 as a novel signaling player that links oxidative stress to pulmonary emphysema.
EXPERIMENTAL PROCEDURES
Lung Histology—The mice were sacrificed at the appropriate time point, and the right ventricle was perfused with normal saline to remove blood. The lungs were then inflated to fixed pressure (25 cm of H2O) with 10% buffered formalin for 10 min. The lungs were fixed overnight before embedding in paraffin. Sections were obtained for morphological and histochemical analysis as described previously (24–27). Briefly, lung sections were stained with hematoxylin and eosin (H&E). For each lung, images from 20 randomly selected ×20 fields were analyzed using the NIH Image software. Images were edited to remove airways, blood vessels, and lymphocytic nodules; they were then thresholded manually, made binary, and inverted. Horizontal and vertical grid lines were sequentially superimposed over the images, and the number of intercepts was measured using NIH Image. The mean linear intercept (Lm) was calculated from the number of intercepts as described previously (25).
RESULTS
Caveolin-1 Activates ATM after Oxidative Stress by Sequestering PP2A-C into Caveolar Membranes—The ATM protein kinase is a key regulator of the p53 pathway in response to genotoxic stress. Reactive oxygen species have been shown to activate ATM (28) and p53 in an ATM-dependent manner in different cell types (29, 30). Activation of ATM occurs when oxidative stress promotes autophosphorylation of multimeric ATM at serine 1981, which dissociates into active monomers and then rapidly phosphorylates and activates numerous substrates, including p53 (31). Because we have previously shown that overexpression of caveolin-1 activates p53 in mouse embryonic fibroblasts (21), we asked whether caveolin-1 may play a role in the activation of p53 after oxidative stress through modulation of ATM.
Using subcytotoxic hydrogen peroxide as a source of free radicals, we first show that oxidative stress activated ATM, up-regulated p21Waf1/Cip1, a downstream target of p53 that regulates cell cycle arrest, and induced premature senescence in WI-38 human lung fibroblasts (supplemental Fig. 1, A–D). Stress-induced premature senescence was dependent upon activation of ATM, as shown by a dramatically reduced number of senescent cells in the presence of caffeine, an ATM inhibitor (supplemental Fig. 1, C and D). Interestingly, oxidative stress induced the translocation of PP2A-C, an ATM inhibitor, into caveolar membranes, away from ATM, and promoted the interaction between PP2A-C and caveolin-1 (supplemental Fig. 2, A and B, respectively). Does the translocation of PP2A-C into caveolar membranes play a role in the activation of ATM? We show in Fig. 1, A and B, that the translocation of PP2A-C into caveolar membranes occurred in wild type but not caveolin-1 null mouse embryonic fibroblasts (MEFs), which do not express caveolin-1. Consistent with this result, we show in supplemental Fig. 3A that PP2A-C and caveolin-1 did not co-localize under resting conditions in WT MEFs; PP2A-C was localized in the nucleus and cytoplasm, whereas caveolin-1 was localized at the plasma membrane. However, PP2A-C partially co-localized with caveolin-1 at the plasma membrane (supplemental Fig. 3A, arrows) and in cytoplasmic membranes (supplemental Fig. 3A, arrowhead) after oxidative stress in WT MEFs. In contrast, PP2A-C was localized in the nucleus and cytoplasm either before or after oxidative stress in caveolin-1 null MEFs (supplemental Fig. 3A). We also show that ATM was localized in the nucleus in both WT and caveolin-1 null MEFs under resting conditions and that oxidative stress did not change its localization (supplemental Fig. 3B).
FIGURE 1.
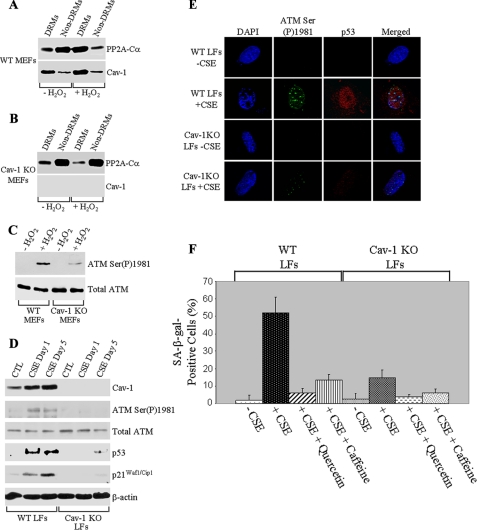
Cigarette smoke extracts induce premature senescence of lung fibroblasts in vitro through a caveolin-1-dependent activation of the ATM/p53/p21 pathway. A–C, WT and Cav-1 KO MEFs were treated with 150 μm H2O2 for 2 h (+H2O2). Cells were washed and recovered in growth medium for 24 h. Untreated cells were used as controls (–H2O2). Cells were then collected, and detergent-resistant microdomains (DRMs) were separated from the bulk of cellular membranes and cytosolic proteins (Non-DRMs) by equilibrium sucrose density gradient centrifugation. Expression of PP2A-Cα and Cav-1 was examined by immunoblotting analysis using specific antibody probes (A and B). In C, cells were treated as in A and B and subjected to immunoblotting analysis using antibody probes specific for phospho-Ser-1981 ATM (ATM Ser(P)1981) and total ATM. D–F, WTLFs and Cav-1 KO LFs were exposed to 2% CSE for 1 (D and E) and 5 (D and F) days. In F, cells were also treated with CSE in the presence of 30 μm quercetin and 200 nm caffeine. Cells were then subjected to immunoblotting (D) and immunofluorescence (E) analysis using specific antibody probes. Cells were also subjected to the senescence-associated β-galactosidase activity assay (F). n = 4; values represent means ± S.E. The total number of cells per field was maintained identically in WT and caveolin-1 null LFs before SA-β-gal staining. CTL, control; DAPI, 4′,6-diamidino-2-phenylindole.
Interestingly, caveolin-1 expression was required for the activation of ATM after hydrogen peroxide treatment, as shown by a dramatically reduced expression of phospho-Ser-1981 ATM in caveolin-1 null MEFs, as compared with wild type MEFs (Fig. 1C). Thus, our data indicate that caveolin-1 activates ATM after oxidative stress by sequestering PP2A-C into caveolar membranes away from ATM.
Activation of the ATM-p53 Pathway by Cigarette Smoke Extracts Requires Caveolin-1—Cigarette smoke is a known source of free radicals, and oxidative stress is a known inducer of the p53 pathway. In addition, ATM is a known activator of p53, and we show above that caveolin-1 mediates activation of ATM after oxidative stress. To translate our findings into a more physiological/pathological context, we hypothesized that cigarette smoke extracts may activate the ATM-p53 pathway in a caveolin-1-dependent manner.
Wild type and caveolin-1 null lung fibroblasts were derived and treated with cigarette smoke extracts. Untreated lung fibroblasts were used as controls. Cells were then collected and subjected to immunoblotting analysis using antibody probes specific for caveolin-1 and autophosphorylated ATM (anti-phospho-Ser-1981 ATM IgGs). Fig. 1D shows that CSE up-regulated caveolin-1 and phospho-Ser-1981 ATM expression in wild type but not caveolin-1 null lung fibroblasts. Consistent with these data, the cigarette smoke extract-induced accumulation of phospho-Ser-1981 ATM into the nucleus was more pronounced in wild type then caveolin-1 null lung fibroblasts (Fig. 1E). Interestingly, phospho-Ser-1981 ATM co-localized in the nucleus of wild type lung fibroblasts with senescence-associated heterochromatic foci, as detected by 4′,6-diamidino-2-phenylindole (DAPI) staining (Fig. 1E). In support of these data, we demonstrate in Fig. 1, D and E, that p53 expression in wild type lung fibroblasts was significantly increased by treatment with cigarette smoke extracts. We also show that CSE up-regulated p21Waf1/Cip1 protein expression, a downstream target of p53, in lung fibroblasts from wild type mice (Fig. 1D). Up-regulation of both p53 and p21Waf1/Cip1 protein expression by cigarette smoke extracts was dramatically prevented in lung fibroblasts derived from caveolin-1 null mice, which lack caveolin-1 expression (Fig. 1, D and E). We conclude that caveolin-1 expression is required for the activation of the ATM-p53 pathway in lung fibroblasts following stimulation with cigarette smoke extracts.
Caveolin-1 Expression Is Required for Induction of Premature Senescence of Lung Fibroblasts by Cigarette Smoke Extracts in Vitro—The p53 pathway responds to a variety of intrinsic and extrinsic stress signals that can disrupt the fidelity of DNA replication (32). Stresses that would compromise genome stability can then be accommodated by either preventing cell cycle progression (senescence) or, alternatively, eliminating the cell from the body (apoptosis) through p53 activation. Thus, p53 is a key regulator of cellular senescence, which is known to play a central role in aging and aging-related diseases.
Because we show in Fig. 1 that caveolin-1 expression is required for the activation of the ATM-p53 pathway after stimulation with cigarette smoke extracts, we asked whether cigarette smoke may induce premature senescence in a caveolin-1-dependent fashion. To this end, we looked at the effect of lack of caveolin-1 on senescence of lung fibroblasts induced by cigarette smoke extracts. WT LFs and caveolin-1 null (Cav-1 KO LFs) lung fibroblasts were isolated and treated with CSEs for 1 and 5 days. Untreated cells were used as controls. Cells were then collected, and cellular senescence was assessed by senescence-associated β-galactosidase (SA-β-gal) staining. We show that cigarette smoke extracts successfully induced cellular senescence of wild type lung fibroblasts, as demonstrated by the increased number of SA-β-gal-positive cells after CSE treatment (Fig. 1F). We also demonstrate that free radicals produced by cigarette smoke extracts caused SIPS, as shown by a dramatically reduced number of senescence-associated β-galactosidase-positive cells when lung fibroblasts were treated with cigarette smoke extracts in the presence of the antioxidant quercetin (Fig. 1F). Importantly, SIPS was significantly inhibited in the presence of the ATM inhibitor caffeine (Fig. 1F), suggesting that SIPS was, at least in part, dependent upon ATM activation. Cigarette smoke extract-induced premature senescence of lung fibroblasts was caveolin-1-dependent, as demonstrated by compromised SIPS in caveolin-1 null lung fibroblasts (Fig. 1F).
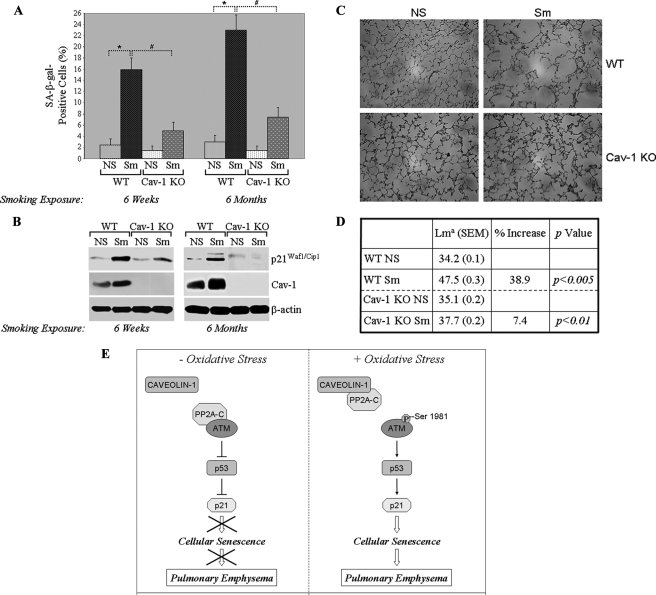
Caveolin-1 Expression Is Necessary for Cigarette Smoking-induced Senescence and Emphysema in Vivo—To investigate the in vivo role of caveolin-1 in cigarette smoking-induced cellular senescence, wild type and caveolin-1 null mice (on a C57Bl6 background) were exposed to cigarette smoking (Fig. 2, A and B, Sm) for either 6 weeks or 6 months. Non-smoking age-matched mice (Fig. 2, A and B, NS) were used as controls. Lung fibroblasts were derived from these mice, and cellular senescence was assessed by SA-β-gal staining and p21Waf1/Cip1 expression. Exposure to cigarette smoking for only 6 weeks was sufficient to up-regulate caveolin-1 expression and induce premature senescence of lung fibroblasts in wild type mice (Fig. 2, A and B). Interestingly, lack of caveolin-1 prevented cellular senescence of lung fibroblasts induced by cigarette smoking, as demonstrated by a dramatically reduced increase of SA-β-gal-positive cells (Fig. 2A) and lack of p21Waf1/Cip1 up-regulation (Fig. 2B), as compared with lung fibroblasts from exposed wild type mice. Exposure to cigarette smoking for 6 months further increased the number of senescent cells by ∼40% (Fig. 2A).
FIGURE 2.
Cigarette smoking induces premature senescence of lung fibroblasts and emphysema in vivo in a caveolin-1-dependent manner. 2-month-old WT and Cav-1 KO mice were exposed to cigarette smoking (Sm) for either 6 weeks or 6 months. Non-smoking WT and Cav-1 null mice were used as controls (NS). Lung tissues were extracted, and primary cultures of lung fibroblasts were derived and subjected to SA β-galactosidase activity staining (A; the total number of cells per field was maintained identical in WT and caveolin-1 null LFs before SA-β-gal staining) and immunoblotting analysis using anti-p21 and anti-caveolin-1 IgGs (B). Immunoblotting with anti-β-actin IgGs was performed to show equal loading. Values in A represent means ± S.E.; n = 6; *, #, p < 0.001. In addition, lung sections were subjected to H&E staining (C) and morphometric analysis (D). Lma in D is mean linear intercept, which measures the average distance between alveolar walls and is proportional to the amount of emphysema. Data are expressed as the mean value ± S.E. (SEM) in each experimental group; n = 20 sections/group. E, schematic diagram summarizing the caveolin-1-dependent activation of the ATM/p53/p21/senescence pathway and induction of pulmonary emphysema after oxidative stress/cigarette smoking.
H&E staining and morphometric analysis of lung tissues showed that cigarette smoking for 6 weeks did not induce airway enlargement in either wild type or caveolin-1 null mice (data not shown). In contrast, cigarette smoking for 6 months promoted pulmonary emphysema in wild type mice, as shown by a significant airway enlargement (∼39%) (Fig. 2, C and D). Interestingly, cigarette smoking-induced emphysema was dramatically prevented in lungs from caveolin-1 null mice, as indicated by only a 7% airway enlargement (Fig. 2, C and D). Taken together, these data indicate that cigarette smoking induces senescence and emphysema in vivo in a caveolin-1-dependent manner.
DISCUSSION
We show that caveolin-1 expression is required for the development of senescent lung fibroblasts and emphysema in vivo following exposure of mice to cigarette smoking. Notably, senescent lung fibroblasts appear in wild type mice after 6 weeks of exposure to cigarette smoking, whereas 6 months of exposure to cigarette smoking are required for pulmonary emphysema to be morphologically detectable, suggesting that the early lung senescence response may be a precursor to the later emphysema development. In addition, we show that lack of caveolin-1 expression prevents the development of both senescent lung fibroblasts and emphysema. Based on these data, we propose a model in which the presence of senescent lung fibroblasts plays a key role in the pathogenesis of emphysema in a caveolin-1-dependent manner. Consistent with this hypothesis, lung fibroblasts from patients with emphysema show a reduced proliferation rate (11, 34), an altered growth factor response (12), and a lower number of population doubling in culture (34). As fibroblasts provide part of the structural support and matrix of the lung that is essential for its integrity, senescent fibroblasts may affect tissue microbalance and the structural maintenance of the lung and contribute to the pathogenesis of emphysema. Interestingly, senescent cells, including fibroblasts, have been shown to secrete matrix metalloproteases (35), which would further contribute to protease/antiprotease imbalance, and mediators of inflammation (36, 37), therefore perpetuating the abnormal inflammatory response. Consistent with this notion, we show in supplemental Fig. 4 that the accumulation of inflammatory cells in response to cigarette smoke exposure was dramatically reduced in caveolin-1 null lungs, as demonstrated by counting inflammatory cells using bronchoalveolar lavage fluid.
At a molecular level, we demonstrate that cigarette smoking up-regulates caveolin-1 expression, supporting our previous results showing increased caveolin-1 expression following treatment with hydrogen peroxide (22). Interestingly, we identify caveolin-1 as a novel upstream activator of ATM. In fact, activation of ATM by cigarette smoke extracts is prevented in caveolin-1 null lung fibroblasts. Why does lack of caveolin-1 prevent activation of ATM? Protein phosphatase 2A belongs to the conserved phosphoprotein phosphatase family of serine/threonine protein phosphatases, which regulates a variety of cellular processes (reviewed in Refs. 38 and 39). PP2A is a holoenzyme composed of a catalytic C subunit (PP2A-C), a scaffolding A subunit (PP2A-A), and a regulatory B subunit (PP2A-B). Goodarzi et al. (40) have shown PP2A as a negative regulator of ATM autophosphorylation and activity in vivo. We demonstrate here that caveolin-1 activates ATM by sequestering the ATM inhibitor PP2A-C into caveolar membranes. Lack of activation of ATM in the absence of caveolin-1 prevents the activation of the p53-p21Waf1/Cip1 pathway and, as a consequence, induction of premature senescence. Thus, our results indicate the existence of a novel signaling pathway that links cigarette smoking to pulmonary emphysema: cigarette smoking → increased caveolin-1 expression → activation of ATM → activation of p53 → up-regulation of p21Waf1/Cip1 → induction of cellular senescence of lung fibroblasts → development of emphysema (Fig. 2E).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant AG022548 (to F. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental methods and four supplemental figures.
Footnotes
The abbreviations used are: MAPK, mitogen-activated protein kinase; ATM, ataxia telangiectasia-mutated; PP2A, protein phosphatase 2A; SIPS, stress-induced premature senescence; DMEM, Dulbecco's modified Eagle's medium; CSE, cigarette smoke extract; WT, wild type; H&E, hematoxylin and eosin; MEF, mouse embryonic fibroblast; LF, lung fibroblast; Cav-1 KO, caveolin-1 null; SA-β-gal, senescence-associated β-galactosidase.
References
- 1.Lundberg, A. S., Hahn, W. C., Gupta, P., and Weinberg, R. A. (2000) Curr. Opin. Cell Biol. 12 705–709 [DOI] [PubMed] [Google Scholar]
- 2.Sherr, C. J., and DePinho, R. A. (2000) Cell 102 407–410 [DOI] [PubMed] [Google Scholar]
- 3.Frippiat, C., Chen, Q. M., Zdanov, S., Magalhaes, J. P., Remacle, J., and Toussaint, O. (2001) J. Biol. Chem. 276 2531–2537 [DOI] [PubMed] [Google Scholar]
- 4.Chen, Q. M., Bartholomew, J. C., Campisi, J., Acosta, M., Reagan, J. D., and Ames, B. N. (1998) Biochem. J. 332 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campisi, J., and d'Adda di Fagagna, F. (2007) Nat. Rev. Mol. Cell Biol. 8 729–740 [DOI] [PubMed] [Google Scholar]
- 6.MacNee, W. (2005) Proc. Am. Thorac. Soc. 2 50–60 [DOI] [PubMed] [Google Scholar]
- 7.Macnee, W., and Rahman, I. (1999) Am. J. Respir. Crit. Care Med. 160 S58–65 [DOI] [PubMed] [Google Scholar]
- 8.Snider, G. L. (1992) Am. Rev. Respir. Dis. 146 1615–1622 [DOI] [PubMed] [Google Scholar]
- 9.Nyunoya, T., Monick, M. M., Klingelhutz, A., Yarovinsky, T. O., Cagley, J. R., and Hunninghake, G. W. (2006) Am. J. Respir. Cell Mol. Biol. 35 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji, T., Aoshiba, K., and Nagai, A. (2004) Am. J. Respir. Cell Mol. Biol. 31 643–649 [DOI] [PubMed] [Google Scholar]
- 11.Nobukuni, S., Watanabe, K., Inoue, J., Wen, F. Q., Tamaru, N., and Yoshida, M. (2002) Respirology 7 217–223 [DOI] [PubMed] [Google Scholar]
- 12.Noordhoek, J. A., Postma, D. S., Chong, L. L., Vos, J. T., Kauffman, H. F., Timens, W., and van Straaten, J. F. (2003) Exp. Lung Res. 29 291–302 [DOI] [PubMed] [Google Scholar]
- 13.Muller, K. C., Welker, L., Paasch, K., Feindt, B., Erpenbeck, V. J., Hohlfeld, J. M., Krug, N., Nakashima, M., Branscheid, D., Magnussen, H., Jorres, R. A., and Holz, O. (2006) Respir. Res. 7 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto, T., Schlegel, A., Scherer, P. E., and Lisanti, M. P. (1998) J. Biol. Chem. 273 5419–5422 [DOI] [PubMed] [Google Scholar]
- 15.Sargiacomo, M., Scherer, P. E., Tang, Z.-L., Kubler, E., Song, K. S., Sanders, M. C., and Lisanti, M. P. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 9407–9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Cardena, G., Oh, P., Liu, J., Schnitzer, J. E., and Sessa, W. C. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart, E., Ying, Y.-S., Conrad, P., and Anderson, R. G. W. (1994) J. Cell Biol. 127 1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldovan, N., Heltianu, C., Simionescu, N., and Simionescu, M. (1995) Exp. Cell Res. 219 309–313 [DOI] [PubMed] [Google Scholar]
- 19.Li, S., Okamoto, T., Chun, M., Sargiacomo, M., Casanova, J. E., Hansen, S. H., Nishimoto, I., and Lisanti, M. P. (1995) J. Biol. Chem. 270 15693–15701 [DOI] [PubMed] [Google Scholar]
- 20.Schnitzer, J. E., Liu, J., and Oh, P. (1995) J. Biol. Chem. 270 14399–14404 [DOI] [PubMed] [Google Scholar]
- 21.Galbiati, F., Volonte, D., Liu, J., Capozza, F., Frank, P. G., Zhu, L., Pestell, R. G., and Lisanti, M. P. (2001) Mol. Biol. Cell 12 2229–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volonte, D., Zhang, K., Lisanti, M. P., and Galbiati, F. (2002) Mol. Biol. Cell 13 2502–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasari, A., Bartholomew, J. N., Volonte, D., and Galbiati, F. (2006) Cancer Res. 66 10805–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houghton, A. M., Quintero, P. A., Perkins, D. L., Kobayashi, D. K., Kelley, D. G., Marconcini, L. A., Mecham, R. P., Senior, R. M., and Shapiro, S. D. (2006) J. Clin. Investig. 116 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hautamaki, R. D., Kobayashi, D. K., Senior, R. M., and Shapiro, S. D. (1997) Science 277 2002–2004 [DOI] [PubMed] [Google Scholar]
- 26.Maeno, T., Houghton, A. M., Quintero, P. A., Grumelli, S., Owen, C. A., and Shapiro, S. D. (2007) J. Immunol. 178 8090–8096 [DOI] [PubMed] [Google Scholar]
- 27.Shapiro, S. D., Goldstein, N. M., Houghton, A. M., Kobayashi, D. K., Kelley, D., and Belaaouaj, A. (2003) Am. J. Pathol. 163 2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shackelford, R. E., Innes, C. L., Sieber, S. O., Heinloth, A. N., Leadon, S. A., and Paules, R. S. (2001) J. Biol. Chem. 276 21951–21959 [DOI] [PubMed] [Google Scholar]
- 29.Chen, K., Albano, A., Ho, A., and Keaney, J. F., Jr. (2003) J. Biol. Chem. 278 39527–39533 [DOI] [PubMed] [Google Scholar]
- 30.Moiseeva, O., Mallette, F. A., Mukhopadhyay, U. K., Moores, A., and Ferbeyre, G. (2006) Mol. Biol. Cell 17 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakkenist, C. J., and Kastan, M. B. (2003) Nature 421 499–506 [DOI] [PubMed] [Google Scholar]
- 32.Vogelstein, B., Lane, D., and Levine, A. J. (2000) Nature 408 307–310 [DOI] [PubMed] [Google Scholar]
- 33.Bernhard, D., Moser, C., Backovic, A., and Wick, G. (2007) Exp. Gerontol. 42 160–165 [DOI] [PubMed] [Google Scholar]
- 34.Holz, O., Zuhlke, I., Jaksztat, E., Muller, K. C., Welker, L., Nakashima, M., Diemel, K. D., Branscheid, D., Magnussen, H., and Jorres, R. A. (2004) Eur. Respir. J. 24 575–579 [DOI] [PubMed] [Google Scholar]
- 35.Campisi, J. (2005) Cell 120 513–522 [DOI] [PubMed] [Google Scholar]
- 36.Boren, E., and Gershwin, M. E. (2004) Autoimmun. Rev. 3 401–406 [DOI] [PubMed] [Google Scholar]
- 37.Chung, H. Y., Kim, H. J., Kim, K. W., Choi, J. S., and Yu, B. P. (2002) Microsc. Res. Tech. 59 264–272 [DOI] [PubMed] [Google Scholar]
- 38.Cohen, P. T. (2002) J. Cell Sci. 115 241–256 [DOI] [PubMed] [Google Scholar]
- 39.Honkanen, R. E., and Golden, T. (2002) Curr. Med. Chem. 9 2055–2075 [DOI] [PubMed] [Google Scholar]
- 40.Goodarzi, A. A., Jonnalagadda, J. C., Douglas, P., Young, D., Ye, R., Moorhead, G. B., Lees-Miller, S. P., and Khanna, K. K. (2004) EMBO J. 23 4451–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.