Abstract
Novel immunomodulatory molecule FTY720 is a synthetic analog of myriocin, but unlike myriocin FTY720 does not inhibit serine palmitoyltransferase. Although many of the effects of FTY720 are ascribed to its phosphorylation and subsequent sphingosine 1-phosphate (S1P)-like action through S1P1,3–5 receptors, studies on modulation of intracellular balance of signaling sphingolipids by FTY720 are limited. In this study, we used stable isotope pulse labeling of human pulmonary artery endothelial cells with l-[U-13C, 15N]serine as well as in vitro enzymatic assays and liquid chromatography-tandem mass spectrometry methodology to characterize FTY720 interference with sphingolipid de novo biosynthesis. In human pulmonary artery endothelial cells, FTY720 inhibited ceramide synthases, resulting in decreased cellular levels of dihydroceramides, ceramides, sphingosine, and S1P but increased levels of dihydrosphingosine and dihydrosphingosine 1-phosphate (DHS1P). The FTY720-induced modulation of sphingolipid de novo biosynthesis was similar to that of fumonisin B1, a classical inhibitor of ceramide synthases, but differed in the efficiency to inhibit biosynthesis of short-chain versus long-chain ceramides. In vitro kinetic studies revealed that FTY720 is a competitive inhibitor of ceramide synthase 2 toward dihydrosphingosine with an apparent Ki of 2.15 μm. FTY720-induced up-regulation of DHS1P level was mediated by sphingosine kinase (SphK) 1, but not SphK2, as confirmed by experiments using SphK1/2 silencing with small interfering RNA. Our data demonstrate for the first time the ability of FTY720 to inhibit ceramide synthases and modulate the intracellular balance of signaling sphingolipids. These findings open a novel direction for therapeutic applications of FTY720 that focuses on inhibition of ceramide biosynthesis, ceramide-dependent signaling, and the up-regulation of DHS1P generation in cells.
FTY7202 is a synthetic analog of sphingosine and is currently being studied as a potent immunosuppressive and immunomodulatory agent (1–3). FTY720-induced immunosuppression is ascribed, in part, to its protective effect on endothelial cell barrier function that results in inhibition of lymphocyte egress from lymph nodes and down-regulation of innate and adaptive immune responses (4). As endothelial cells predominantly express the sphingosine 1-phosphate 1 (S1P1) receptor and its activation initiates signaling that results in the assembly of VE-cadherin-based adherens junctions (5), it is thought that the phosphorylation of FTY720 and the binding of FTY720-P to the S1P1 receptor determine its effect on vasculature (1). Recently it became evident that the action of FTY720 is more complex as several other direct protein targets were identified. Thus, FTY720 was found to bind to and inhibit the cannabinoid CB1 receptor (6), to inhibit cytosolic phospholipase A2 (cPLA2), and to counteract ceramide 1-phosphate-induced cPLA2 activation (7). Additionally FTY720 but not FTY720-P was shown to inhibit S1P lyase (8), which degrades S1P to ethanolamine phosphate and (E)-2-hexadecenal and regulates the removal of sphingoid bases from the cumulative pool of sphingolipids. These findings characterize FTY720 as a molecule with a multitargeted mode of action whose cellular effects are complicated by its metabolic transformation to FTY720-P, a structural and functional analog of S1P.
Phosphorylation of FTY720 to FTY720-P by sphingosine kinases (SphKs) is the only reported metabolic transformation of FTY720 and has been actively explored because of its link to S1P-mediated signaling (1, 2, 9, 10). Recent studies suggest that the endogenous balance between S1P and ceramide molecules regulates prosurvival and proapoptotic signaling cascades, which determine the outcome of cellular response to different stress conditions (11, 12) or the efficiency of anticancer therapy (12–14). However, despite the fact that FTY720 resembles sphingosine (Sph) and is a substrate of SphK2 (15–17), there are no reported studies on the effect of FTY720 on the intrinsic balance of signaling sphingolipids. Metabolic interconnections between proapoptotic (ceramides) and prosurvival (dihydrosphingosine 1-phosphate (DHS1P)) molecules are expected because it is known that fumonisin B1 (FB1), an inhibitor of (dihydro)ceramide synthases, not only blocks the formation of ceramides and up-regulates the intracellular content of dihydrosphingosine (DHSph) but also increases the cellular level of DHS1P (19, 20).
In view of these considerations, it is important to know how compounds with a potential ability to interfere with the sphingolipidome turnover affect the DHS1P-S1P/ceramide balance in cells. To address this question we have investigated the effect of FTY720 on metabolic pathways leading to ceramide and sphingoid base 1-phosphate generation in human pulmonary artery endothelial cells (HPAECs) by using a stable isotope pulse labeling approach and quantitative liquid chromatography-tandem mass spectrometry of signaling sphingolipids. We demonstrate that treatment of HPAECs with FTY720 results in the inhibition of de novo ceramide formation with a concomitant increase in DHSph and DHS1P content in cells. Moreover FTY720 showed a direct inhibition of ceramide synthases in an in vitro assay, albeit it was less efficient compared with the classical inhibitor of ceramide synthases, FB1. Our present findings have identified ceramide synthase isozymes as a novel molecular target for FTY720 action, opening a new direction for its potential therapeutic application through the inhibition of ceramide biosynthesis, ceramide-dependent signaling, and the up-regulation of DHS1P generation in cells.
EXPERIMENTAL PROCEDURES
Standards and Reagents—Sph, DHSph, a 17-carbon analog of Sph (C17-Sph), S1P, DHS1P, a 17-carbon analog of S1P (C17-S1P), N-myristoyl (14:0), N-palmitoyl (16:0), N-oleoyl (18:1), N-stearoyl (18:0), N-arachidoyl (20:0), N-nervonoyl (24:1), N-lignoceroyl (24:0) sphingosines (ceramides (Cer)), N-palmitoyl (16:0), N-oleoyl (18:1), N-stearoyl (18:0), N-arachidoyl (20: 0), N-behenoyl (22:0), N-nervonoyl (24:1), N-lignoceroyl (24:0), DHSph (dihydroceramides (DHCer)), N-heptadecanoyl sphingosine (17:0-Cer), and the palmitoyl (16:0), stearoyl (18:0), arachidoyl (20:0), behenoyl (22:0), nervonoyl (24:1), and lignoceroyl (24:0) coenzyme A substrates were obtained from Avanti Polar Lipids (Alabaster, AL). 3-Keto-DHSph was purchased from Matreya (Pleasant Gap, PA). The nonphosphorylated lipid standards were dissolved in methanol, whereas the sphingoid base phosphates were dissolved in methanol containing a trace amount of concentrated HCl and were stored at –20 °C. FTY720 and FTY720-P were synthesized as described previously (21). l-[U-13C(98%), 15N(98%)]serine was purchased from Cambridge Isotope Laboratories (Andover, MA). SphK1 antibodies were from Abcam (Cambridge, MA). SphK2 antibodies were kindly provided by Dr. Taro Okada (Kobe University, Kobe, Japan).
Cell Culture—HPAECs were from Lonza (Walkersville, MD) and were cultured in complete EGM-2 medium (Lonza). Passages between 5 and 7 were used for experiments. Cells were grown at 37 °C in a humidified atmosphere with 5% CO2, 95% air.
Transfection of HPAECs with SphK1 and SphK2 siRNAs—siGENOME SMART pool siRNAs targeting SphK1 (5′-CGACGAGGACUUUGUGCUA-3′, 5′-GAACCAUUAUGCUGGCUAU-3′, 5′-GAGAAGGGCAGGCAUAUGG-3′, and 5′-GCUAUGAGCAGGUCACCAA-3′) and SphK2 (5′-CAAGGCAGCUCUACACUCA-3′, 5′-GAGACGGGCUGCUCCAUGA-3′, 5′-GCUCCUCCAUGGCGAGUUU-3′, and 5′-CCACUGCCCUCACCUGUCU-3′) were purchased from Dharmacon (Lafayette, CA). HPAECs grown to ∼50% confluence in 6-well plates were transfected with Gene Silencer® (Gene Therapy Systems, San Diego, CA) transfecting agent with 25 nm SphK1 or 10 nm SK2 siRNA in serum-free EBM-2 medium according to the manufacturer's recommendation. After 3 h post-transfection, 1 ml of fresh complete EGM-2 medium containing 10% fetal bovine serum was added, and the cells were cultured for an additional 72 h before treatment with FTY720 and lipid analysis by LC/MS/MS. The efficiency of silencing was confirmed by real time PCR and by Western blotting.
Quantitative Reverse Transcription-PCR and Real Time Reverse Transcription-PCR—RNA (1 μg) was reverse transcribed using a cDNA synthesis kit (Bio-Rad), and real time PCR and quantitative PCR were performed to assess expression of SphK1 and SphK2 using primers designed for the human mRNA sequences. The following sequences were used for mRNA quantitation: SphK1 sense, 5′-TCCTTCACGCTGATGCTCACTG-3′; SphK1 antisense, 5′-CAGACGCCGATACTTCTCACTCTC-3′; SphK2 sense, 5′-CCCGGTTGCTTCTATTGGT; and SphK2 antisense, 5′-GACAGCCCAGCTTCAGAGAT. Amplicon expression in each sample was normalized to its 18 S RNA content. The relative abundance of target mRNA in each sample was calculated as 2 raised to the negative of its threshold cycle value times 106 after being normalized to the abundance of its corresponding 18 S (2–(primer Threshold Cycle)/2–(18 S Threshold Cycle) × 106). Negative controls, consisting of reaction mixtures containing all components except target RNA, were included with each reverse transcription-PCR run. To verify that amplified products were derived from mRNA and do not represent genomic DNA contamination, representative PCR mixtures for each gene were run in the absence of the reverse transcriptase enzyme after first being cycled to 95 °C for 15 min.
Lipid Extraction and Sample Preparation for LC/MS/MS—Cellular lipids were extracted by a modified Bligh and Dyer (22) procedure with the use of 0.1 n HCl for phase separation. C17-S1P (40 pmol), C17-Sph (30 pmol), and 17:0-Cer (30 pmol) were used as internal standards and were added during the initial step of lipid extraction. The extracted lipids were dissolved in methanol/chloroform (4:1, v/v), and aliquots were taken to determine the total phospholipid content as described previously (23). Samples were concentrated under a stream of nitrogen, redissolved in methanol, transferred to autosampler vials, and subjected to consecutive LC/MS/MS analysis of sphingoid bases, ceramides, and sphingoid base 1-phosphates.
Analysis of Sphingoid Bases, Sphingoid Base 1-Phosphates, Ceramides, FTY720, and FTY720 Phosphate—Analyses of the sphingolipids were performed by combined LC/MS/MS. The instrumentation used was an API4000 Q-trap hybrid triple quadrupole linear ion trap mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a TurboIonSpray ionization source interfaced with an automated Agilent 1100 series liquid chromatograph and autosampler (Agilent Technologies, Wilmington, DE). The sphingolipids were ionized via electrospray ionization (ESI) with detection via multiple reaction monitoring (MRM). Analysis of sphingoid bases and the molecular species of ceramides used ESI in positive ions with MRM analysis using a minor modification of published methods (24, 25). Briefly resolution of sphingoid bases was achieved with a Discovery C18 column (2.1 × 50 mm, 5-μm particle size; Supelco, Bellefonte, PA) and a gradient from methanol/water/formic acid (61:38:1, v/v) with 5 mm ammonium formate to methanol/acetonitrile/formic acid (39:60:1, v/v) with 5 mm ammonium formate at a flow rate of 0.5 ml/min. The MRM transitions used for detection of sphingoid bases were as follows: m/z 286 → 268 (C17-Sph, internal standard), m/z 300 → 282 (Sph), and m/z 302 → 284 (DHSph).
Ceramide molecular species were resolved using a 3 × 100-mm X-Terra XDB-C8 column (3.5-μm particle size; Waters, Milford, MA) and a gradient from methanol/water/formic acid (61:39:0.5, v/v) with 5 mm ammonium formate to acetonitrile/chloroform/water/formic acid (90:10:0.5:0.5, v/v) with 5 mm ammonium formate at a flow rate of 0.5 ml/min. MRM transitions monitored for the elution of ceramide molecular species were as follows: m/z 510 → 264, 14:0-Cer; m/z 538 → 264, 16:0-Cer; m/z 540 → 284, 16:0-DHCer; m/z 552 → 264, 17:0-Cer (internal standard); m/z 564 → 264, 18:1-Cer; m/z 566 → 284, 18:1-DHCer; m/z 566 → 264, 18:0-Cer; m/z 568 → 284, 18:0-DHCer; m/z 594 → 264, 20:0-Cer; m/z 596 → 284, 20:0-DHCer; m/z 624 → 284, 22:0-DHCer; m/z 650 → 264, 24:1-Cer; m/z 652 → 284, 24:1-DHCer; m/z 652 → 264, 24:0-Cer; m/z 654 → 284, 24:0-DHCer; m/z 680 → 264, 26:1-Cer; m/z 682 → 264, 26:0-Cer; m/z 708 → 264, 28:1-Cer; and m/z 710 → 264, 28:0-Cer.
S1P and DHS1P were quantified as bisacetylated derivatives with C17-S1P as the internal standard using reverse-phase high pressure liquid chromatography separation, negative ion ESI, and MRM analysis. Details of this approach were described previously (26).
FTY720 was analyzed along with sphingoid bases using positive ion ESI and MRM analysis of the transition from m/z 308 to m/z 255. C17-Sph was used as the internal standard for the quantitation of FTY720. FTY720-P was analyzed along with S1P and DHS1P as a bisacetylated derivative using negative ion ESI and C17-S1P as the internal standard. The m/z 470 → 410 transition was used to detect and quantify FTY720-P.
Standard curves for each of the sphingoid bases, sphingoid base 1-phosphates, ceramide molecular species, FTY720, and FTY720-P were constructed via the addition of increasing concentrations of the individual analyte to 30 pmol of the structural analogs of the sphingolipid classes used as the internal standards. Linearity and the correlation coefficients of the standard curves were obtained via a linear regression analysis. The standard curves were linear over the range of 0.0–300 pmol of each of the sphingolipid analytes with correlation coefficients (R2) > 0.98.
Pulse Labeling Experiments—After HPAECs were treated with FTY720 or FB1 in complete EBM-2 medium for 3 h, the cells were pulse-labeled for 2 h with l-[U-13C, 15N]serine (50 mg/liter) dissolved in EBM-2 complete medium containing the same concentrations of FTY720 or FB1. The medium was removed, and the lipids were extracted as described above. Quantitation of the label incorporated into the sphingolipids was achieved by the use of LC/MS/MS by monitoring the [M + 3] isotopes in precursor and product ions used in corresponding MRM programs to detect sphingoid bases, ceramides, and sphingoid base 1-phosphates as described previously (27).
Ceramide Synthase and Serine Palmitoyltransferase (SPT) Assays—The ceramide synthase assay was performed using the total HPAEC lysate as described previously (28) with DHSph and different acyl-CoA substrates. Briefly assays were performed with 0.01–10 μm DHSph; 5 μm 16:0-, 18:0-, 20:0-, 22:0-, or 24:1-CoA (all as a solution in 0.1% BSA); and 50 μg of total protein/assay for 15 min at 37 °C in a reaction buffer consisting of 20 mm HEPES, pH 7.4, 2 mm MgCl2, 25 mm KCl, and 0.1% BSA. FTY720 (0.1–30 μm) was added as a solution in 0.1% BSA to the reaction buffer 2 min before the addition of DHSph solubilized in 0.1% BSA. The reaction was stopped by lipid extraction with 17:0-Cer as the internal standard, and product formation (16:0-, 18:0-, 20:0-, 22:0-, and 24:1-DHCer) was quantified by LC/MS/MS.
The serine palmitoyltransferase assay was performed as described previously (29) with 1 mm stable isotope-labeled l-[U-13C, 15N]serine and 0.4 mm 16:0-CoA as substrates. The reaction was carried out with 100 μg of total lysate protein/reaction for 20 min at 37 °C in a buffer consisting of 20 mm HEPES, pH 8.0, containing 5 mm EDTA, 10 mm dithiothreitol, and 50 μm pyridoxal 5′-phosphate. The reaction was stopped by lipid extraction with C17-Sph as the internal standard. The stable isotope-labeled [M + 3] analog of 3-keto-DHSph was quantified by LC/MS/MS by detecting a specific transition from m/z 303 to m/z 285, which corresponds to the M + 3 isotope analogs of molecular and product ions of 3-keto-DHSph. The standard curve of response of variable amounts of 3-keto-DHSph versus fixed amounts of C17-Sph was created to perform a proper quantitative determination of the formed product.
Statistical Analyses—Each experiment was performed in triplicate. At least three independent experiments were carried out, and the data for one representative experiment are presented as the mean ± S.E. Two-tailed unpaired t tests were performed as post hoc tests of significance. Kinetic analysis of the inhibition of ceramide synthases by FTY720 was performed using the GraphPad Prism enzyme kinetic module (GraphPad Software, La Jolla, CA).
RESULTS
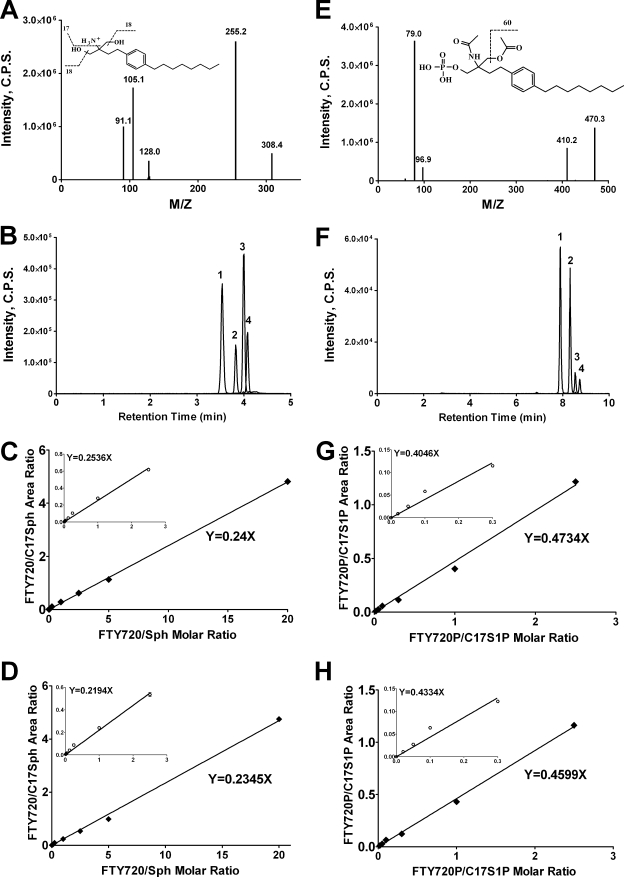
LC/MS/MS Quantitation of FTY720 and FTY720-P Using C17-Sph and C17-S1P as Internal Standards—To integrate the analyses of FTY720 and FTY720-P into the existing and already used protocols of LC/MS/MS quantitation of sphingoid bases and sphingoid base 1-phosphates we developed an LC/MS/MS approach with synthetic C17-Sph and C17-S1P as the internal standards. First we determined the breakdown pattern of FTY720 and found that the transition from m/z 308 to m/z 255 due to the loss of two water molecules and the amino group is the most characteristic for FTY720 in the positive ion mode (Fig. 1A). Then we confirmed that FTY720 can be resolved from all analyzed sphingoid bases using the column and solvent protocol we used for the sphingoid base analysis (Fig. 1B). Finally we determined the linearity of the standard curve with increasing amounts of FTY720 (1–300 pmol) and a fixed amount (30 pmol) of C17-Sph without (Fig. 1C) and with (Fig. 1D) the background of total lipids extracted from HPAECs (∼200 pmol of total lipid phosphorus on the column) (Fig. 1C). The data demonstrate a linear relationship of the response over a wide range of analyte concentrations with correlation coefficients greater than 0.995.
FIGURE 1.
Development of LC/MS/MS methods for quantitation of FTY720 and FTY720-P. A, positive product ion spectrum of FTY720. B, chromatographic separation of FTY720 and sphingoid bases. 1, FTY720; 2, C17-Sph; 3, Sph; 4, DHSph. C and D, standard curves for FTY720 with C17-Sph as the internal standard without interfering lipid background (C) and in the presence of total lipid extract (D). E, negative product ion spectrum of FTY720-P. F, chromatographic separation of FTY720-P and sphingoid base 1-phosphates. 1, FTY720-P; 2, C17-S1P; 3, S1P; 4, DHS1P. G and H, standard curves for FTY720-P with C17-S1P as the internal standard without interfering lipid background (G) and in the presence of total lipid extract (H). Refer to text for further details. C.P.S., counts/s.
Similarly we found that FTY720-P can be analyzed in the negative ion mode as its bisacetylated derivative along with S1P and DHS1P. The amino and the hydroxy groups of FTY720-P are easily acetylated, and the loss of an acetyl moiety gives rise to a characteristic m/z 470 to m/z 410 transition (Fig. 1E). Chromatographically FTY720-P bisacetate behaves similarly to the S1P and DHS1P bisacetylated derivatives (Fig. 1F), and most importantly, the standard curve of the response of variable amounts of FTY720-P versus a fixed amount of C17-S1P (the internal standard) is linear (R2 > 0.995) over a wide range of FTY720-P concentrations in the presence or absence of total lipid extracts (Fig. 1, G and H).
Integration of FTY720 and FTY720-P quantitation into the existing protocols of LC/MS/MS analysis of sphingoid bases and sphingoid base 1-phosphates is a logical and chemically rational approach. FTY720 and FTY720-P are structurally very similar to DHSph and DHS1P, respectively, and their reactivity and chromatographic behavior are very similar to those of DHSph and DHS1P. Hence the use of synthetic C17 analogs of Sph and S1P as the internal standards for the quantitation of FTY720 and FTY720-P is a convenient approach. Because of the structural similarities between FTY720-P and DHS1P, both compounds undergo acetylation of one amino group and one hydroxyl group and lose one acetyl moiety in the most characteristic transition during collision-induced decomposition. We found that C17-Sph and C17-S1P are good substitutions for stable isotope-labeled analogs of FTY720 and FTY720-P, which would be optimal choices but are not yet available. An ether analog of FTY720 (compound Y-32919, Novartis Pharma AG, Basel, Switzerland) and its phosphorylated derivative were used previously as internal standards to quantify FTY720 and FTY720-P, respectively (15, 30). However, because these compounds are not readily available, the choice of C17-Sph and C17-S1P as internal standards for quantitation of FTY720 and FTY720-P is a simple and convenient substitute strategy.
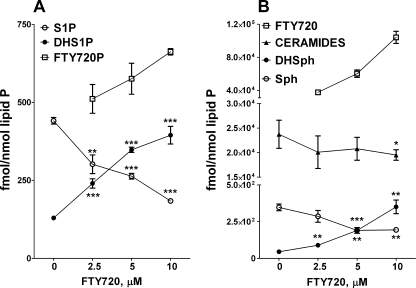
FTY720 Up-regulates DHS1P Biosynthesis through the Inhibition of Ceramide Synthase(s)—Treatment of HPAECs with FTY720 affected intracellular levels of all of the major signaling sphingolipids. Thus, increasing concentrations of FTY720 gradually decreased S1P while increasing the DHS1P content in the cells (Fig. 2A). The shift in S1P-DHS1P ratio was mirrored by similar changes in the content of sphingoid bases (Fig. 2B), suggesting that FTY720 interferes with ceramide biosynthesis and/or catabolism. Indeed FTY720 decreased the ceramide level in cells (Fig. 2B) but had no effect on dihydroceramides (data not shown). This observation suggests a potential inhibition of DHSph conversion to dihydroceramides by (dihydro)ceramide synthase(s) rather than the inhibition of dihydroceramide desaturase.
FIGURE 2.
The effect of FTY720 on intracellular level of signaling sphingolipids in HPAEC. Cells were treated with increasing concentrations of FTY720 for 2 h, and S1P, DHS1P, and FTY720-P (A) and Sph, DHSph, ceramides, and FTY720 (B) were quantified by LC/MS/MS. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. Error bars represent S.E.
Next we investigated the fate of the exogenously added FTY720 to HPAECs. Quantitative LC/MS/MS analysis of both FTY720 and FTY720-P in lipid extracts revealed that ∼1% of the added FTY720 was converted to FTY720-P, whereas >98% of the added FTY720 was recovered from cells at 2 h after the beginning of treatment (Fig. 2).
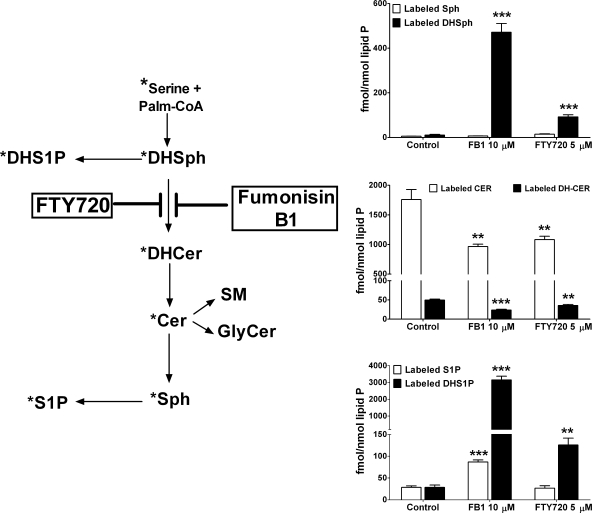
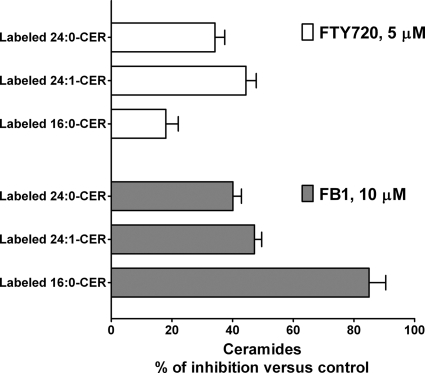
To identify the metabolic step in the sphingolipid biosynthetic pathway affected by FTY720, we used stable isotope labeling in conjunction with LC/MS/MS quantitation of labeled sphingolipids, an approach recently adopted by us for sphingolipid metabolic studies related to DHS1P and S1P formation in SphK1-overespressing cells (27). This approach consists of pulse labeling of cells with l-[U-13C, 15N]serine and quantitation of the [M + 3] mass analogs of natural sphingolipids that allows one to establish which metabolic step of sphingolipid de novo biosynthesis is affected. As shown in Fig. 3, treatment of HPAECs with FTY720 led to a substantial accumulation of stable isotope-labeled DHSph in cells, but no change in the content of labeled Sph was observed. FB1, a classical inhibitor of ceramide synthases (30–32), had a very similar effect on the content of sphingoid bases (Fig. 3). Furthermore FTY720 was as effective as FB1 in decreasing the incorporation of l-[U-13C, 15N]serine into total dihydroceramides and ceramides (Fig. 3), suggesting a close efficiency for both compounds in affecting ceramide biosynthesis in cultured HPAECs. However, analyses of l-[U-13C, 15N]serine incorporation into individual ceramide molecular species showed significant differences in the actions of FTY720 and FB1. FB1 was more effective in inhibiting l-[U-13C, 15N]serine incorporation into 16:0-ceramide than into 24:1- and C24:0-ceramides, whereas FTY720 was more potent in inhibiting de novo biosynthesis of 24:1- and 24:0-ceramides (Fig. 4). Both FTY720 and FB1 up-regulated label incorporation into DHS1P (Fig. 3), although FTY720 was less effective compared with FB1. Surprisingly FB1, but not FTY720, also increased the cellular levels of labeled (Fig. 3) and non-labeled (data not shown) S1P.
FIGURE 3.
The effects of FTY720 and FB1 on the incorporation of l-[U-13C, 15N]serine into signaling sphingolipids. HPAECs were pretreated with the indicated concentrations of FTY720 or FB1 for 3 h, and then the cells were labeled with l-[U-13C, 15N]serine for 2 h. Label-incorporated sphingoid bases, sphingoid base 1-phosphates, ceramides, and dihydroceramides were quantified by LC/MS/MS. The point of interference for both compounds with sphingolipidde novo biosynthesis is indicated in the scheme (star indicates labeled molecules). **, p < 0.01; ***, p < 0.001 versus control. Palm., palmitoyl; GlyCer, glycosylceramide; SM, sphingomyelin. Error bars represent S.E.
FIGURE 4.
The effects of FTY720 and FB1 on the de novo generation of individual ceramide molecular species. HPAECs were pretreated with FTY720 (5 μm) or FB1 (10 μm) for 3 h at 37 °C, and then the cells were labeled with l-[U-13C, 15N]serine for 2 h. Label-incorporated 16:0-, 24:1-, and 24:0-ceramides were quantified by LC/MS/MS. Data are presented as percentage of control label incorporation into the same ceramide molecular species in vehicle-treated cells. Error bars represent S.E.
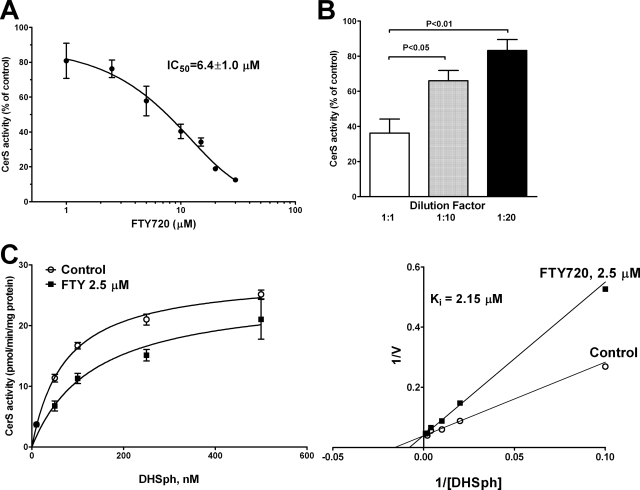
To further study the inhibition of ceramide synthases by FTY720, we performed an in vitro assay of ceramide synthase activity essentially as described previously (28) using the total cell lysate; DHSph; and 16:0-, 18:0-, 20:0-, 22:0-, or 24:1-CoA as substrates and LC/MS/MS to quantitate corresponding dihydroceramide formation. First we determined the type of inhibition of ceramide synthase 2 (CerS2) by FTY720 using 22:0-CoA as one of its preferred substrates (28). FTY720 proved to be an inhibitor of CerS2 with an apparent IC50 of 6.4 μm when 0.1 μm DHSph was used as the substrate (Fig. 5A). The inhibition of CerS2 activity was reversible as the potency of inhibition diminished with the dilution of the enzyme-inhibitor preparation (Fig. 5B). The analysis of the kinetic of inhibition with increasing substrate (DHSph) concentrations revealed that FTY720 (2.5 μm) does not change the apparent Vmax of the reaction (25.8 ± 0.8 pmol/min/mg of total protein) but shifts the apparent Km from 67.7 to 135.6 nm. This typifies a competitive type of CerS2 inhibition by FTY720 with an apparent Ki of 2.15 μm (Fig. 5C).
FIGURE 5.
FTY720 is a competitive inhibitor of ceramide synthase 2. A, concentration range of FTY720 was tested in an in vitro ceramide synthase assay using 5 μm 22:0-coenzyme A, 100 nm DHSph, and 50 μg of total cell lysate protein/assay as described under “Experimental Procedures.” Data are plotted as percentage of the activity in the presence of vehicle (0.1% BSA). 22:0-Dihydroceramide was quantified by LC/MS/MS. B, total cell lysate proteins were incubated with 10 μm FTY720 for 2 min. Then part of the protein preparation was diluted 10- or 20-fold with 0.1% BSA in the reaction buffer, and the ceramide synthase reaction was initiated by the addition of 5 μm 22:0-CoA and 100 nm DHSph. 22:0-DHCer was quantified by LC/MS/MS. Data are expressed as the percentage of the activity in the presence of vehicle for each corresponding protein concentration and demonstrate the reversibility of enzyme inhibition on FTY720 dilution. C, left, inhibition of ceramide synthase 2 activity by FTY720 (2.5 μm) with increasing concentrations of DHSph, 5 μm 22:0-CoA, and 50 μg of total cell lysate protein/reaction. 22:0-DHCer was quantified by LC/MS/MS. Right, the same experimental data are presented as a Lineweaver-Burk plot; the results typify a competitive inhibition of DHSph binding to ceramide synthase by FTY720. Error bars represent S.E.
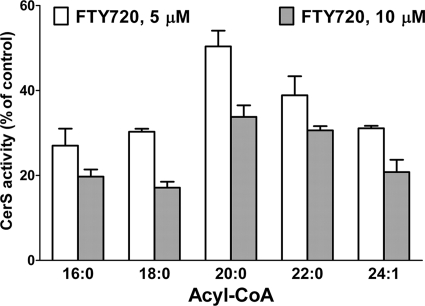
Next we compared the efficacy of FTY720 to inhibit the formation of the major molecular species of dihydroceramides in the total cell lysate by using substrates specific for CerS1 (18:0-CoA), CerS2 (22:0-CoA), CerS4 (20:0-CoA), and CerS5/6 (16: 0-CoA). As shown in Fig. 6, FTY720 exhibited a similar efficacy in inhibiting all of the ceramide synthase isozymes with CerS4 being the least inhibited.
FIGURE 6.
Effect of FTY720 on inhibition of CerS isozymes in the cell lysate assay. FTY720 (5 and 10 μm) was tested in the in vitro ceramide synthase assay with 100 nm DHSph, 50 μg of total cell lysate protein, and 5 μm 16:0-, 18:0-, 20:0-, 22:0-, or 24:1-CoA as described under “Experimental Procedures.” Dihydroceramides were quantified by LC/MS/MS. Data are expressed as a percentage of the activity in the presence of vehicle (0.1% BSA in the reaction buffer). Error bars represent S.E.
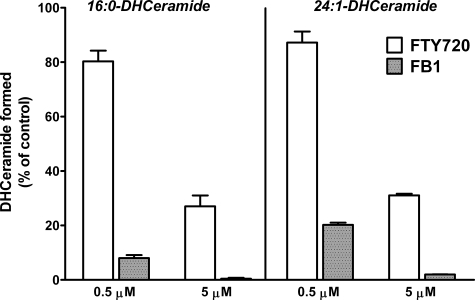
The ability of FTY720 to inhibit l-[U-13C, 15N]serine incorporation into 24:1- and 24:0-ceramides in HPAECs (Fig. 4) was similar to that of FB1; therefore, we compared both compounds in an in vitro assay using 16:0-CoA and 24:1-CoA as the acyl donor. In contrast to the labeling studies with l-[U-13C, 15N]serine carried out in intact HPAECs (Fig. 4), the experiments with the cell lysates revealed that FB1 was more potent than FTY720 in inhibiting the formation of both 16:0- and 24:1-dihydroceramides (Fig. 7). This indicates the complexity of FB1-mediated modulation of ceramide biosynthesis and catabolism in intact cells.
FIGURE 7.
FB1 is more potent than FTY720 in inhibiting ceramide synthases in the in vitro assay. CerS activity was determined using 5 μm 16:0- or 24:1-CoA, 100 nm DHSph, and 50 μg of total cell lysate protein with the indicated concentrations of FTY720 or FB1 as described under “Experimental Procedures.” 16:0- and 24:1-DHCer were quantified by LC/MS/MS. Error bars represent S.E.
FTY720 and FB1 Do Not Directly Modulate SPT Activity in HPAECs—FTY720 does not inhibit SPT activity (33), but FB1 is able to up-regulate SPT expression as a result of prolonged animal treatment (34). To test whether FTY720 and FB1 modulate SPT activity in HPAECs, we compared the effects of FTY720 (5 μm), FB1 (5 μm), and myriocin (0.5 μm; a classical SPT inhibitor) on SPT activity in the total HPAEC homogenate containing palmitoyl coenzyme A and stable isotope-labeled l-[U-13C, 15N]serine. The LC/MS/MS-based methodology of quantitation of [M + 3]-labeled 3-keto-DHSph was used. Neither FB1 nor FTY720 at 5 μm had any significant effect on SPT activity, whereas myriocin (0.5 μm) completely abolished (>99.5% inhibition) the formation of 3-keto-DHSph (data not shown). These data indicate that FTY720 and FB1 do not modulate SPT activity in HPAECs.
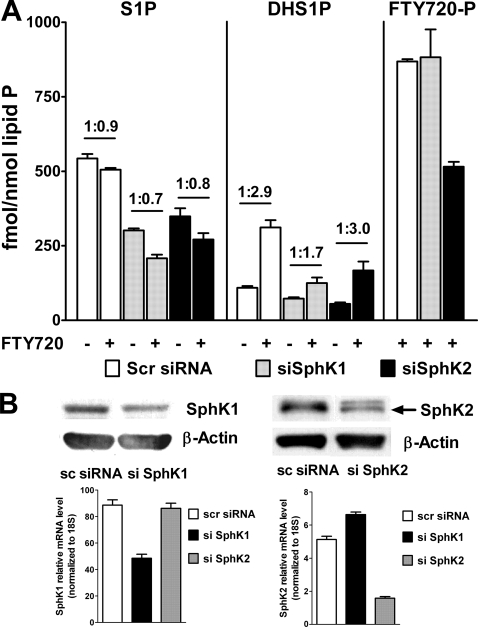
SphK1, but Not SphK2, Is Involved in FTY720-induced DHS1P Biosynthesis—As FTY720 increased DHS1P formation, we next investigated which isoform of sphingosine kinase is involved in the FTY720-induced DHS1P up-regulation as well as in FTY720-P formation in HPAECs. Expression of endogenous SphK1 and SphK2 was down-regulated by siRNA specific to SphK1 and SphK2. Knockdown of SphK1, but not SphK2, attenuated the up-regulating effect of FTY720 on DHS1P formation but had no effect on FTY720 conversion to FTY720-P. On the contrary, knockdown of SphK2, but not SphK1, decreased FTY720-P formation in HPAECs (Fig. 8). The conversion of FTY720 to FTY720-P was ∼1% of the total FTY720 present in cells (Figs. 2 and 8); this is consistent with published work showing very low expression of SphK2 in HPAECs (which is mostly localized in the nucleus) (35). These results indicate differential roles for SphK1 and SphK2 in utilizing DHSph and FTY720 as substrates for phosphorylation.
FIGURE 8.
SphK1 but not SphK2 is involved in FTY720-mediated DHS1P generation in HPAECs. A, SphK1 or SphK2 were silenced by siRNA, and then HPAECs were treated with 5 μm FTY720 for 2 h. The lipids were extracted and analyzed for the presence of S1P, DHS1P, and FTY720-P. Values above bars represent -fold increase versus corresponding controls. Statistical significance is not shown for better presentation of the data. B, silencing of SphK1 and SphK2 by siRNA for 72 h decreases protein (top) and mRNA (bottom) levels of SphK1 and SphK2 in HPAECs. Protein and real time PCR data are matched with lipid data presented in A. Scr (or sc), scrambled. Error bars represent S.E.
DISCUSSION
FTY720 is an effective immunomodulatory molecule and is currently in Phase III clinical trials as an immunosuppressive agent and for the treatment of multiple sclerosis (2, 3). Additionally FTY720 or its analogs have been demonstrated to inhibit tumor angiogenesis (36, 37) and vascular permeability (38, 39), suggesting that FTY720 has utility in regulating tumor metastasis, inflammation, and vascular edema. The conversion of FTY720 to FTY720-P and its extracellular action through S1P1,3–5 receptors has attracted a great deal of attention because of the potent receptor-mediated S1P-like effects of FTY720-P. Surprisingly the potential functional contributions of the intracellular effects of FTY720 through the modulation of the balance of signaling sphingolipids have not been fully considered as part of its mechanism of action. Even in a recent review, FTY720 was claimed not to interfere with sphingolipid biosynthesis (2). However, the newly discovered inhibition of S1P lyase (8) and the previously reported inhibition of SphK1 (16, 40) by FTY720 suggest that some effects of FTY720 may be attributed to its action on intracellular enzymes affecting S1P metabolism.
This study provides novel data on the modulation of ceramide and S1P-DHS1P levels by FTY720 by inhibition of ceramide synthases in HPAECs. Our results demonstrate for the first time that FTY720 inhibits ceramide synthase activity in intact endothelial cells as well as in cell-free preparations. LC/MS/MS quantitation of sphingoid bases, sphingoid base 1-phosphates, ceramides, and dihydroceramides demonstrated a profound effect of FTY720 on their intrinsic intracellular balance. An intriguing observation that came from the LC/MS/MS methodology was a “precursor-product” effect of FTY720 on the content of DHS1P and S1P that was mirrored by similar changes in DHSph and Sph levels. These findings suggest that FTY720 may inhibit the conversion of DHSph to DHCer in these cells. This hypothesis was proven by the use of LC/MS/MS methodology in combination with a pulse labeling of cells with l-[U-13C, 15N]serine. This approach clearly demonstrated ceramide synthase(s) inhibition by FTY720 in cells (Figs. 3 and 4) similar to that of FB1, a known inhibitor of ceramide synthases (30), but with a different efficiency of inhibition of biosynthesis of short-chain versus long-chain ceramides. Our kinetic analysis of CerS2 inhibition in cell lysates showed that the FTY720-induced inhibition was reversible and competitive toward DHSph (Fig. 5). FTY720 inhibited CerS2 in vitro with an IC50 value of 6.4 μm and also inhibited most of the other ceramide synthase isozymes with a similar potency as probed by using different acyl-CoA substrates (Figs. 6 and 7). This novel and crucial finding suggests that FTY720 or its analogs may have utility in regulating ceramide production and ceramide-driven signaling cascades involved in endothelial cell apoptosis.
Ceramides are key intermediates in the biosynthesis of all complex sphingolipids and also key signaling molecules controlling multiple cellular processes including mitochondria function and the decision of cell death versus survival paradigm (41, 42). Several pathological conditions have been reported to be linked either to increased ceramide de novo biosynthesis or to sphingomyelinase-mediated ceramide generation (11, 41–44). Hence the ability to control ceramide formation in cells, in particular to control the formation of selective ceramide molecular species and at different intracellular compartments, is of great physiological/pathological significance. There is compelling evidence for differential functional roles of individual ceramide molecular species. In particular, de novo generated 16:0-ceramide mediated p53-dependent apoptosis in prostate cancer cells (45) and in EB-1 colon cancer and Molt-4 LXSN leukemia cells (46). Similarly 18:0-ceramide generated via ceramide synthase but not via sphingomyelinase caused ischemia/reperfusion-induced mitochondrial dysfunction in mouse brain (47); moreover de novo formed 16:0- and 24:0-ceramides contributed to spontaneous neutrophil apoptosis (48).
There are six known mammalian ceramide synthases; they all show selectivity toward the chain length of the fatty acyl CoA they use for N-acylation of sphingoid bases (28, 49). A direct comparison between FTY720 and FB1 demonstrated that both compounds have a similar efficacy for inhibiting the formation of long-chain (24:0 and 24:1) ceramides (mediated by CerS2), but FB1 is more potent in inhibiting the formation of 16:0-ceramide (mediated by CerS5 and CerS6) in HPAECs (Fig. 4). However, in cell lysates, FB1 was found to be a much more potent inhibitor of both CerS2 as well as CerS5/6 (Fig. 7). This indicates a potential importance of FB1 interaction with other enzymes of sphingolipid biosynthesis and catabolism that is supported in part by the known up-regulation of SPT by FB1 in vivo (34).
Inhibition of ceramide synthases by FTY720 brings an additional but important element of complexity to the anti-inflammatory action of FTY720. Inhibition of ceramide biosynthesis may result in decreased ceramide kinase-dependent ceramide 1-phosphate formation in cells. Ceramide 1-phosphate is an important allosteric activator of form IV cPLA2 (50–52). This crucial enzyme releases arachidonic acid from glycerophospholipids and is thus involved in proinflammatory eicosanoid biosynthesis. FTY720 has been shown to down-regulate arachidonic acid release and eicosanoid generation in RBL-2H3 cells through a direct inhibition of cPLA2 and counteraction with ceramide 1-phosphate allosteric regulation of cPLA2 but not through an S1P receptor-dependent mechanism (7). Our data suggest that an additional mechanism of cPLA2 inhibition by FTY720 may be operative, i.e. through the regulation of the de novo biosynthesis of ceramide and ceramide 1-phosphate.
Another unique observation in the present study was the up-regulation of the intracellular level of DHS1P by FTY720. Although no definitive role for intracellular DHS1P is demonstrated, SphK1 has again been shown to be responsible for DHS1P biosynthesis. Previously we demonstrated that overexpression of SphK1, but not SphK2, up-regulates intracellular accumulation of DHS1P through the de novo biosynthesis (27). Our present results suggest that the supplementary availability of newly formed DHSph for SphK1 to generate DHS1P as a result of a metabolic block at the level of ceramide synthases appears to be a common mechanism for both FTY720 and FB1 action in cells. In fact, DHS1P rather than DHSph was proposed to be a better indicator of fumonisin toxicity (20, 53). Our data support the notion that native SphK1, similar to overexpressed FLAG-tagged SphK1 (27), has access to the de novo formed DHSph to form DHS1P. Conversely SphK2 appears not to be involved in DHS1P generation because silencing of SphK2, in contrast to silencing of SphK1, had no effect on FTY720-induced up-regulation of DHS1P content in cells (Fig. 7). Our data also suggest that the inhibition of S1P lyase by FTY720 (8) is not a major factor contributing to DHS1P accumulation in cells as the content of S1P was decreased, instead of being increased, by treatment of HPAECs with FTY720. The same is true for a potential inhibition of S1P phosphatases and lipid phosphate phosphatases by FTY720 as neither of these enzymes can discriminate between S1P and DHS1P (35, 54).
The human lung endothelial cells used in this study convert FTY720 to FTY720-P. We confirmed that SphK2 but not SphK1 mediates phosphorylation of FTY720; this is consistent with published information on SphK2-dependent phosphorylation of FTY720 in mammalian cells (15–17). However, the rate of FTY720 conversion to FTY720-P in HPAECs was low, and only ∼1% of added FTY720 was present in its phosphorylated form irrespective of the duration of treatment (data not shown) or FTY720 concentration (Fig. 2). Despite the low rate of formation of FTY720-P in HPAECs, our data cannot exclude the possibility that some effects of FTY720 on sphingolipid metabolism are determined by FTY720-P. However, our data do not support a role for FTY720-P in DHS1P accumulation in cells because silencing of SphK1 had no effect on the intracellular level of FTY720-P but decreased the ratio of DHS1P up-regulation in response to FTY720 treatment (Fig. 7). On the contrary, silencing of SphK2 decreased the intracellular content of FTY720-P but had no effect on DHS1P up-regulation by FTY720. These results suggest a minimal role for intracellular FTY720-P compared with FTY720 in regulating DHS1P accumulation in HPAECs. Furthermore our results support the significance of ceramide synthase inhibition by FTY720 as a mechanism of up-regulation of DHS1P in lung endothelial cells.
In conclusion, we have demonstrated a novel pathway for the regulation of the intracellular balance of signaling sphingolipids via ceramide synthases by FTY720. This modality of FTY720 action adds to the already complex picture of the role of FTY720 in cellular biochemistry and opens a new aspect of its interference with sphingolipid balance and signaling (Fig. 9). Our findings present a new potential direction for applications of FTY720 and its analogs through the targeting of ceramide de novo biosynthesis. An understanding of the mechanisms by which FTY720 and its analogs regulate sphingoid base 1-phosphate/ceramide balance via ceramide synthases may provide new targets for pharmacological and therapeutic interventions.
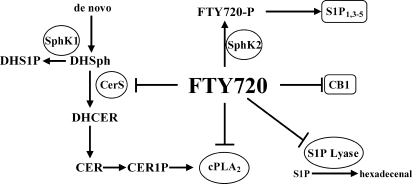
FIGURE 9.
Multiple points of FTY720 interference with sphingolipid metabolism and signaling. FTY720 is phosphorylated by SphK2, and the formed FTY720-P activates S1P1,3–5 receptors. Also FTY720 can inhibit the cannabinoid receptor 1 (CB1). Intracellularly FTY720 inhibits S1P lyase, cPLA2, and ceramide synthases. Overall FTY720 action favors anti-inflammatory responses. Cer1P, ceramide 1-phosphate.
Acknowledgments
We thank Dr. Taro Okada (Kobe University, Kobe, Japan) for providing the SphK2 antibody.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-079396 (to V. N.) and HL-083187 (to R. B.). This work was also supported by the Lipidomics Facility of the Department of Medicine at the University of Chicago. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: FTY720, 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol; FTY720-P, (S)-FTY720 phosphate; SphK, sphingosine kinase; Sph, sphingosine; DHSph, dihydrosphingosine; S1P, sphingosine 1-phosphate; DHS1P, dihydrosphingosine 1-phosphate; Cer, ceramides; DHCer, dihydroceramides; SPT, serine palmitoyltransferase; CerS, ceramide synthase; cPLA2, cytosolic phospholipase A2; LC/MS/MS, liquid chromatography-tandem mass spectrometry; HPAEC, human pulmonary artery endothelial cell; FB1, fumonisin B1; siRNA, small interfering RNA; ESI, electrospray ionization; MRM, multiple reaction monitoring; BSA, bovine serum albumin.
References
- 1.Zhang, Z., and Schluesener, H. J. (2007) Mini Rev. Med. Chem. 7 845–850 [DOI] [PubMed] [Google Scholar]
- 2.Hiestand, P. C., Rausch, M., Meier, D. P., and Foster, C. A. (2008) Prog. Drug Res. 66 363–381 [DOI] [PubMed] [Google Scholar]
- 3.Mansoor, M., and Melendez, A. J. (2008) Rev. Recent Clin. Trials 3 62–69 [DOI] [PubMed] [Google Scholar]
- 4.Schwab, S. R., and Cyster, J. G. (2007) Nat. Immunol. 8 1295–1301 [DOI] [PubMed] [Google Scholar]
- 5.Lee, M. J., Thangada, S., Claffey, K. P., Ancellin, N., Liu, C. H., Kluk, M., Volpi, M., Sha'afi, R. I., and Hla, T. (1999) Cell 99 301–312 [DOI] [PubMed] [Google Scholar]
- 6.Paugh, S. W., Cassidy, M. P., He, H., Milstien, S., Sim-Selley, L. J., Spiegel, S., and Selley, D. E. (2006) Mol. Pharmacol. 70 41–50 [DOI] [PubMed] [Google Scholar]
- 7.Payne, S. G., Oskeritzian, C. A., Griffiths, R., Subramanian, P., Barbour, S. E., Chalfant, C. E., Milstien, S., and Spiegel, S. (2007) Blood 109 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandhuvula, P., Tam, Y. Y., Oskouian, B., and Saba, J. D. (2005) J. Biol. Chem. 280 33697–33700 [DOI] [PubMed] [Google Scholar]
- 9.Oo, M. L., Thangada, S., Wu, M. T., Liu, C. H., Macdonald, T. L., Lynch, K. R., Lin, C. Y., and Hla, T. (2007) J. Biol. Chem. 282 9082–9089 [DOI] [PubMed] [Google Scholar]
- 10.Kharel, Y., Lee, S., Snyder, A. H., Sheasley-O'neill, S. L., Morris, M. A., Setiady, Y., Zhu, R., Zigler, M. A., Burcin, T. L., Ley, K., Tung, K. S., Engelhard, V. H., Macdonald, T. L., Pearson-White, S., and Lynch, K. R. (2005) J. Biol. Chem. 280 36865–36872 [DOI] [PubMed] [Google Scholar]
- 11.Petrache, I., Natarajan, V., Zhen, L., Medler, T. R., Richter, A. T., Cho, C., Hubbard, W. C., Berdyshev, E. V., and Tuder, R. M. (2005) Nat. Med. 11 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales, A., and Fernandez-Checa, J. C. (2007) Mini Rev. Med. Chem. 7 371–382 [DOI] [PubMed] [Google Scholar]
- 13.Baran, Y., Salas, A., Senkal, C. E., Gunduz, U., Bielawski, J., Obeid, L. M., and Ogretmen, B. (2007) J. Biol. Chem. 282 10922–10934 [DOI] [PubMed] [Google Scholar]
- 14.Huwiler, A., and Pfeilschifter, J. (2006) Curr. Pharm. Des. 12 4625–4635 [DOI] [PubMed] [Google Scholar]
- 15.Zemann, B., Kinzel, B., Müller, M., Reuschel, R., Mechtcheriakova, D., Urtz, N., Bornancin, F., Baumruker, T., and Billich, A. (2006) Blood 107 1454–1458 [DOI] [PubMed] [Google Scholar]
- 16.Paugh, S. W., Payne, S. G., Barbour, S. E., Milstien, S., and Spiegel, S. (2003) FEBS Lett. 554 189–193 [DOI] [PubMed] [Google Scholar]
- 17.Billich, A., Bornancin, F., Dévay, P., Mechtcheriakova, D., Urtz, N., and Baumruker, T. (2003) J. Biol. Chem. 278 47408–47415 [DOI] [PubMed] [Google Scholar]
- 18.Deleted in proof
- 19.Suzuki, H., Riley, R. T., and Sharma, R. P. (2007) Toxicology 229 42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, D. H., Yoo, H. S., Lee, Y. M., Kie, J. H., Jang, S., and Oh, S. (2006) J. Toxicol. Environ. Health Part A 69 2071–2082 [DOI] [PubMed] [Google Scholar]
- 21.Lu, X., and Bittman, R. (2006) Tetrahedron Lett. 47 825–827 [Google Scholar]
- 22.Bligh, E. G., and Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37 911–917 [DOI] [PubMed] [Google Scholar]
- 23.Vaskovsky, V. E., Kostetsky, E. Y., and Vasendin, I. M. (1975) J. Chromatogr. 114 129–141 [DOI] [PubMed] [Google Scholar]
- 24.Murphy, R. C., Fiedler, J., and Hevko, J. (2001) Chem. Rev. 101 479–526 [DOI] [PubMed] [Google Scholar]
- 25.Merrill, A. H., Caligan, T. B., Wang, E., Peters, K., and Ou, J. (2000) Methods Enzymol. 312 3–9 [DOI] [PubMed] [Google Scholar]
- 26.Berdyshev, E. V., Gorshkova, I. A., Garcia, J. G., Natarajan, V., and Hubbard, W. C. (2005) Anal. Biochem. 339 129–136 [DOI] [PubMed] [Google Scholar]
- 27.Berdyshev, E. V., Gorshkova, I. A., Usatyuk, P., Zhao, Y., Saatian, B., Hubbard, W., and Natarajan, V. (2006) Cell. Signal. 18 1779–1792 [DOI] [PubMed] [Google Scholar]
- 28.Laviad, E. L., Albee, L., Pankova-Kholmyansky, I., Epstein, S., Park, H., Merrill, A. H., Jr., and Futerman, A. H. (2008) J. Biol. Chem. 283 5677–5684 [DOI] [PubMed] [Google Scholar]
- 29.Yamaji-Hasegawa, A., Takahashi, A., Tetsuka, Y., Senoh, Y., and Kobayashi, T. (2005) Biochemistry 44 268–277 [DOI] [PubMed] [Google Scholar]
- 30.Salm, P., Warnholtz, C. R., Lynch, S. V., and Taylor, P. J. (2006) J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 843 157–163 [DOI] [PubMed] [Google Scholar]
- 31.Desai, K., Sullards, M. C., Allegood, J., Wang, E., Schmelz, E. M., Hartl, M., Humpf, H. U., Liotta, D. C., Peng, Q., and Merrill, A. H., Jr. (2002) Biochim. Biophys. Acta 1585 188–192 [DOI] [PubMed] [Google Scholar]
- 32.Merrill, A. H., Jr., Wang, E., Vales, T. R., Smith, E. R., Schroeder, J. J., Menaldino, D. S., Alexander, C., Crane, H. M., Xia, J., Liotta, D. C., Meredith, F. I., and Riley, R. T. (1996) Adv. Exp. Med. Biol. 392 297–306 [DOI] [PubMed] [Google Scholar]
- 33.Kiuchi, M., Adachi, K., Kohara, T., Minoguchi, M., Hanano, T., Aoki, Y., Mishina, T., Arita, M., Nakao, N., Ohtsuki, M., Hoshino, Y., Teshima, K., Chiba, K., Sasaki, S., and Fujita, T. (2000) J. Med. Chem. 43 2946–2961 [DOI] [PubMed] [Google Scholar]
- 34.He, Q., Suzuki, H., Sharma, N., and Sharma, R. P. (2006) Toxicol. Sci. 94 388–397 [DOI] [PubMed] [Google Scholar]
- 35.Zhao, Y., Kalari, S. K., Usatyuk, P. V., Gorshkova, I., He, D., Watkins, T., Brindley, D. N., Sun, C., Bittman, R., Garcia, J. G., Berdyshev, E. V., and Natarajan, V. (2007) J. Biol. Chem. 282 14165–14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid, G., Guba, M., Papyan, A., Ischenko, I., Brückel, M., Bruns, C. J., Jauch, K. W., and Graeb, C. (2005) Transplant. Proc. 37 110–111 [DOI] [PubMed] [Google Scholar]
- 37.Schmid, G., Guba, M., Ischenko, I., Papyan, A., Joka, M., Schrepfer, S., Bruns, C. J., Jauch, K. W., Heeschen, C., and Graeb, C. (2007) J. Cell. Biochem. 101 259–270 [DOI] [PubMed] [Google Scholar]
- 38.Sanchez, T., Estrada-Hernandez, T., Paik, J. H., Wu, M. T., Venkataraman, K., Brinkmann, V., Claffey, K., and Hla, T. (2003) J. Biol. Chem. 278 47281–47290 [DOI] [PubMed] [Google Scholar]
- 39.LaMontagne, K., Littlewood-Evans, A., Schnell, C., O'Reilly, T., Wyder, L., Sanchez, T., Probst, B., Butler, J., Wood, A., Liau, G., Billy, E., Theuer, A., Hla, T., and Wood, J. (2006) Cancer Res. 66 221–231 [DOI] [PubMed] [Google Scholar]
- 40.Vessey, D. A., Kelley, M., Zhang, J., Li, L., Tao, R., and Karliner, J. S. (2007) J. Biochem. Mol. Toxicol. 21 273–279 [DOI] [PubMed] [Google Scholar]
- 41.Taha, T. A., Mullen, T. D., and Obeid, L. M. (2006) Biochim. Biophys. Acta 1758 2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siskind, L. J. (2005) J. Bioenerg. Biomembr. 37 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannun, Y. A., and Obeid, L. M. (2008) Nat. Rev. Mol. Cell Biol. 9 139–150 [DOI] [PubMed] [Google Scholar]
- 44.Thevissen, K., François, I. E., Winderickx, J., Pannecouque, C., and Cammue, B. P. (2006) Mini Rev. Med. Chem. 6 699–709 [DOI] [PubMed] [Google Scholar]
- 45.Eto, M., Bennouna, J., Hunter, O. C., Lotze, M. T., and Amoscato, A. A. (2006) Int. J. Urol. 13 148–156 [DOI] [PubMed] [Google Scholar]
- 46.Panjarian, S., Kozhaya, L., Arayssi, S., Yehia, M., Bielawski, J., Bielawska, A., Usta, J., Hannun, Y. A., Obeid, L. M., and Dbaibo, G. S. (2008) Prostaglandins Other Lipid Mediat. 86 41–48 [DOI] [PubMed] [Google Scholar]
- 47.Yu, J., Novgorodov, S. A., Chudakova, D., Zhu, H., Bielawska, A., Bielawski, J., Obeid, L. M., Kindy, M. S., and Gudz, T. I. (2007) J. Biol. Chem. 282 25940–25949 [DOI] [PubMed] [Google Scholar]
- 48.Seumois, G., Fillet, M., Gillet, L., Faccinetto, C., Desmet, C., François, C., Dewals, B., Oury, C., Vanderplasschen, A., Lekeux, P., and Bureau, F. (2007) J. Leukoc. Biol. 81 1477–1486 [DOI] [PubMed] [Google Scholar]
- 49.Pewzner-Jung, Y., Ben-Dor, S., and Futerman, A. H. (2006) J. Biol. Chem. 281 25001–25005 [DOI] [PubMed] [Google Scholar]
- 50.Subramanian, P., Stahelin, R. V., Szulc, Z., Bielawska, A., Cho, W., and Chalfant, C. E. (2005) J. Biol. Chem. 280 17601–17607 [DOI] [PubMed] [Google Scholar]
- 51.Pettus, B. J., Kitatani, K., Chalfant, C. E., Taha, T. A., Kawamori, T., Bielawski, J., Obeid, L. M., and Hannun, Y. A. (2005) Mol. Pharmacol. 68 330–335 [DOI] [PubMed] [Google Scholar]
- 52.Stahelin, R. V., Subramanian, P., Vora, M., Cho, W., and Chalfant, C. E. (2007) J. Biol. Chem. 282 20467–20474 [DOI] [PubMed] [Google Scholar]
- 53.Kim, D. H., Lee, Y. S., Lee, Y. M., Oh, S., Yun, Y. P., and Yoo, H. S. (2007) Arch. Pharm. Res. 30 962–969 [DOI] [PubMed] [Google Scholar]
- 54.Le Stunff, H., Peterson, C., Thornton, R., Milstien, S., Mandala, S. M., and Spiegel, S. (2002) J. Biol. Chem. 277 8920–8927 [DOI] [PubMed] [Google Scholar]