Abstract
Collagen type V/XI is a minor but essential component of collagen fibrils in vertebrates. We here report on age- and tissue-related variations in isoform usage in cartilages. With maturation of articular cartilage, the α1(V) chain progressively replaced the α2(XI) chain. A mix of the molecular isoforms, α1(XI)α1(V)α3(XI) and α1(XI)α2(XI)α3(XI), best explained this finding. A prominence of α1(V) chains is therefore characteristic and a potential biomarker of mature mammalian articular cartilage. Analysis of cross-linked peptides showed that the α1(V) chains were primarily cross-linked to α1(XI) chains in the tissue and hence an integral component of the V/XI polymer. From nucleus pulposus of the intervertebral disc (in which the bulk collagen monomer is type II as in articular cartilage), type V/XI collagen consisted of a mix of five genetically distinct chains, α1(XI), α2(XI), α3(XI), α1(V), and α2(V). These presumably were derived from several different molecular isoforms, including α1(XI)α2(XI)α3(XI), (α1(XI))2α2(V), and others. Meniscal fibrocartilage shows yet another V/XI phenotype. The findings support and extend the concept that the clade B subfamily of COL5 and COL11 gene products should be considered members of the same collagen subfamily, from which, in combination with clade A gene products (COL2A1 or COL5A2), a range of molecular isoforms has evolved into tissue-dependent usage. We propose an evolving role for collagen V/XI isoforms as an adaptable polymeric template of fibril macro-architecture.
The collagen framework of hyaline cartilages is based on a covalently cross-linked heteropolymeric network of types II, IX, and XI collagens. During development, collagen type IX molecules are covalently linked to the surface of thin, new fibrils of type II collagen polymerized on a template of type XI collagen (1–5). In fetal cartilage, type XI collagen is a heterotrimer of three genetically distinct chains, α1(XI), α2(XI), and α3(XI) in a 1:1:1 ratio (6–9). The α3(XI) chain has the same primary sequence as α1(II), but the chains differ in their post-translational processing and cross-linking properties (7–9). All three collagen subunits, II, IX, and XI, are heavily cross-linked in the same fibril through a lysyl oxidase-mediated mechanism (2, 5, 9). The location of the cross-links determined by sequence analysis of peptides prepared from proteolytically degraded fibrils reveals a high degree of chain specificity (9). Collagen XI molecules are linked to each other in a head-to-tail fashion by N-telopeptide2 to helix cross-links and laterally to type II collagen molecules through α1(II) C-telopeptides (9). Isolated from mature articular cartilage, type XI collagen includes a significant pool of α1(V) chains (6), implying the presence of V/XI hybrid molecules. The ratio of type XI collagen to type II collagen is about 1 to 10 in fetal bovine and human epiphyseal cartilage when compared with 1 to 30 in adult articular cartilage. Similarly, the ratio of collagen IX to collagen II falls from about 1 to 10 to 1 to 100 between fetal and adult. In adult articular cartilage, most of the collagen IX is located in the immediate pericellular matrix (10–12).
The intervertebral disc has a unique collagen architecture that combines features of ligament and cartilage in its morphology, function, and matrix biochemistry. The lamellar fabric of the outer annulus fibrosus combines collagens I and II fibrils in a complex weave with a radial gradient from mostly type I in the outermost layers and mostly type II in the interior. Nucleus pulposus, the gel-like center of the young intervertebral disc, has a similar collagen molecular phenotype to hyaline cartilage in which types II, IX, and XI collagens are the principal cross-linked fibrillar components (13–16). Collagen IX in the disc has a different protein isoform to that of hyaline cartilages. The α1(IX) chain is expressed as a short form that lacks the amino-terminal NC4 domain (16). One of the aims of the present study was to determine whether a unique pattern of type V/XI hybrid molecules is present in disc tissue when compared with articular cartilage and a more typical fibrocartilage, the knee meniscus.
The results show an accumulation of collagen α1(V) chains as articular cartilage matures. A related but distinct complexity in chain usage in the type V/XI collagen of nucleus pulposus is also revealed. Such tissue diversity suggests that the different molecular isoforms produce functional differences in the type V/XI polymeric template on which the bulk fibril architecture of a tissue is built.
EXPERIMENTAL PROCEDURES
Preparation of Type XI Collagen—Cartilage samples were dissected from the epiphyseal ends of fetal bovine long bones from 18-month steer articular cartilage (knee) and 4-year cow articular cartilage (knee). Nucleus pulposus was dissected from the lumbar spines and menisci from the knees of 3-month postnatal calves. Tissue samples were powdered by Spex mill under liquid N2 and extracted in 4 m guanidine HCl, 0.05 m Tris-HCl, pH 7.4, containing protease inhibitors (2 mm EDTA, 5 mm benzamidine, 2 mm phenylmethylsulfonyl fluoride, and 5 mm phenanthroline) at 4 °C for 48 h to remove proteoglycans and other matrix proteins and then washed thoroughly with water and freeze-dried. Cross-linked collagens were solubilized by digesting the washed residues with pepsin in 3% acetic acid using a ratio of 1:10 (w/w) pepsin:tissue for 24 h at 4 °C. Pepsin-solubilized collagens were fractionated into types II, XI, and IX by precipitation at 0.8, 1.2, and 2.0 m NaCl, respectively. The type XI collagen recovered in the 1.2 m NaCl fraction was redissolved in 3% acetic acid and reprecipitated at 1.2 m NaCl after removing residual type II collagen at 0.9 m NaCl (17).
Quantitation of Collagen Yield on Pepsin Digestion—The yields of collagens II, IX, and XI on pepsin digestion at the three stages of cartilage development were quantified in a further experiment using 100-mg aliquots of guanidine-HCl tissue residues. Tissue samples were powdered by Spex mill under liquid N2. The dry weights of each collagen fraction (0.8, 1.2, and 2.0 m NaCl) recovered after precipitation, dialysis, and freeze-drying were expressed as a percentage of the starting material. In addition, the small amount of insoluble material from the pepsin digest was weighed, digested with CNBr, and analyzed by SDS-PAGE to rule out enrichment of collagen V/XI in the insoluble residue.
Reduction of Divalent Cross-links with Tritiated Borohydride—The purified type V/XI collagen preparation from 4-year cow articular cartilage was reacted with NaB3H4 (10 mCi/mm) in 0.1 m sodium phosphate, pH 7.4, to label the reducible cross-linking residues. After 1 h at 25 °C, the reaction mixture was acidified, dialyzed against 0.1 m (v/v) acetic acid, and freeze-dried as described previously (9).
Column Chromatography—The individual type XI/V chains were resolved by reverse-phase HPLC on a Vydac C4 column (4.6 mm × 25 cm; 5-μm particle size) with a linear gradient of acetonitrile:n-propyl alcohol (3:1, v/v) in aqueous 0.1% (v/v) trifluoroacetic acid from 18 to 28% in 40 min at a flow rate of 1 ml/min. Eluent was monitored for 220 nm absorbance, and aliquots (100 μl) of collected fractions were assayed for 3H activity. Tryptic peptides containing 3H-labeled reducible cross-links were purified by sequential DEAE ion-exchange and C8 reverse-phase HPLC (9).
To resolve various molecular forms of type V/XI collagen under non-denaturing conditions, ion-exchange HPLC was performed on a Bio-Gel DEAE-5-PW column (75 × 7.5 mm, Bio-Rad), eluting with a linear gradient of 0–0.8 m NaCl in 40 ml of 0.025 m Tris-HCl, pH 7.5, containing 2.0 m urea. Eluent was monitored for 220 nm absorbance.
Trypsin Digestion—Samples of purified type XI collagen were dissolved in 0.2 m NH4HCO3 at 2 mg/ml, heat-denatured at 70 °C for 10 min, and digested with trypsin (1:50 w/w; Roche Applied Science sequencing grade) for 20 h at 37 °C.
Gel Electrophoresis and Electroblotting of Proteins—Collagens recovered by differential salt fractionation and column chromatography fractions were routinely examined by SDS-PAGE (18). For protein microsequencing, the proteins were transblotted after electrophoresis to polyvinylidene difluoride membrane (Bio-Rad) using a MilliBlot-SDE electroblotting apparatus (5).
Antisera and Western Blotting—Polyclonal antisera were raised in rabbits against synthetic peptides, DGSKGPTISA and GGDKGPVVAA, the human homologues of the N-telopeptide sequences found in the amino-terminal cross-linking region of the bovine α1(XI) and α2(XI) chains, respectively (9, 19). The peptides were conjugated to keyhole limpet hemocyanin with glutaraldehyde. For Western blotting, the protein samples were resolved by SDS-PAGE and transblotted to polyvinylidene difluoride membrane (Bio-Rad). Western blot analysis was performed with polyclonal antiserum and probed with an alkaline phosphatase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Avondale, PA). The blots were developed using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium as substrate for alkaline phosphatase.
Amino-terminal Protein Sequence Analysis—Purified component α-chains and cross-linked peptides were identified by amino-terminal sequence analysis on a Porton 2090E gas phase sequencer with on-line phenylthiohydantoin-derivative analysis.
Mass Spectrometry—Mass spectrometry was performed on a ThermoFinnigan LCQ Deca XP with electrospray ionization source and in-line C8 reverse-phase HPLC (19). Individual protein bands after Coomassie Blue staining on SDS-PAGE were digested in-gel by trypsin (20). The resulting peptides were subjected to microbore column liquid chromatography (Vydac C8 mass spectrometry 0.3 mm × 15 cm) interfaced directly to a tandem mass spectrometer equipped with a micro-electrospray ionization source. For protein identification, peptide fragments were compared with the National Center for Biotechnology Information (NCBI) non-redundant protein data base (nr.fasta) using SEQUEST, an automated data base search algorithm designed for use with tandem mass spectrometry data.
RESULTS
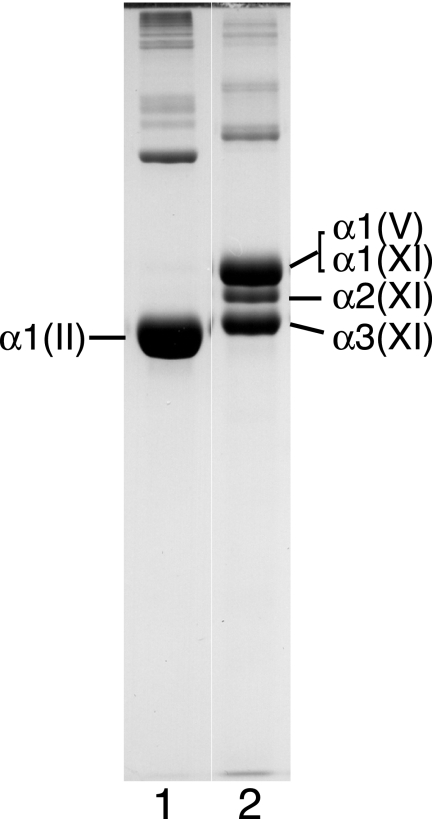
Fig. 1 shows that the three α-chains of type XI collagen isolated from 4-year bovine articular cartilage were not in a 1:1:1 ratio. The intensity of the α2(XI) chain was greatly reduced relative to the intensity of the α1(XI) chain by Coomassie Blue staining (lane 2).
FIGURE 1.
SDS-PAGE of pepsin-extracted types II and XI collagens from 4-year bovine articular cartilage. Samples were run on SDS-6% polyacrylamide gel and stained with Coomassie Brilliant Blue R250. Lane 1, type II collagen (0.8 m NaCl fraction). Lane 2, type XI collagen (1.2 m NaCl fraction). Further analysis by reverse-phase HPLC showed that α1(V) chains co-migrated with α1(XI) (Figs. 2 and 3).
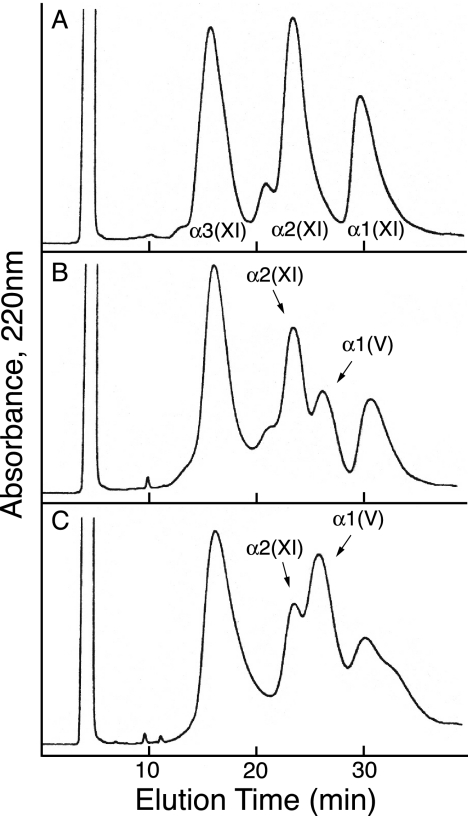
Using an established HPLC method to resolve α1(V) from α1(XI), α2(XI), and α3(XI) chains (9), we determined that a component eluting in the position of the α1(V) chain was prominent in the mature cartilage extracts. Fig. 2 compares the elution profiles of type XI collagen α-chain components in preparations from tissue at three different ages. The results show that type XI collagen molecules from fetal cartilage consist primarily of (α1(XI)α2(XI)α3(XI)) heterotrimers with only trace amounts of α1(V) detected (Fig. 2A) consistent with previous findings (9). The leading shoulder on the α2(XI) peak contains α3(XI) dimers. With increasing articular cartilage maturity, the type XI collagen fraction contained increasing amounts of α1(V), and the level of α2(XI) decreased relative to α1(XI) and α3(XI). With increasing tissue maturity, the α1(XI) peak also appeared to decrease in height, but this was due to broadening and a trailing shoulder, and no decrease was evident from SDS-PAGE results across the fractions (Fig. 3). This heterogeneity in α1(XI) can be explained by the increase in the proportion of chains with an α1(V) N-telopeptide cross-linked to the helix altering their chromatographic behavior. The identity of the α1(V) chain was confirmed by amino-terminal sequencing and tryptic peptide analysis by tandem mass spectrometry. Neither type I collagen (Fig. 1, lane 1) nor the α2(V) chain (Fig. 2) was seen in any of the collagen preparations from articular cartilage. The appearance of the α1(V) chain in the adult tissue therefore was not due simply to a switch in basic collagen phenotype from collagen II to collagen I with accompanying collagen V, a molecular phenotype that occurs for example in fibrocartilages (21).
FIGURE 2.
Reverse-phase HPLC of α1(V), α1(XI), α2(XI), and α3(XI) collagen chains extracted by pepsin from bovine articular cartilage at different developmental ages. A, fetal cartilage; B, 18-month cartilage; C, 4-year cartilage. Purified type XI collagen preparations were denatured and chromatographed on a C4 column to resolve chain components (see “Experimental Procedures” for details).
FIGURE 3.
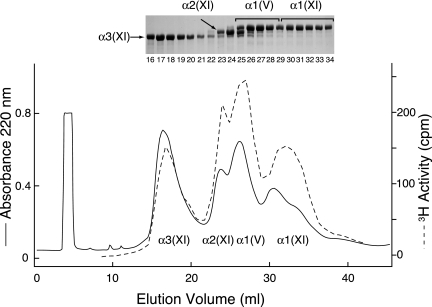
Reverse-phase HPLC resolution of the α-chains of pepsin-extracted type XI collagen from adult (4-year) bovine articular cartilage. The [3H]NaBH4-treated type XI collagen molecules were denatured and chromatographed on a C4 column to resolve chain components. Aliquots of collected fractions (100 μl) were assayed for tritium activity and by SDS-6% PAGE (inset).
Results of the quantitative experiment to determine collagen yields on pepsin digestion at the three stages of articular cartilage development and maturity were as follows. From 100 mg of starting dry weight of guanidine-HCl extracted tissue, insoluble material was 4, 9, and 2 mg from fetal, 18-month steer, and 4-year cow cartilage. The recovered yields of the three collagen fractions were: type II, 57, 68, and 76 mg; type V/XI, 15, 3, and 4 mg; and type IX, 9, 2, and 1.5 mg, respectively, from fetal, steer, and 4-year cow tissue. In summary, 90–98% of the collagen was solubilized from powdered fetal, steer, and cow tissue. In addition, the insoluble residues from the pepsin digests showed essentially only type II collagen peptides on SDS-PAGE for steer and cow and predominantly type I collagen peptides from fetal tissue (data not shown). Therefore, no enrichment of collagen V/XI was evident in the residual insoluble material after pepsin digestion. The changing proportions of α1(V), α1(XI), and α2(XI) chains observed with cartilage maturation in the 1.2 m NaCl fraction therefore reflect a change in overall tissue matrix composition.
Tritium-labeled divalent cross-linking residues were prominent in all four chains of the borotritide-treated type XI collagen from 4-year bovine articular cartilage (Fig. 3). Pyridinoline cross-links were minor components when compared with type II collagen from the same tissue (latter data not shown, see also Ref. 9).
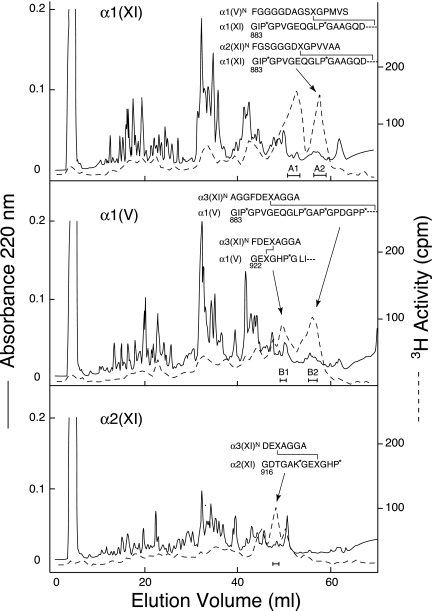
Fig. 4 shows the results of tryptic peptide fractionation to isolate tritium-labeled divalent cross-linked peptides from the individual chains resolved in Fig. 3. Sequence analysis of the cross-linked tryptic peptides showed that α1(V) chains were covalently cross-linked in the tissue to type XI collagen chains. The cross-linking interaction specificities of the α1(V) chain and α2(XI) are similar. Their carboxyl-terminal helical sites both link to α3(XI) N-telopeptides. Their N-telopeptides link to α1(XI) at its C-helical site (Fig. 4). Peak A1 containing tritium activity in the uppermost chromatogram (Fig. 4) is from the same site of cross-linking as peak A2, except that the helical fragment is shorter beginning at residue 922 (9).
FIGURE 4.
Reverse-phase HPLC fractionation of tryptic peptides prepared from α1(XI), α2(XI), and α1(V) chains of 4-year bovine articular cartilage. Samples (1 mg) were eluted from a C8 column (Brownlee Aquapore R-300; 25 cm × 4.6 mm) with a linear gradient (0–35%) of solvent B in solvent A over 70 min at a flow rate of 1 ml/min. Solvent A was 0.1% trifluoroacetic acid (v/v) in water, and solvent B was 0.085% trifluoroacetic acid (v/v) in acetonitrile:n-propyl alcohol (3:1, v/v). Also shown are the amino-terminal sequences of the isolated tritiated peptides. P*, 4-hydroxyproline; K*, hydroxylysine; and X, cross-linking hydroxylysine residue.
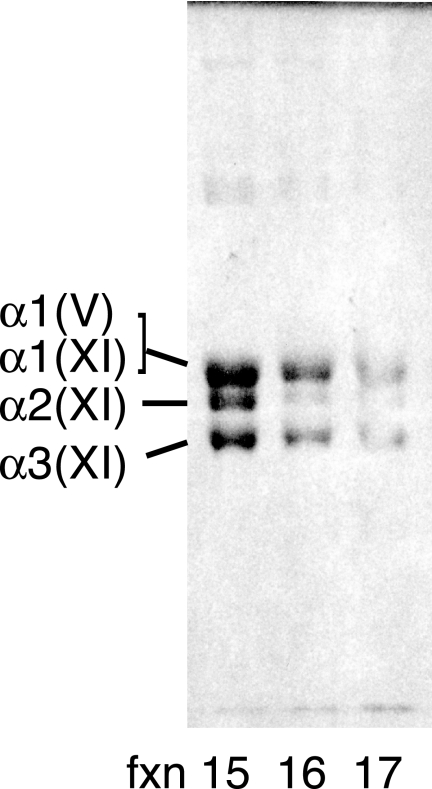
At least two molecular forms of type V/XI collagen could be partially resolved when the pepsin-extracted type XI collagen fraction from adult articular cartilage was run on anion-exchange HPLC under non-denaturing conditions (Fig. 5). An earlier eluting molecular form that gave three resolved chains in about a 1:1:1 ratio (an (α1(XI)α2(XI)α3(IX)) heterotrimer) was partially separated from a later fraction containing a molecular form that lacked the α2(XI) chain. We can interpret this finding based on mass spectral analysis of in-gel trypsin digests of individual chains and results from varying the chromatography conditions and running different collagen V/XI preparations. The earlier fraction (Fig. 5, fxn 15) is a mixture of (α1(XI)α2(XI)α3(XI)) and (α1(XI)α1(V)α3(XI)) native molecules, and the later fraction (Fig. 5, fxn 16 and fxn 17) is enriched in (α1(XI)α1(V)α3(XI)) native molecules.
FIGURE 5.
DEAE-HPLC fractionation of native type V/XI collagen molecules from adult bovine articular cartilage. Molecular forms of type XI/V collagen were partially resolved on a Bio-Gel DEAE-5-PW column under non-denaturing conditions. Fractions (fxn) were desalted and analyzed by SDS-6% PAGE.
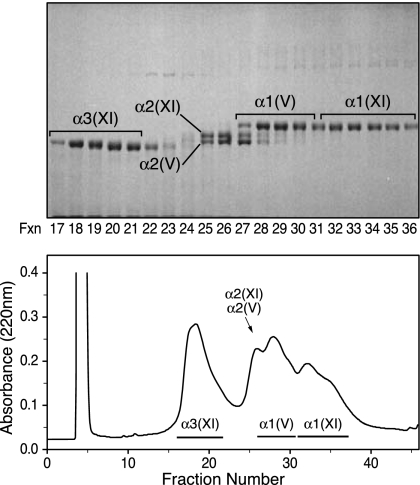
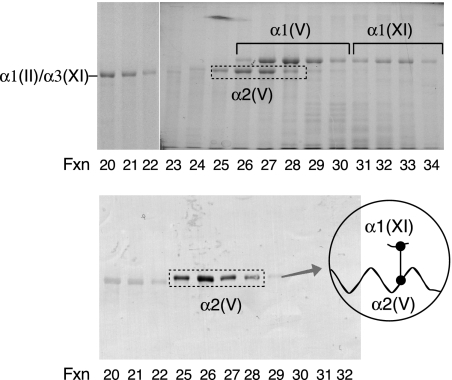
From nucleus pulposus, the type V/XI component gave an unexpectedly different pattern of α-chains when compared with articular cartilage, despite both tissues having type II collagen as the sole clade A bulk monomer. In addition to the α1(XI), α2(XI), and α3(XI) chains found in developing hyaline cartilage, two collagen V chains, α1(V) and α2(V), were also prominent in the pepsin-digest of nucleus pulposus (Fig. 6). Chain identities, indicated by their elution on reverse-phase HPLC and migration on SDS-PAGE, were established beyond doubt by in-gel trypsin digestion and microbore liquid chromatography/mass spectrometry with data base matching (Table 1).
FIGURE 6.
Reverse-phase HPLC and SDS-PAGE two-dimensional resolution of chain components of the collagen V/XI fraction from pepsin-digested calf nucleus pulposus. The sample (1 mg) was denatured and resolved on a C4 column monitoring for UV absorbance (lower panel). Fractions were analyzed by SDS-6% PAGE (upper panel). The conditions were as in Fig. 4. Individual collagen chains were identified by in-gel trypsin digestion and mass spectrometry.
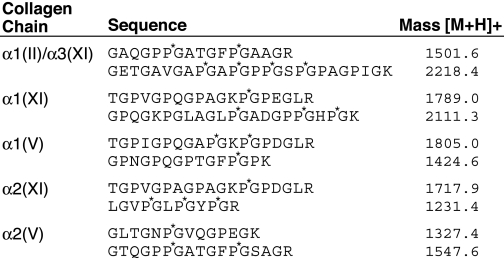
TABLE 1.
Representative tryptic peptides identified by in-gel trypsin digestion and mass spectrometry
The individual collagen bands on SDS-PAGE stained by Coomassie Brilliant Blue shown in Fig. 6 were cut out and digested in-gel by trypsin and analyzed by mass spectrometry. P*, 4-hydroxyproline residue; K*, hydroxylysine residue.
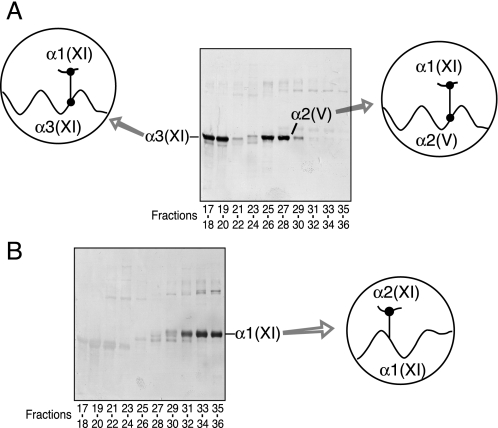
Two polyclonal antisera made against synthetic peptides matching the telopeptide sequences that contain the cross-linking lysine at the amino terminus of human α1(XI) and α2(XI) chains were used to probe replicate SDS-PAGE gels. The antiserum to the α1(XI) N-telopeptide cross-linking domain stained both α3(XI) and α2(V) bands, indicating that the α1(XI) N-telopeptide was covalently linked to α3(XI) and α2(V) chains in the tissue collagen (Fig. 7A). The antiserum against the α2(XI) N-telopeptide cross-linking domain stained only the pepsin-solubilized α1(XI) chain, indicating that a fragment of the α2(XI) N-telopeptide cross-linking domain remained covalently linked exclusively to the helical cross-linking site in the α1(XI) chain (Fig. 7B). These results imply that α2(V) chains were present exclusively in polymers of triple helical molecules containing α1(XI), α3(XI), and α2(V) chains but do not define the chain composition of the molecules containing α2(V).
FIGURE 7.
SDS-PAGE/Western blot analysis of type V/XI collagen chains resolved by HPLC from nucleus pulposus. Aliquots of pooled fractions in Fig. 6 were resolved by SDS-6% PAGE and transblotted to polyvinylidene difluoride membrane. The blots were reacted with polyclonal antisera specific to the N-telopeptide cross-linking sequences of α1(XI) (A) and α2(XI) (B) to detect α-chains with these telopeptides still attached through cross-linking.
Similar to nucleus pulposus, α1(V), α1(XI), and α2(V) chains are the main chain components of the type V/XI collagen molecules isolated from bovine meniscus (Fig. 8). Western blot analysis using an antiserum to the α1(XI) N-telopeptide cross-linking domain also revealed that the α1(XI) N-telopeptide was covalently linked to the α2(V) triple helix (Fig. 8, lower panel). Faint staining is evident in the position of the α3(XI) chain consistent with α3(XI) chains being a component of the collagen V/XI pool prepared from meniscus tissue but only in trace amounts when compared with α1(V), α1(XI), and α2(V) chains. This suggests underlying small amounts of (α1(XI)α2(XI)α3(XI)) molecules. This explanation is consistent with the absence of any band on a Western blot using the antiserum against the α2(XI) N-telopeptide cross-linking domain (data not shown).
FIGURE 8.
SDS-PAGE and Western blot analysis of type V/XI collagen chains resolved by HPLC from calf meniscus. The conditions are as in Fig. 6. Upper panel, Coomassie Brilliant Blue-stained gel. Lower panel, Western blot using a polyclonal antiserum specific against α1(XI) N-telopeptide cross-linking domain. fxn, fraction.
DISCUSSION
Type XI collagen from fetal cartilage consisted of (α1(XI)-α2(XI)α3(XI)) heterotrimers, with little or no α1(V) detected (9). With increasing articular cartilage maturity, the α1(V) chain increased in the type XI collagen fraction proportionally at the expense of α2(XI) (Figs. 1, 2, 3). Purified type XI collagen contained only traces of pyridinoline cross-links, the principal type of cross-link in types II and IX collagens of cartilage, even when isolated from the adult articular tissue. Instead, the α-chains from borotritide-treated type V/type XI collagen from the 4-year bovine tissue were enriched in tritium-labeled divalent cross-linking residues. This implies that whatever the molecular packing arrangement of the V/XI component in the mature fibrils in association with the bulk type II monomer, it does not allow maturation of its initial divalent cross-links (keto-imines) to trivalent pyridinolines.
Type XI collagen is cross-linked by lysyl oxidase-mediated bonds (9). The pattern of type XI collagen cross-linking in fetal cartilage exhibits strict chain specificity (9). Each α-chain is cross-linked through its triple helix (approximately residue 930) to a specific N-telopeptide hydroxylysine aldehyde in an adjacent type XI molecule staggered from it by 4D periods (where D is 1/4.4 of the molecular length or 67 nm, a characteristic feature of the molecular packing in collagen fibrils). This was shown by structural analysis of cross-linked peptides, i.e. α1(XI) is linked to an α2(XI) N-telopeptide, α2(XI) is linked to an α3(XI) N-telopeptide, andα3(XI) is linked to anα1(XI) N-telopeptide (9). From the present results, the α1(V) chain shows the same cross-linking preferences as α2(XI), with the α1(V) N-telopeptide linked to an α1(XI) helix and the α1(V) helix linked to an α3(XI) N-telopeptide. Together with the observed decrease in α2(XI) in exchange for an increase inα1(V), we conclude thatα1(V) replacesα2(XI) isomorphically in an increasing proportion and eventual majority of newly synthesized V/XI hybrid collagen molecules as articular cartilage matures. Some molecules with other chain combinations are also possible, but this explanation best fits the results. The findings imply that the expression of type XI collagen by chondrocytes is under a more complex regulation than previously appreciated. In a related but opposite phenomenon, the α1(XI) chain accumulates in the type V collagen component of bone with maturational age (22). It remains to be seen whether these changes reflect anatomically uniform metabolic changes throughout the tissues or, for example, surface-to-deep or pericellular, territorial, and interterritorial micro-anatomical variations in articular cartilage. To speculate, α1(V)-containing type V/XI molecules may provide a polymeric filamentous template for the thicker collagen II fibrils (200–500 nm diameter) that accumulate in adult articular cartilage (23) as opposed to the uniformly thin type II collagen fibrils (10–20 nm) associated with the (α1(XI)α2(XI)α3(XI)) template of epiphyseal growth cartilages.
Similar to the findings with articular cartilage, the N-telopeptide of α2(XI) was shown to be linked exclusively to α1(XI) in young nucleus pulposus (Fig. 7). However, α1(XI) in the nucleus pulposus was shown to be linked to either α3(XI) or α2(V) but not to α1(XI), α2(XI), or α1(V). Based on the observed strict chain specificities in the cross-linking properties of the individual type V/XI chains found in articular cartilage, the results from disc tissue strongly suggest that in addition to forming heterotrimers with α2(XI) or α1(V) and α3(XI), α1(XI) chains can also form native heterotrimers that include α2(V) chains. The most likely molecular form of the latter is (α1(XI))2α2(V), which is the same V/XI isoform found in bovine vitreous in association with collagens II and IX (24). In addition to (α1(XI)α2(XI)α3(XI)) and (α1(XI))2α2(V), it is possible that other heterotrimeric combinations are represented in the type V/XI collagen pool of the disc. The governing mechanism for these variants may largely be the interaction preferences of the different C-propeptide domains of the various procollagen chains (25) and their relative expression levels into the endoplasmic reticulum. Differences in cell phenotype within the nucleus pulposus both spatially and temporally, for example chondrocytes versus notochordal cells, may also be involved.
Collagen type V/XI gene products are best considered as members of the same collagen subclass, although two gene clades contribute α-chains, clade A represented by α1(II) (i.e. α3(XI)) and α2(V) and clade B represented by α1(XI), α2(XI), and α1(V) (26, 27). In cartilage, the present results show that the α1(V) chain becomes an integral fibril component, increasing in proportion at the expense of α2(XI) with increasing tissue maturity. In a reverse manner in bone, the α1(XI) chain accumulates with developmental age at the expense of α1(V) forming (α1(V)α1(XI)α2(V)) triple helical molecule (22, 28). The type V/XI collagen isoforms in nucleus pulposus are more complex than in hyaline cartilage. Five genetically distinct chains, α1(XI), α2(XI), α3(XI), α1(V), and α2(V), are present in different heterotrimers. Whether this reflects contributions by different cell types, for example the chondrocyte-like cells of mature disc and the notochordal cells that persist in young disc (29), is unknown. The presence of hybrid (α1(XI))2α2(V) molecules in vitreous humor (24) is a notable similarity because nucleus pulposus and vitreous share a similar type II collagen phenotype (30), gel-like texture, low concentrations of very thin fibrils, and lack of physical constraints between the fibrils when the tissue is removed from the eye or disc and swollen osmotically (31). It is also notable that the collagen IX isoforms of vitreous and disc are also the same (i.e. no NC4 domain) and distinct from that of cartilage (NC4 present) (16). Because type I collagen was absent from the samples of nucleus pulposus analyzed, so the α1(V) and α2(V) chains do not simply reflect a superimposed collagen type I-containing fibrocartilage phenotype. In considering collagen V/XI as a filamentous template for the assembly of the banded collagen fibrils that characterize higher vertebrate connective tissues, it is attractive to view the different chain isoforms, and perhaps splice forms, as protein template variants.
In summary, the type V/XI collagen component of adult articular cartilage comprises four genetically distinct chains, α1(XI), α2(XI), α1(V), and α3(XI), assembled into at least two distinct heterotrimeric molecules, (α1(XI)α2(XI)α3(XI)) and (α1(XI)α1(V)α3(XI)). There is a shift in chain isotype usage as the hyaline cartilage precursor matures postnatally. With increasing tissue maturity, the type XI collagen fraction contains more α1(V) and less α2(XI) in proportion to α1(XI) and α3(XI). In contrast, type V/XI collagen from nucleus pulposus contains five genetically distinct chains, α1(XI), α2(XI), α3(XI), α1(V), and α2(V), which are distributed among several distinct heterotrimeric molecules. The findings support an evolving role for tissue-specific molecular variants of type V/XI collagen in regulating fibril form and function across vertebrate connective tissues.
This work was supported, in whole or in part, by National Institutes of Health Grants AR37318 and AR36794. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: N- and C-telopeptides, short sequences that lack the (Gly-XY)n repeat and from the amino and carboxyl ends of various collagen chains; HPLC, high performance liquid chromatography.
References
- 1.Müller-Glauser, W., Humbel, B., Glatt, M., Strauli, P., Winterhalter, K. H., and Bruckner, P. (1986) J. Cell Biol. 102 1931–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eyre, D. R., Apone, S., Wu, J. J., Ericsson, L. H., and Walsh, K. A. (1987) FEBS Lett. 220 337–341 [DOI] [PubMed] [Google Scholar]
- 3.Vaughan, L., Mendler, M., Huber, S., Bruckner, P., Winterhalter, K. H., Irwin, M. I., and Mayne, R. (1988) J. Cell Biol. 106 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendler, M., Eich-Bender, S. G., Vaughan, L., Winterhalter, K. H., and Bruckner, P. (1989) J. Cell Biol. 108 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu, J. J., Woods, P. E., and Eyre, D. R. (1992) J. Biol. Chem. 267 23007–23014 [PubMed] [Google Scholar]
- 6.Eyre, D. R., and Wu, J. J. (1987) in Structure and Function of Collagen Types (Mayne, R., and Burgeson, R. E., eds) pp. 261–281, Academic Press, Orlando, FL
- 7.Burgeson, R. E., and Hollister, D. W. (1979) Biochem. Biophys. Res. Commun. 87 1124–1131 [DOI] [PubMed] [Google Scholar]
- 8.Morris, N. P., and Bächinger, H. P. (1987) J. Biol. Chem. 262 11345–11350 [PubMed] [Google Scholar]
- 9.Wu, J. J., and Eyre, D. R. (1995) J. Biol. Chem. 270 18865–18870 [DOI] [PubMed] [Google Scholar]
- 10.Eyre, D. R., Wu, J.-J., and Niyibizi, C. (1990) in Calcium Regulation and Bone Metabolism (Cohn, D. V., Glorieux, F. H., and Martin, T. J., eds) pp. 188–194, Elsevier Science Publishers, New York, NY
- 11.Poole, C. A., Gilbert, R. T., Herbage, D., and Hartmann, D. J. (1997) Osteoarthritis Cartilage 5 191–204 [DOI] [PubMed] [Google Scholar]
- 12.Eyre, D. R., Pietka, T., Weis, M. A., and Wu, J. J. (2004) J. Biol. Chem. 279 2568–2574 [DOI] [PubMed] [Google Scholar]
- 13.Eyre, D. R., and Muir, H. (1976) Biochem. J. 157 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayad, S., Marriott, A., Morgan, K., and Grant, M. E. (1989) Biochem. J. 262 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyre, D. R., Matsui, Y., and Wu, J. J. (2002) Biochem. Soc. Trans. 30 844–848 [DOI] [PubMed] [Google Scholar]
- 16.Wu, J. J., and Eyre, D. R. (2003) J. Biol. Chem. 278 24521–24525 [DOI] [PubMed] [Google Scholar]
- 17.Wu, J. J., and Eyre, D. R. (1984) Biochem. Biophys. Res. Commun. 123 1033–1039 [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. (1970) Nature 277 680–685 [DOI] [PubMed] [Google Scholar]
- 19.Eyre, D. R., Weis, M. A., and Wu, J. J. (2008) Methods (Amst.) 45 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinter, M., and Sherman, N. E. (2000) in Protein Sequencing and Identification Using Tandem Mass Spectrometry (Desiderio, D. M., and Nibbering, N. M. M., eds) pp. 152–157, John Wiley & Sons, New York, NY
- 21.Eyre, D. R., and Wu, J. J. (1983) FEBS Lett. 158 265–270 [DOI] [PubMed] [Google Scholar]
- 22.Niyibizi, C., and Eyre, D. R. (1989) FEBS Lett. 242 314–318 [DOI] [PubMed] [Google Scholar]
- 23.Lane, J. M., and Weiss, C. (1975) Arthritis Rheum. 18 553–562 [DOI] [PubMed] [Google Scholar]
- 24.Mayne, R., Brewton, R. G., Mayne, P. M., and Baker, J. R. (1993) J. Biol. Chem. 268 9381–9386 [PubMed] [Google Scholar]
- 25.Malone, J. P., Alvares, K., and Veis, A. (2005) Biochemistry 44 15269–15279 [DOI] [PubMed] [Google Scholar]
- 26.Boot-Handford, R. P., Tuckwell, D. S., Plumb, D. A., Rock, C. F., and Poulsom, R. (2003) J. Biol. Chem. 278 31067–31077 [DOI] [PubMed] [Google Scholar]
- 27.Huxley-Jones, J., Robertson, D. L., and Boot-Handford, R. P. (2007) Matrix Biol. 26 2–11 [DOI] [PubMed] [Google Scholar]
- 28.Niyibizi, C., and Eyre, D. R. (1994) Eur. J. Biochem. 224 943–950 [DOI] [PubMed] [Google Scholar]
- 29.Oegema, T. R., Jr. (2002) Biochem. Soc. Trans. 30 839–844 [DOI] [PubMed] [Google Scholar]
- 30.Bishop, P. N., Crossman, M. V., McLeod, D., and Ayad, S. (1994) Biochem. J. 299 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snowden, J. M., and Swann, D. A. (1980) Investig. Ophthalmol. Vis. Sci. 19 610–618 [PubMed] [Google Scholar]