Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP and protein kinase A (PKA)-regulated Cl– channel in the apical membrane of epithelial cells. The metabolically regulated and adenosine monophosphate-stimulated kinase (AMPK) is colocalized with CFTR and attenuates its function. However, the sites for CFTR phosphorylation and the precise mechanism of inhibition of CFTR by AMPK remain obscure. We demonstrate that CFTR normally remains closed at baseline, but nevertheless, opens after inhibition of AMPK. AMPK phosphorylates CFTR in vitro at two essential serines (Ser737 and Ser768) in the R domain, formerly identified as “inhibitory” PKA sites. Replacement of both serines by alanines (i) reduced phosphorylation of the R domain, with Ser768 having dramatically greater impact, (ii) produced CFTR channels that were partially open in the absence of any stimulation, (iii) significantly augmented their activation by IBMX/forskolin, and (iv) eliminated CFTR inhibition post AMPK activation. Attenuation of CFTR by AMPK activation was detectable in the absence of cAMP-dependent stimulation but disappeared in maximally stimulated oocytes. Our data also suggest that AMP is produced by local phosphodiesterases in close proximity to CFTR. Thus we propose that CFTR channels are kept closed in nonstimulated epithelia with high baseline AMPK activity but CFTR may be basally active in tissues with lowered endogenous AMPK activity.
The cystic fibrosis transmembrane regulator (CFTR)2 gene is mutated in patients with cystic fibrosis. CFTR has an adapted ABC transporter structural motif thereby creating an anion channel at the apical surface of secretory epithelia (1). The consequent CFTR-mediated ion transport is tightly controlled by ATP binding and phosphorylation by protein kinase A (PKA). However, a number of other protein kinases including PKC, Ca2+/calmodulin-dependent kinase, and cGMP-dependent kinase also control the activity of CFTR (2–4). These kinases converge on the regulatory domain of CFTR that is unique not only within the large ABC transporter family but among all known sequences, and may be considered as a “phosphorylation control module” (3). Regulation of CFTR by an inhibitory kinase, the adenosine monophosphate-dependent kinase (AMPK), has been described recently but the regulatory sites within CFTR, the mechanism of regulation, and the physiological relevance have all remained obscure (5–8). Additionally, CFTR mutation is linked to inflammation and a lack of functional CFTR expression has itself been suggested to up-regulate AMPK activity in epithelial cells carrying the cystic fibrosis (CF) defect. Pharmacologic AMPK activation was shown to inhibit secretion of inflammatory mediators (9). Thus AMPK may play multiple roles in CF pathophysiology making the mechanism of interaction an important problem in biology.
AMPK is a ubiquitous serine/threonine kinase that exists as a heterotrimer with a catalytic α subunit and regulatory β and γ subunits, each with multiple isoforms. In response to metabolic depletion and a consequent increase in the cellular AMP to ATP ratio, AMPK phosphorylates numerous proteins and activates catabolic pathways that generate ATP, whereas inhibiting cell growth, protein biosynthesis, and a number of other ATP-consuming processes, thereby operating as a cellular “low-fuel” sensor (10, 11). AMPK also controls signaling pathways involved in apoptosis, cell cycle, and tissue inflammation (12). Because AMPK is a cellular metabolic sensor that inhibits CFTR and limits cAMP activated Cl– secretion, a coupling of membrane transport by CFTR to the cellular metabolism has been proposed (13). However, AMPK activity can also increase without detectable changes in the cytosolic AMP to ATP ratio, suggesting a contribution of additional AMP-independent signals for regulation of CFTR by AMPK (14). Drugs used to combat type 2 diabetes, such as phenformin and metformin, act in this manner to activate AMPK, AMP-independently. It is also likely that cytosolic AMP is compartmentalized depending on the distribution of AMP generating enzymes such as phosphodiesterases that convert cAMP to AMP. The concept of spatiotemporal control of cAMP signaling by anchored protein complexes is well established (15). CFTR is known to form such macromolecular complexes with a number of interacting partners (16–18). For example, competitive interaction of EBP50-PKA and Shank2-PDE4D with CFTR has been demonstrated recently (19). In addition, Barnes and co-workers (20) demonstrated that phosphodiesterase 4D generates a cAMP diffusion barrier local to the apical membrane of the airway epithelium. It is therefore likely that activator pathways through cAMP and inhibitory AMP/AMPK signaling occur in a local CFTR-organized compartment. Here we explore the functional links between CFTR, inhibition of phosphodiesterases, and AMPK focusing on the effects of mutating putative AMPK targets within the R domain on CFTR function.
EXPERIMENTAL PROCEDURES
cRNAs for CFTR and Double Electrode Voltage Clamp—Oocytes were injected with cRNA (10 ng, 47 nl of double-distilled water) encoding wtCFTR, L1430A/L1431A, S573A, S1248A, F508del-CFTR, G551D-CFTR, S768A, S737A, S768D, S737D, E1474X, and AMPKα1. All mutants were generated by PCR and correct sequences were confirmed by restriction digest and by sequencing. Water-injected oocytes served as controls. 2–4 days after injection, oocytes were impaled with two electrodes (Clark Instruments Ltd., Salisbury, UK), which had a resistance of <1 mΩ when filled with 2.7 mol/liter KCl. Using two bath electrodes and a virtual-ground head stage, the voltage drop across the serial resistance was effectively zero. Membrane currents were measured by voltage clamping (oocyte clamp amplifier, Warner Instruments LLC, Hamden CT) in intervals from –60 to +40 mV, in steps of 10 mV, each 1 s. The bath was continuously perfused at a rate of 5 ml/min. All experiments were conducted at room temperature (22 °C).
Oocyte Staining—Oocytes were incubated for 60 min in ND96 solution (in mm: 96 NaCl, 2.0 KCl, 1.8 CaCl2, 1.0 MgCl2, 5.0 HEPES, pH 7.4), fixed for 60 min with 3% paraformaldehyde (in TBS, pH 8.0), and washed in TBS. After embedding in optimum cutting temperature compound (Sakura Finetek Europe, Zoeterwoude, NL), oocytes were cut to 20-μm slices with a cryostat (Leica CM3050 S, Wetzlar, Germany). Sections were put in either TBS or phosphate-buffered saline (PBS (mm): 137 NaCl, 1.8 KH2PO4, 10.3 Na2HPO4, pH 7.4), incubated for 5 min in 0.1% (w/v) SDS in PBS and washed 2 times with either TBS or PBS. Sections were incubated for 60 min in TBS or PBS (5% bovine serum albumin) and for 60 min at 37 °C with the anti-FLAG M2 antibody diluted 1:50 in 2% bovine serum albumin/TBS or a goat polyclonal casein kinase IIα antibody (Santa Cruz Biotechnology, Heidelberg, Germany) diluted 1:25 in 2% bovine serum albumin/PBS. Afterward sections were washed twice in PBS and incubated for 1 h with secondary antibodies (donkey anti-mouse IgG-Alexa Fluor 488 conjugated and donkey anti-goat IgG-Alexa Fluor 546 conjugated; Molecular Probes, Eugene, OR) at a dilution of 1:1000 in 2% bovine serum albumin/PBS. Sections were washed 2 times with PBS for 5 min and covered with DakoCytomation fluorescent mounting medium (DakoCytomation, Inc., Carpinteria, CA). Images were obtained using a Zeiss Axiovert 200M microscope with a ×63 objective (Carl Zeiss, Inc., Jena, Germany).
Immunofluorescence—Nasal ciliated epithelial cells harvested from the inferior turbinate of patients undergoing unrelated surgery (approved by local ethical committee) were maintained in cell culture medium M199 prior to fixation in 4% paraformaldehyde. Cells were permeabilized using 1% Triton X-100, washed 3 times in PBS, then blocked in 1 mm glycine for 15 min, followed by 5% donkey serum for 15 min. Pelleted cells were resuspended in PBS containing primary antibodies (goat anti-CK2α (Santa Cruz) and mouse anti-CFTR NBD1 (Neomarkers) at a 1:100 dilution) and incubated at room temperature, with shaking, overnight. After 3 washes in PBS, pelleted cells were resuspended in PBS containing fluorescein isothiocyanate-labeled anti-goat and rhodamine-labeled anti-mouse IgG secondary antibodies (Jackson, 1:100). After 2 h incubation with shaking the cells were washed five times in PBS and resuspended in 15 μl of anti-fade mountant (6% n-propyl gallate in 70% glycerol, 100 mm Tris/HCl, pH 7.4) for mounting on glass slides. Coverslips were sealed with nail varnish for image capture using a Zeiss 510 laser scanning confocal microscope.
In Vitro Phosphorylation—Recombinant R domain was phosphorylated with recombinant AMPK (Calbiochem 171536) or PKA (New England Biolabs P6000S) and peptides were separated subsequently by two-dimensional electrophoresis as described earlier (21). In brief, for AMPK phosphorylation, onto approximately a 20-μl packed volume of substrate beads (approximately 2 μg of protein), 20 μl of kinase reaction buffer was added. 32 mm HEPES (pH 7.4), 0.65 mm dithiothreitol, 0.012% Brij-35, 200 μm AMP (if included in that particular assay), 100 μm ATP, 10 mm MgAc, and approximately 500–800 cpm/pmol of [γ-32P]ATP. 0.1 unit of enzyme activity was added per reaction (as defined by the manufacturer's SAMS assay) and incubated at 30 °C for 15 min before being washed 3 times with 1 ml of ice-cold 50 mm Tris (pH 7.5). The beads were then resuspended in SDS sample buffer and separated on 10% SDS-PAGE, Coomassie stained to visualize protein, dried, and analyzed via autoradiography. For PKA reactions, the manufacturer's 10 times buffer was diluted by adding 0.1 unit of PKA with 100 μm ATP (∼500–800 counts/min). For mapping purposes the [γ-32P]ATP concentration was increased 10-fold and the ATP concentration reduced to 50 μm to increase the incorporation of radioactive signal in the tryptic peptides. The S768A mutant of CFTR abrogated almost entirely by phosphorylation of AMPK.
Materials and Statistical Analysis—All compounds used were of highest available grade of purity (Sigma or Calbiochem). The R domain construct was kindly supplied by Dr. J. Hanrahan (Department of Physiology, McGill University, Montréal, Canada). The construct encodes for a His-tagged R domain of human CFTR containing amino acids 635 to 837. Compounds were applied after fully activating CFTR unless otherwise stated. Alternatively oocytes were stimulated with IBMX/forskolin in the absence or presence of compounds. Student's t test was used for statistical analysis. A p value of <0.05 was regarded as significant.
RESULTS
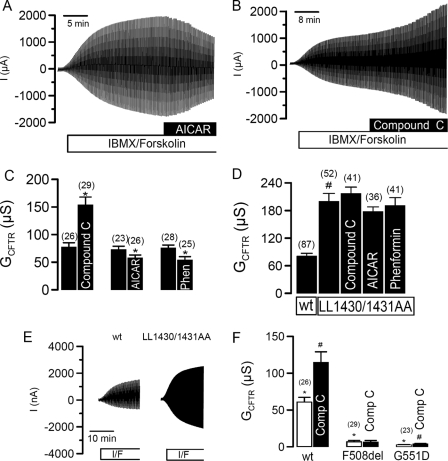
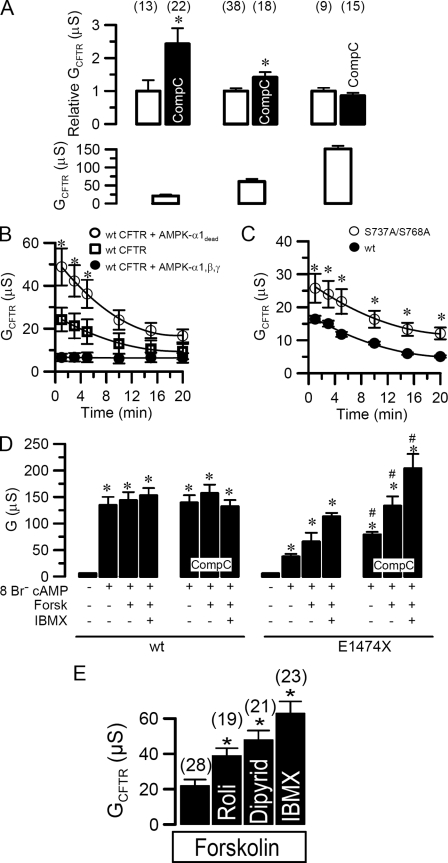
CFTR Is Inhibited by Baseline Activity of AMPK—Fig. 1 shows that cyclic AMP/PKA activated whole cell Cl– currents in Xenopus oocytes expressing CFTR. The CFTR current was slightly but significantly inhibited by activators of AMPK such as membrane permeable 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR, 80 μm for 1 h) (22) or phenformin (1 mm for 1 h) (Fig. 1, A and C). In contrast to these small inhibitory effects of AICAR and phenformin, CFTR whole cell conductance was doubled by the AMPK-inhibitor compound C, suggesting the presence of a latent constitutive inhibition of CFTR by a tonic baseline AMPK activity (Fig. 1, B and C). This is consistent with the recognized and substantial activity of AMPK in the absence of AMP (note the designation of the kinase is AMP-activated and not AMP-dependent). Interestingly, the application of compound C alone also activated CFTR without the need for PKA stimulation. To exclude a nonspecific effect of compound C, we expressed a CFTR mutant (L1430A/L1431A), which has been proposed to eliminate binding of AMPKα1 to a C-terminal region of CFTR (5). This mutant showed a much higher conductance (Fig. 1, D and E), which was not further augmented by compound C or inhibited by the two recognized AMPK activators, AICAR or phenformin (Fig. 1D). Also two common CFTR mutants, F508del-CFTR and G551D-CFTR, could not be further activated by inhibition of AMPK with compound C (Fig. 1F).
FIGURE 1.
Effect of AMPK on CFTR. A and B, whole cell currents activated by IBMX (1 mm) and forskolin (2 μm) in CFTR expressing oocytes showing the small effects of the AMPK activator AICAR (1 mm)(left) and compound C (right). C, summary of the effects of compound C (80 mm), AICAR (1 mm), and phenformin (1 mm) on CFTR whole cell conductances activated by IBMX and forskolin. D, summary of whole cell conductances generated by wtCFTR and L1430A/L1431A-CFTR and effects of activators and inhibitors of AMPK. E, whole cell currents activated by IBMX and forskolin in wtCFTR and L1430A/L1431A-CFTR expressing oocytes. F, comparison of the effects of compound C (80 μm) on whole conductances generated by wtCFTR, F508del-CFTR, and G551D-CFTR. Membrane currents were measured by voltage clamping in intervals from –60 to +40 mV, in steps of 10 mV. *, significant difference when compared with control. #, significant difference when compared with wtCFTR. Data are shown as mean ± S.E. (number of experiments).
This enhanced activity of L1430A/L1431A-CFTR was similar to that seen with wild-type CFTR first exposed to compound C and then activated by PKA. Thus local AMPK bound to CFTR is likely to be essential for inhibition of the maximum CFTR current post PKA stimulation. The proximity of the two proteins was further confirmed as shown in Fig. 2A. CFTR (red) and AMPKα1 (green) are colocalized in an apical compartment of human nasal epithelial cells, thus confirming previous results (5). Activation of AMPK in Xenopus oocytes by phenformin did not change the expression of CFTR in membranes of Xenopus oocytes (supplemental Fig. S1), confirming previous results indicating inhibition of the open probability of CFTR by AMPK (6).
FIGURE 2.
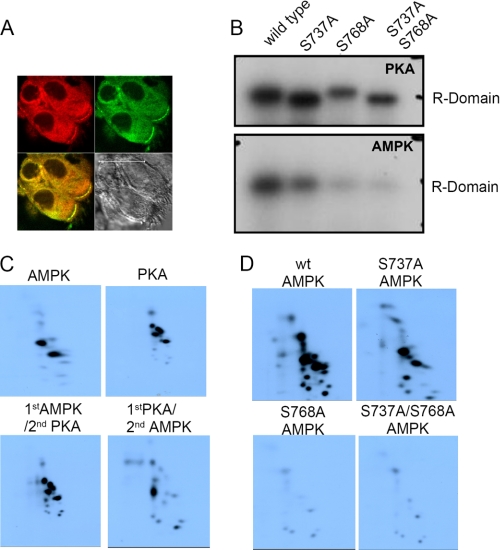
Expression and phosphorylation of CFTR. A, immunostaining of CFTR (red) and AMPKα1 (green) in human nasal epithelial cells. B, phosphorylation of the R domain with PKA and AMPK. AMPK phosphorylation was largely reduced or abolished in S737A and S768A, respectively (lower panel). When PKA alone was used to phosphorylate the R domain, a complex gel shift with some diminution of phosphorylation was found after mutation of the two serines. This shift was not observed when AMPK alone was the phosphorylating kinase but serine mutation abrogated the phosphorylation (lower panel). C, order dependence of AMPK and PKA in R domain phosphorylation. PKA- and AMPK-dependent phosphorylation of R domain creates distinct peptide patterns. Recombinant R domain phosphorylated with recombinant AMPK in the presence of unlabeled ATP (lower left) followed by PKA and labeled ATP gives rise to some measurable incorporation. However, when the PKA was added first, AMPK could only phosphorylate to a level ∼4-fold less than that with PKA or AMPK alone. D, effect of mutating serines 737 and 768 at AMPK phosphorylation sites. The S768A mutant abrogates almost all the phosphorylation. Electronic autoradiography was done at identical gain settings.
Inhibitory R Domain Sites Are Targeted by AMPK—It is not known where AMPK phosphorylates the CFTR protein (5) but the R domain of CFTR contains multiple phosphorylation sites for PKA. Interestingly two of these sites, namely Ser737 and Ser768, have been identified as “inhibitory” R domain sites, i.e. when mutated to alanines they augment the open probability of CFTR relative to wild type (23, 24). We hypothesized that these sites might be phosphorylated by AMPK (rather than “inhibited” by PKA) given some sequence homology with the expected consensus sequence for AMPK in local amino acids and therefore examined in vitro phosphorylation of isolated single or double mutants or wild type R domain protein. Fig. 2B demonstrates that AMPK indeed phosphorylates the R domain in vitro and this AMPK phosphorylation is largely reduced in R domain mutants S737A and S768A (compare lanes 2 and 3 in Fig. 2B, lower panel). The data also suggest that serine 768 has a much greater reductive impact because S768A almost abolished all the phosphorylation, whereas some were preserved with S737A. Crucially, the double mutant labeling was similar to S768A alone. We quantified data (supplemental Fig. S2) from three independent experiments and found that the S737A mutation leads to a small 23 ± 8% reduction in counts, whereas S768A leads to a major 81 ± 5% (mean ± range) reduction similar to the double mutant (87% ± 4%) when compared with the wild type (100%). Thus the data confirm that AMPK targets Ser768 preferentially and also phosphorylates Ser737. The specificity of this finding was confirmed toward AMPK because no such dramatic inhibition of labeling of the R domain was found when PKA was used to phosphorylate the R domain in vitro (compare upper and lower panels in Fig. 2B, quantitation in supplement Fig. S2). The gel shifts observed with PKA (but not AMPK) in the presence of these mutants are unexplained but are consistent with a selective change in R domain structure after PKA phosphorylation (24, 25). Correspondingly, Fig. 2C reveals that PKA- and AMPK-dependent phosphorylation of the R domain created distinct peptide patterns, as described below in detail.
The combined biochemical and physiological data shown below suggest a complex relationship between the PKA and AMPK driven phosphorylation of the R domain. To test this we undertook phosphopeptide mapping of the AMPK- and PKA-dependent phosphorylation of the R domain. In such experiments, it must be remembered that the interpretation rests on dramatic changes in phosphorylation because it is not possible to match exactly the conditions that might pertain in vivo. First, we tested the effects of each kinase separately and found only two major phosphopeptide spots with AMPK but 4 phosphopeptide spots with PKA (Fig. 2C, left uppermost panel pair). Next, we added the kinases sequentially in an attempt to mimic the physiological protocols used in the oocytes to stimulate or inhibit CFTR ion transport. These experiments were undertaken using unlabeled ATP in each first kinase run. To ease interpretation, we washed out residual kinase between runs to prevent any spillover effects or kinase-kinase interactions (Fig. 2C, lower panels). First, note the remarkable similarity between AMPK first, PKA second when compared with PKA alone (compare upper right with lower left in Fig. 2, panel C). We conclude that AMPK (as first cold-label) cannot abrogate the subsequent 4-spot “hot” PKA-mediated pattern. AMPK when added second, cannot generate the expected two-spot pattern observed with AMPK alone (Fig. 2C, lower right compared with upper left panels) suggesting either inaccessibility of the necessary site(s) based on the gel shift data (Fig. 2B, upper panel) that was either suggestive of a structural alteration or the unknown effects of some other phosphorylated site. Thus in vitro rank order matters for these kinases where specific site(s) on the R domain labeling is concerned. We interpret these data to imply that a selective, major (>80%) reduction in R domain phosphorylation occurs when the Ser768 OH group is no longer available for phosphorylation, which could either mean that this is a key AMPK site by itself or that Ser768 is “permissive,” i.e. is involved in docking/orienting AMPK or, alternatively is required for labeling some other site on CFTR.
Next we tried to explore the different roles of the two serines with respect to AMPK but found that attempts to recreate the wild type pattern of spots in the presence of serine-mutated R domain always showed a profound loss of label incorporation at short incubation times despite identical R domain protein loading, suggesting that these two serines were the major R domain targets for AMPK. We therefore used prolonged incubation times to maximize the counts and these data are shown in Fig. 2D. It is clear that S768A (lower left panel) has a more profound inhibitory effect on R domain phosphorylation compared with S737A (upper right) given that all these experiments were run simultaneously with similar concentrations of R domain protein and kinase, and each imaged for identical lengths of time. Broadly, prolonged incubation that previously created two major spots found with AMPK alone was now supplemented by multiple small spots that almost disappeared after S768A mutation, but much less so after S737A mutation (Fig. 2D, compare lower two panels with upper). Once again the critical role of Ser768 is reiterated relative to Ser737.
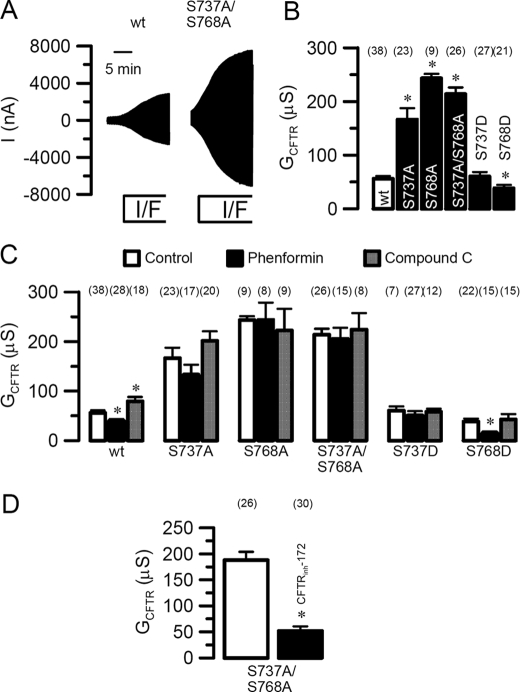
When expressed in oocytes, CFTR bearing the R domain mutants S737A and S768A as well as the double mutant S737A/S768A (Fig. 3A) produced dramatically enhanced conductances (S768A > S737A; Fig. 3B) upon stimulation with forskolin (2 μm) and IBMX (1 mm). In contrast, the S768D mutation, mimicking phosphorylation at Ser768, produced a whole cell conductance that was significantly smaller than even wtCFTR (note that conductance not lowered with S737D; see also Fig. 3C for phenformin sensitivity of these mutants). The large Cl– conductances generated by S737A/S768A were inhibited by 5 μm of the specific chloride channel blocker CFTRinh-172 (Fig. 3D), or alternatively, the PKA inhibitor KT5720 and Cl– replacement by gluconate (not shown). Once again the differential roles of these two serines were observed because the conductance observed with S737A was almost 100 μS lower than that found with S768A. Furthermore, there remained a nonsignificant but interesting trend toward the retention of either inhibition or stimulation (with phenformin and compound C, respectively) with the S573A but not with the S768A (compare second and third panels of Fig. 3C with the corresponding wild type result in the first panel of the figure). Overall the data suggest that S737A, S768A, and the double mutant S737A/S768A were no longer sensitive to stimulation or inhibition of AMPK indicating that phosphorylation at both serines is required (Fig. 3C, middle panels). In contrast the residual CFTR conductances generated by S768D (but not S737D) were not only further inhibited by phenformin, but neither phosphomimic mutant could be augmented by the AMPK inhibitor compound C. Thus the presence of a fixed negative charge at Ser768 maintains sensitivity to AMPK-mediated inhibition, whereas this is lost when Ser737 has a similar negative charge. Yet, unlike wild type CFTR, neither Asp mutant is able to enhance their conductance when AMPK is inhibited (Fig. 3C). Thus the negative charge at serine 768 is discriminant between inhibition and activation by AMPK.
FIGURE 3.
Effects of AMPK on wtCFTR and R domain mutants. A, whole cell currents activated by IBMX (1 mm) and forskolin (2 μm) in wtCFTR and S737A/S768A-CFTR expressing oocytes. B, summary of whole cell conductances generated by wtCFTR and different CFTR mutants. C, summary of CFTR whole cell conductances generated by wtCFTR and different CFTR mutants, and effects of phenformin and compound C. D, summary of the whole cell conductance activated by S737A/S768A-CFTR and inhibition by the CFTR blocker CFTRinh-172. Membrane currents were measured by voltage clamping in intervals from –60 to +40 mV, in steps of 10 mV. *, significant difference when compared with control. #, significant difference when compared with wtCFTR. Data are shown as mean ± S.E. (number of experiments).
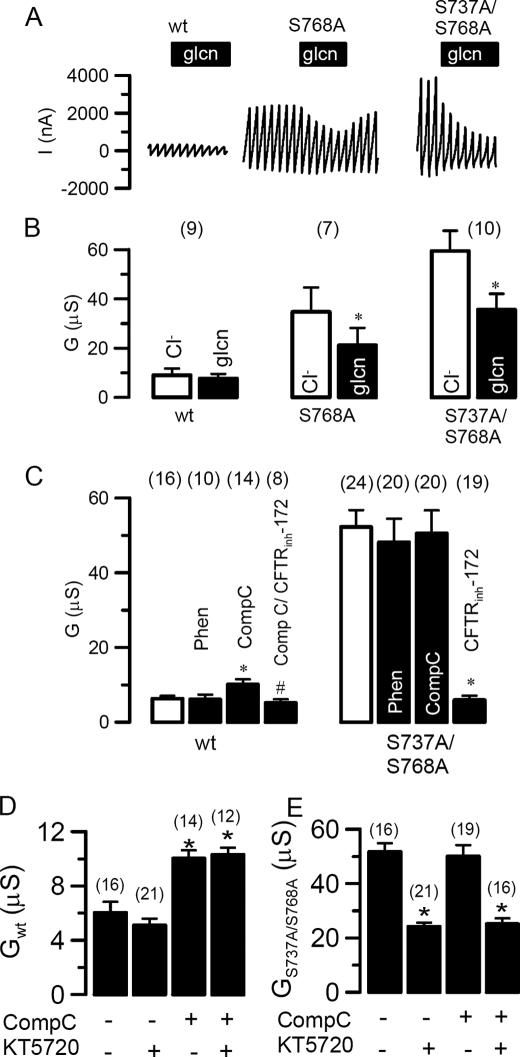
In contrast to wtCFTR the CFTR mutants S737A (not shown), S768A, and S737A/S768A produced a high Cl– conductance under basal conditions, i.e. in the absence of IBMX and forskolin (Fig. 4, A and B). Enhanced basal conductance of the mutants (but not wild type) was inhibited by replacement of extracellular Cl– with gluconate (Fig. 4B). Yet both the basal conductance of the mutants and the basal conductance of wtCFTR after compound C were blocked by CFTRinh-172 (Fig. 4C, rightmost columns) suggesting both were indeed CFTR mediated. As described above for activated whole cell Cl– conductance, the enhanced baseline conductance exhibited by these mutants was also insensitive to phenformin and compound C, consistent with a loss of AMPK sensitivity (Fig. 4C). Moreover, the PKA inhibitor KT5720 (50 μm) inhibited this enhanced baseline CFTR conductance generated by S737A/S768A irrespective of the presence of compound C (Fig. 4E). In contrast, KT5720 did not affect the very low conductance produced by unstimulated wtCFTR (Fig. 4D), suggesting this was PKA-independent (note also the corresponding insensitivity to gluconate replacement, Fig. 4, A and B, left panels). We interpret this finding to suggest that the sensitivity of S737A/S768A-CFTR toward PKA inhibition is retained, which implies that PKA is now active after the loss of AMPK sensitivity. The combined data posit a feedback loop between PKA and AMPK.
FIGURE 4.
S737A/S768A-CFTR generates a baseline conductance. A, effect of extracellular Cl– replacement by gluconate (glcn) on whole cell currents generated by wtCFTR, S768A-CFTR, and S737A/S768A-CFTR in the absence of IBMX and forskolin. B, summary of the corresponding whole cell conductances. C, summary of the whole cell conductances generated by wtCFTR and S737A/S768A-CFTR in the absence of stimulation with IBMX and forskolin and effects of phenformin, compound C, and CFTRinh-172. D and E, baseline whole cell conductances generated by wild type CFTR (D) and S737A/S768A-CFTR (E) and effects of compound C and the PKA inhibitor KT520. Membrane currents were measured by voltage clamping in intervals from –60 to +40 mV, in steps of 10 mV. *, significant difference when compared with control. Data are shown as mean ± S.E. (number of experiments).
Physiological Role of AMPK Regulation—We further examined the conditions under which regulation of CFTR by AMPK occurs. To that end we gradually increased CFTR activity by stimulating wtCFTR expressing oocytes either leaving phosphodiesterases intact or by inhibiting them differentially (Fig. 5A, left to right) as follows: (i) 2 μm forskolin (21 ± 3.7 μS), (ii) 2 μm forskolin and 1 mm IBMX (61 ± 6.8 μS), or (iii) 2 μm forskolin, 5 mm IBMX, and 3 mm 8Br–-cAMP (120 ± 9.8 μS), in the presence or absence of compound C to detect inhibitory effects of AMPK (Fig. 5A). Interestingly, at increased PKA stimulation, the compound C effect was reduced and was undetectable at maximal stimulation, confirming interference between phosphorylation by AMPK and PKA (upper panel). Thus the data suggest that one physiological role of the tonic basal AMPK activity may be to keep the CFTR channel shut, as evidenced by the rise in basal conductance with compound C alone in the absence of secretagogue stimulation (Fig. 4), rather than limiting excessive activation as suggested previously. The initial recovery from PKA stimulation was equal in S737A/S768A (1.1 ± 0.3 μS/min) and wtCFTR (1.1 ± 0.1 μS/min) (Fig. 5C), but was enhanced in ooctyes coexpressing kinase-dead AMPKα1-K45R (2.8 ± 0.4 μS/min), whereas overexpression of wtAMPKα1β1γ1 literally eliminated CFTR currents (Fig. 5B). In these experiments we stimulated oocytes with only 20 μmol/liter forskolin that creates a transient pulse of cAMP capable of being degraded by phosphodiesterases. Although AMPK largely antagonizes activation of CFTR, the mutated serines S737A and S768A do not seem to influence the recovery time from forskolin stimulation.
FIGURE 5.
Functional role of AMPK-regulation of CFTR. A, activation of CFTR whole cell conductance by stimulation with 20 μm forskolin (left column pair, raw baseline data in lower panel), 1 mm IBMX, 80 μm forskolin (middle column), and 5 mm IBMX, 2 μm forskolin, and 3 mm 8Br–-cAMP (right column). Increase of CFTR whole cell conductance by compound C (80 μm) relative to CFTR baseline conductance (upper panels). B, inactivation of CFTR whole cell conductances in oocytes expressing CFTR, CFTR, and AMPKα1β1γ1, or the catalytically dead mutant AMPKα1-K45R. C, inactivation of conductances generated by wtCFTR and S737A/S768A-CFTR. D, CFTR whole cell conductances activated by 3 mm Br–-cAMP, 3 mm Br–-cAMP, and 2 μm forskolin, or 3 mm Br–-cAMP and 2 μm forskolin and 1 mm IBMX, and effects of compound C. E, activation of CFTR by forskolin (2 μm) and additional stimulation by rolipram (50 μm), dipyridamol (80 μm), and IBMX (1 mm). Membrane currents were measured by voltage clamping in intervals from –60 to +40 mV, in steps of 10 mV. *, significant difference when compared with control. #, significant difference when compared with wtCFTR. Data are shown as mean ± S.E. (number of experiments).
AMP may be generated in close proximity of CFTR, probably by local phosphodiesterases. It has been shown that PDE4D is translocated to CFTR via the PDZ protein Shank2 that binds to the C-terminal PDZ binding domain of CFTR (19). Thus negative regulation of CFTR via Shank2 occurs in competition with positive regulation by other PDZ proteins, such as NHERF1, which translocates protein kinase A close to the R domain (26). We eliminated the PDZ binding domain of CFTR (E1474X-CFTR) and gradually increased stimulation of the oocytes as shown in Fig. 5D. As expected, we found that stimulation with 3 mm 8Br–-cAMP (which acts both through stimulation of PKA and by blocking PDE activity) was able by itself to activate wtCFTR maximally, i.e. no further activation by additional stimulation with forskolin (2 μm) or IBMX (5 mm). Also, consistent with Fig. 5A, right panel, inhibition of AMPK by compound C had no further enhancing effects on wtCFTR activity. In marked contrast 3 mm 8Br–-cAMP alone was unable to promote full activation of E1474X-CFTR but instead required co-stimulation by 8Br–-cAMP, forskolin, and IBMX, and compound C further augmented whole cell conductance (Fig. 5D) suggesting AMPK sensitivity was retained by this mutant. The data suggest that translocation of signaling cascades in close proximity to CFTR might be required for graded regulation of CFTR. This notion was supported by injection of oocytes with AMP to reach a final cytosolic concentration of 1 mm, which did not inhibit CFTR. Although diffusion barriers for AMP could exist within the oocyte, the result suggests that global cytosolic AMP increase is unlikely to influence the membrane compartment. Furthermore, the inhibitors of the CFTR-specific PDE4 and PDE5 rolipram and dipyridamole also augmented CFTR whole cell conductance suggesting that CFTR-localized phosphodiesterase activity may generate environmental AMP that enhances AMPK activity in close proximity of CFTR (Fig. 5E).
DISCUSSION
Regulation of CFTR by AMPK—The present results demonstrate that baseline CFTR activity is tonically inhibited by high baseline AMPK activity but after PKA stimulation, AMPK only has a modest inhibitory effect on wild type CFTR. This might explain why data reported previously by Hallows and colleagues (5) found only modest inhibition of pre-activated CFTR by post-activation of AMPK in Xenopus oocytes. It is not widely appreciated outside the specialist kinase field that AMPK has a substantial and constitutive baseline activity in most tissues that is further stimulated by increments in the intracellular AMP/ATP ratio or pharmacological activation by drugs such as phenformin. Additionally, independent regulation by the calmodulin-dependent kinase (CAMKK) has been shown previously (27, 28). It may be of relevance to our findings in oocytes that PKA was shown to inhibit AMPK activity by attenuating phosphorylation through upstream calcium/calmodulin-dependent protein kinase (29). Thus multisite phosphorylation controls AMPK activity and the cross-talk between PKA and AMPK may be relevant for the competitive regulation of CFTR by PKA and AMPK as demonstrated in the present paper.
AMPK Phosphorylates the R Domain—We provide biochemical and electrophysiological data consistent with the hypothesis that AMPK regulation involves two so-called inhibitory PKA sites at Ser737 and Ser768 in the R domain (23, 24). Correspondingly, but with the caveat that phosphorylation of these serines happens at a biochemical level in vitro, we nevertheless, observe a dramatic difference in the consequent pattern of phosphopeptides in the R domain when the rank order in which these kinases are applied is reversed (as shown in Fig. 2) and note the differential effect of serine 768 in this respect. Binding of the α1-subunit of AMPK to residues 1420–1457 in the C terminus of CFTR seems to be essential for AMPK regulation of CFTR, as demonstrated by a failure of AMPK to inhibit CFTR in the absence of this binding motif (5). The present results (see supplemental Fig. S1) also confirm those previous studies in that they demonstrate inhibition of the open probability of CFTR by phosphorylation through AMPK, rather than effects on membrane expression (6). The actions of AMPK are complex as this kinase regulates a number of transport proteins involved in secretion or absorption of electrolytes in epithelia. Thus epithelial Na+ channels (30–32), the renal NKCC2 (33, 34), CFTR (5–7), and probably the secretory Na+/2Cl–/K+-cotransporter (NKCC1) (34) can all be controlled by AMPK. Regulation occurs either indirectly, as in the case of epithelial Na+ channels (ENaC), or directly through AMPK phosphorylation as for CFTR, NKCC2, and probably NKCC1. AMPK is inhibitory on the ion transport generated by ENaC, CFTR, and NKCC1, however, the functional impact of AMPK on NKCC2 still remains to be determined (34). It is also of interest that AMPK activation reduces inflammation in airway epithelial cells of CF patients (9) and we note that AMPK and CFTR are co-localized at the apical membrane in such tissues.
CFTR is the only ABC transporter that has a regulatory (R) domain, containing multiple consensus sites for phosphorylation by PKA and PKC (35). Phosphorylation of the R domain by PKA is a prerequisite, but is not sufficient to gate the Cl– channels. Additional binding of ATP to both nucleotide binding domains is required to open the channel (25, 36). Phosphorylation at individual PKA sites has additive effects on the open probability of CFTR, and so phosphorylation of the individual serines occurs independently. Moreover, none of the PKA sites is absolutely necessary for activation of CFTR (23). Notably, two of the PKA consensus sites (Ser737 and Ser768) have been suggested to be inhibitory because elimination of these sites largely augments activation of CFTR (23, 24). Our present results suggest that it might be AMPK rather than PKA that is phosphorylating Ser737 and Ser768 under baseline conditions, i.e. in the absence of any stimulation by secretagogues. This novel idea is consistent with the inhibition of CFTR in the presence of Asp mutants at one of these sites, namely Ser768.
Physiological Relevance—It has been assumed that the inhibitory effects mediated by Ser737 and Ser768 can be overcome by stimulation with supermaximal concentrations of IBMX (23). The present data clearly demonstrate that this is not the case. Even maximal stimulation of wtCFTR with a mixture of 8-Br–-cAMP, IBMX, and forskolin does not produce the same high level of conductance as S737A-CFTR or S768A-CFTR. A detailed single channel analysis by the Gadsby group arrived at a related conclusion that early phosphorylation of Ser768 in CFTR impairs subsequent phosphorylation of stimulatory R domain serines. They further postulated that the observed reduced sensitivity to activation by PKA imparted by Ser768 might ensure activation of CFTR during strong stimulation but attenuating responses to weak signals (24). Further data will be needed to test this idea but our phosphorylation data concur to the extent that they demonstrate that a complex interaction occurs through this critical serine.
In the present study we found that AMPK phosphorylation of Ser737 and Ser768 does not appear to affect activation or deactivation kinetics of CFTR. However, our data strongly suggest that increasing PKA activity “competes out” AMPK sensitivity, so that only PKA will control CFTR. An antagonistic regulation of CFTR by AMPK under control conditions will guarantee that the channel is kept closed in the absence of secretagogues. Overall our data are consistent with the notion that AMPK activity acts as a “biological rheostat” toward CFTR. As some epithelial tissues such as the sweat duct epithelium and the submucosal gland epithelium show a high baseline CFTR activity (37, 38), it will be interesting to examine if these tissues lack AMPKα1 expression or have otherwise a reduced AMPK activity or sensitivity toward CFTR.
CFTR Localized AMPK—Around 40% of resting energy expenditure in humans is used to control ion gradients across cells and cross-talk between transport and metabolism has been proposed that would adjust transport activity to the cellular energy supply (13). CFTR is ideally localized to perform such a sensing role because it lies in a macromolecular complex together with receptors, adenylate cyclase, kinases, scaffolding proteins, and phosphodiesterases. Thus CFTR can integrate various proteins to a “channelsome,” a functional unit that operates independently of other cellular compartments (16). Receptor-mediated compartmental activation of CFTR is probably disconnected from the cytosol, i.e. intracellular signaling by cAMP is spatially restricted and compartmentalized (reviewed in Refs. 39 and 40). In this model, regulation of CFTR is predicted to occur without any detectable changes in cytosolic second messenger concentration, because CFTR is intimately connected to signaling elicited by stimulation of A2B adenosine and β2-adrenergic receptors (41–43). Moreover, type 2 lysophosphatidic acid receptors also form macromolecular complexes with CFTR that are mediated through a PDZ scaffolding protein (NHERF2). In contrast to adenosine and β2-adrenergic stimulation, lysophosphatidic acid inhibits CFTR through a lysophosphatidic acid 2 receptor-mediated Gi pathway (44). Thus compartmentalized receptor-CFTR coupling appears to be critical for a rapid and specific signal transduction from the receptor to the channel. PDEs provide the means to avoid spreading of intracellular signals by degrading cAMP, and therefore play a vital role in shaping intracellular gradients of the second messenger (39). Particularly PDE4D, which couples to CFTR via the PDZ adaptor Shank2, precludes cAMP/PKA signals (19). However, not only PDEs but also other mechanisms contribute to compartmentalization such as the cAMP transporter MRP4. This multidrug resistance protein also belongs to the large family of ABC transporters, and physically couples to CFTR via PDZK1 (43). By providing an efflux pathway for cAMP, it constitutes an additional way of regulating cAMP levels in a microdomain below the surface membrane. In the present study both the PDE4 inhibitor rolipram and the PDE5 inhibitor dipyridamole augmented CFTR activity, confirming that both PDE subtypes degrade cAMP locally, i.e. in close proximity to CFTR. In analogy to the well anticipated localized cAMP signaling, we hypothesize that local generation of AMP, perhaps in some instances generated by the very same phosphodiesterases from cAMP, controls the activity of AMPK, rather than global cytosolic changes in the ATP/AMP ratio. Thus AMPK may serve as a local controller of CFTR activity rather than coupling global cellular metabolism to the transport activity of CFTR (13). The data show that enhanced CFTR activity can be observed by mutating critical serines that negate inhibition by AMPK, such as serine 768. Our failure to reverse the gating defects in two common disease causing mutants (G551D and F508delCFTR) suggests that their mechanism of dysfunction is not due to an overactive AMPK but further work will have to establish whether inhibition of AMPK might be a therapeutic option in other mutations.
Supplementary Material
Acknowledgments
We acknowledge the expert technical assistance by E. Tartler and A. Paech. We thank D. G. Hardie (Dundee) and A. Woods and D. Carling (London) for reagents, antibodies, and advice.
Author's Choice—Final version full access.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB699 A6 and DFG KU756/8-1 and Wellcome Trust Grants 079965/z/06/z (to K. J. T.) and 075237/z/04/z (to D. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: CFTR, cystic fibrosis transmembrane conductance regulator; PDE4D, phosphodiesterases 4D; PKA, protein kinase A; AMPK, adenosine monophosphate-stimulated kinase; CF, cystic fibrosis; TBS, Tris-buffered saline; PBS, phosphate-buffered saline; AICAR, 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside; IBMX, isobutylmethylxanthine.
References
- 1.Riordan, J. R. (2005) Annu. Rev. Physiol. 67 701–718 [DOI] [PubMed] [Google Scholar]
- 2.Dahan, D., Evagelidis, A., Hanrahan, J. W., Hinkson, D. A., Jia, Y., Luo, J., and Zhu, T. (2001) Pflugers Arch. 443 Suppl. 1, S92–S96 [DOI] [PubMed] [Google Scholar]
- 3.Aleksandrov, A. A., Aleksandrov, L. A., and Riordan, J. R. (2007) Pflugers Arch. 453 693–702 [DOI] [PubMed] [Google Scholar]
- 4.Kunzelmann, K., and Mall, M. (2002) Physiolog. Rev. 82 245–289 [DOI] [PubMed] [Google Scholar]
- 5.Hallows, K. R., Raghuram, V., Kemp, B. E., Witters, L. A., and Foskett, J. K. (2000) J. Clin. Investig. 105 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallows, K. R., McCane, J. E., Kemp, B. E., Witters, L. A., and Foskett, J. K. (2003) J. Biol. Chem. 278 998–1004 [DOI] [PubMed] [Google Scholar]
- 7.Walker, J., Jijon, H. B., Churchill, T., Kulka, M., and Madsen, K. L. (2003) Am. J. Physiol. 285 G850–G860 [DOI] [PubMed] [Google Scholar]
- 8.Hallows, K. R., Kobinger, G. P., Wilson, J. M., Witters, L. A., and Foskett, J. K. (2003) Am. J. Physiol. 284 C1297–C1308 [DOI] [PubMed] [Google Scholar]
- 9.Hallows, K. R., Fitch, A. C., Richardson, C. A., Reynolds, P. R., Clancy, J. P., Dagher, P. C., Witters, L. A., Kolls, J. K., and Pilewski, J. M. (2006) J. Biol. Chem. 281 4231–4241 [DOI] [PubMed] [Google Scholar]
- 10.Motoshima, H., Goldstein, B. J., Igata, M., and Araki, E. (2006) J. Physiol. 574 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardie, D. G., Hawley, S. A., and Scott, J. W. (2006) J. Physiol. 574 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie, D. G. (2004) J. Cell Sci. 117 5479–5487 [DOI] [PubMed] [Google Scholar]
- 13.Hallows, K. R. (2005) Curr. Opin. Nephrol. Hypertens. 14 464–471 [DOI] [PubMed] [Google Scholar]
- 14.Mehta, A. (2007) Pflugers Arch. 455 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarnaess, E., and Tasken, K. (2007) Biochem. Soc. Trans. 35 931–937 [DOI] [PubMed] [Google Scholar]
- 16.Guggino, W. B., and Stanton, B. A. (2006) Nat. Rev. Mol. Cell. Biol. 7 426–436 [DOI] [PubMed] [Google Scholar]
- 17.Li, C., and Naren, A. P. (2005) Pharmacol. Ther. 108 208–223 [DOI] [PubMed] [Google Scholar]
- 18.Guerra, L., Fanelli, T., Favia, M., Riccardi, S. M., Busco, G., Cardone, R. A., Carrabino, S., Weinman, E. J., Reshkin, S. J., Conese, M., and Casavola, V. (2005) J. Biol. Chem. 280 40925–40933 [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. H., Richter, W., Namkung, W., Kim, K. H., Kim, E., Conti, M., and Lee, M. G. (2007) J. Biol. Chem. 282 10414–10422 [DOI] [PubMed] [Google Scholar]
- 20.Barnes, A. P., Livera, G., Huang, P., Sun, C., O'Neal, W. K., Conti, M., Stutts, M. J., and Milgram, S. L. (2005) J. Biol. Chem. 280 7997–8003 [DOI] [PubMed] [Google Scholar]
- 21.Treharne, K. J., Riemen, C. E., Marshall, L. J., Muimo, R., and Mehta, A. (2001) Pflugers Arch. 443 Suppl. 1, S97–S102 [DOI] [PubMed] [Google Scholar]
- 22.Corton, J. M., Gillespie, J. G., Hawley, S. A., and Hardie, D. G. (1995) Eur. J. Biochem. 229 558–565 [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson, D. J., Strong, T. V., Mansoura, M. K., Wood, D. L., Smith, S. S., Collins, F. S., and Dawson, D. C. (1997) Am. J. Physiol. 273 L127–L133 [DOI] [PubMed] [Google Scholar]
- 24.Csanady, L., Seto-Young, D., Chan, K. W., Cenciarelli, C., Angel, B. B., Qin, J., McLachlin, D. T., Krutchinsky, A. N., Chait, B. T., Nairn, A. C., and Gadsby, D. C. (2005) J. Gen. Physiol. 125 171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadsby, D. C., and Nairn, A. C. (1999) Physiol. Rev. 79 S77–S107 [DOI] [PubMed] [Google Scholar]
- 26.Sun, F., Hug, M. J., Lewarchik, C. M., Yun, C., Bradbury, N. A., and Frizzell, R. A. (2000) J. Biol. Chem. 275 29539–29546 [DOI] [PubMed] [Google Scholar]
- 27.McAlroy, H. L., Ahmed, S., Day, S. M., Baines, D. L., Wong, H. Y., Yip, C. Y., Ko, W. H., Wilson, S. M., and Collett, A. (2000) Br. J. Pharmacol. 131 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo, X., Merlin, D., Harvey, R. D., Laboisse, C., and Hopfer, U. (1997) J. Membr. Biol. 155 239–246 [DOI] [PubMed] [Google Scholar]
- 29.Hurley, R. L., Barre, L. K., Wood, S. D., Anderson, K. A., Kemp, B. E., Means, A. R., and Witters, L. A. (2006) J. Biol. Chem. 281 36662–36672 [DOI] [PubMed] [Google Scholar]
- 30.Bhalla, V., Oyster, N. M., Fitch, A. C., Wijngaarden, M. A., Neumann, D., Schlattner, U., Pearce, D., and Hallows, K. R. (2006) J. Biol. Chem. 281 26159–26169 [DOI] [PubMed] [Google Scholar]
- 31.Carattino, M. D., Edinger, R. S., Grieser, H. J., Wise, R., Neumann, D., Schlattner, U., Johnson, J. P., Kleyman, T. R., and Hallows, K. R. (2005) J. Biol. Chem. 280 17608–17616 [DOI] [PubMed] [Google Scholar]
- 32.Woollhead, A. M., Scott, J. W., Hardie, D. G., and Baines, D. L. (2005) J. Physiol. 566 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser, S., Mount, P., Hill, R., Levidiotis, V., Katsis, F., Stapleton, D., Kemp, B. E., and Power, D. A. (2005) Am. J. Physiol. 288 F578–F586 [DOI] [PubMed] [Google Scholar]
- 34.Fraser, S. A., Gimenez, I., Cook, N., Jennings, I., Katerelos, M., Katsis, F., Levidiotis, V., Kemp, B. E., and Power, D. A. (2007) Biochem. J. 405 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riordan, J. R., Rommens, J. M., Kerem, B.-S., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Plavsic, S. L. N., Chou, J.-L., Drumm, M. L., Iannuzzi, C. M., Collins, F. S., and Tsui, L.-C. (1989) Science 245 1066–1072 [DOI] [PubMed] [Google Scholar]
- 36.Sheppard, D. N., and Welsh, M. J. (1999) Physiol. Rev. 79 S23–S45 [DOI] [PubMed] [Google Scholar]
- 37.Quinton, P. M. (2007) Physiol. (Bethesda) 22 212–225 [DOI] [PubMed] [Google Scholar]
- 38.Wine, J. J. (2007) Auton. Neurosci. 133 35–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg, S. F., and Brunton, L. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41 751–773 [DOI] [PubMed] [Google Scholar]
- 40.Cooper, D. M. (2005) Biochem. Soc. Trans. 33 1319–1322 [DOI] [PubMed] [Google Scholar]
- 41.Huang, P., Lazarowski, E. R., Tarran, R., Milgram, S. L., Boucher, R. C., and Stutts, M. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 14120–14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naren, A. P., Cobb, B., Li, C., Roy, K., Nelson, D., Heda, G. D., Liao, J., Kirk, K. L., Sorscher, E. J., Hanrahan, J., and Clancy, J. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, C., Krishnamurthy, P. C., Penmatsa, H., Marrs, K. L., Wang, X. Q., Zaccolo, M., Jalink, K., Li, M., Nelson, D. J., Schuetz, J. D., and Naren, A. P. (2007) Cell 131 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, C., Dandridge, K. S., Di, A., Marrs, K. L., Harris, E. L., Roy, K., Jackson, J. S., Makarova, N. V., Fujiwara, Y., Farrar, P. L., Nelson, D. J., Tigyi, G. J., and Naren, A. P. (2005) J. Exp. Med. 202 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.