Abstract
Rab2 requires glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and atypical protein kinase Cι (aPKCι) for retrograde vesicle formation from vesicular tubular clusters that sort secretory cargo from recycling proteins returned to the endoplasmic reticulum. However, the precise role of GAPDH and aPKCι in the early secretory pathway is unclear. GAPDH was the first glycolytic enzyme reported to co-purify with microtubules (MTs). Similarly, aPKC associates directly with MTs. To learn whether Rab2 also binds directly to MTs, a MT binding assay was performed. Purified Rab2 was found in a MT-enriched pellet only when both GAPDH and aPKCι were present, and Rab2-MT binding could be prevented by a recombinant fragment made to the Rab2 amino terminus (residues 2-70), which directly interacts with GAPDH and aPKCι. Because GAPDH binds to the carboxyl terminus of α-tubulin, we characterized the distribution of tyrosinated/detyrosinated α-tubulin that is recruited by Rab2 in a quantitative membrane binding assay. Rab2-treated membranes contained predominantly tyrosinated α-tubulin; however, aPKCι was the limiting and essential factor. Tyrosination/detyrosination influences MT motor protein binding; therefore, we determined whether Rab2 stimulated kinesin or dynein membrane binding. Although kinesin was not detected on membranes incubated with Rab2, dynein was recruited in a dose-dependent manner, and binding was aPKCι-dependent. These combined results suggest a mechanism by which Rab2 controls MT and motor recruitment to vesicular tubular clusters.
The small GTPase Rab2 is essential for membrane trafficking in the early secretory pathway and associates with vesicular tubular clusters (VTCs)2 located between the endoplasmic reticulum (ER) and the cis-Golgi compartment (1, 2). VTCs are pleomorphic structures that sort anterograde-directed cargo from recycling proteins and trafficking machinery retrieved to the ER (3-6). Rab2 bound to a VTC microdomain stimulates recruitment of soluble factors that results in the release of vesicles containing the recycling protein p53/p58 (7). In that regard, we have previously reported that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and atypical PKC ι (aPKCι) are Rab2 effectors that interact directly with the Rab2 amino terminus and with each other (8, 9). Their interaction requires Src-dependent tyrosine phosphorylation of GAPDH and aPKCι (10). Moreover, GAPDH is a substrate for aPKCι (11). GAPDH catalytic activity is not required for ER to Golgi transport indicating that GAPDH provides a specific function essential for membrane trafficking from VTCs independent of glycolytic function (9). Indeed, phospho-GAPDH influences MT dynamics in the early secretory pathway (11).
GAPDH was the first glycolytic enzyme reported to co-purify with microtubules (MTs) (12) and subsequently was shown to interact with the carboxyl terminus of α-tubulin (13). The binding of GAPDH to MTs promotes formation of cross-linked parallel MT arrays or bundles (14, 15). GAPDH has also been reported to possess membrane fusogenic activity, which is inhibited by tubulin (16). Similarly, aPKC associates directly with tubulin and promotes MT stability and MT remodeling at specific intracellular sites (17-21). It may not be coincidental that these two Rab2 effectors influence MT dynamics because recent studies indicate that the cytoskeleton plays a central role in the organization and operation of the secretory pathway (22).
MTs are dynamic structures that grow or shrink by the addition or loss of α- and β-tubulin heterodimers from the ends of protofilaments (23). Their assembly and stability is regulated by a variety of proteins traditionally referred to as microtubule-associated proteins (MAPs). In addition to the multiple α/β isoforms that are present in eukaryotes, MTs undergo an assortment of post-translational modifications, including acetylation, glycylation, glutamylation, phosphorylation, palmitoylation, and detyrosination, which further contribute to their biochemical heterogeneity (24, 25). It has been proposed that these tubulin modifications regulate intracellular events by facilitating interaction with MAPs and with other specific effector proteins (24). For example, the reversible addition of tyrosine to the carboxyl terminus of α-tubulin regulates MT interaction with plus-end tracking proteins (+TIPs) containing the cytoskeleton-associated protein glycine-rich (CAP-Gly) motif and with dynein-dynactin (27-29). Additionally, MT motility and cargo transport rely on the cooperation of the motor proteins kinesin and dynein (30). Kinesin is a plus-end directed MT motor, whereas cytoplasmic dynein is a minus-end MT-based motor, and therefore the motors transport vesicular cargo toward the opposite end of a MT track (31).
Although MT assembly does not appear to be directly regulated by small GTPases, Rab proteins provide a molecular link for vesicle movement along MTs to the appropriate target (22, 32-34). In this study, the potential interaction of Rab2 with MTs and motor proteins was characterized. We found that Rab2 does not bind directly to preassembled MTs but does associate when both GAPDH and aPKCι are present and bound to MTs. Moreover, the MTs predominantly contained tyrosinated α-tubulin (Tyr-tubulin) suggesting that a dynamic pool of MTs that differentially binds MAPs/effector proteins/motors associates with VTCs in response to Rab2. To that end, we determined that Rab2-promoted dynein/dynactin binding to membranes and that the recruitment required aPKCι.
EXPERIMENTAL PROCEDURES
Quantitative Membrane Binding Assay—HeLa membranes were prepared as described previously (10). Membranes (∼30 μg of total protein) were added to a reaction mixture that contained 27.5 mm Hepes (pH 7.4), 2.75 mm MgOAc, 65 mm KOAc, 5 mm EGTA, 1.8 mm CaCl2, 1 mm ATP, 5 mm creatine phosphate, and 0.2 IU rabbit muscle creatine phosphokinase (35). Purified recombinant Rab2, aPKCι, and aPKCι (K274W) (7, 36) or purified recombinant Rab2 amino-terminal fragment, prepared as described below, was added at the concentrations indicated under “Results,” and the reaction mix was incubated on ice for 10 min. Rat liver cytosol (∼25 μg of total protein) and 2.0 μm GTPγS (Sigma) was then added, and the reactions were shifted to 32 °C and incubated for 12 min. The binding reaction was layered onto a 20% sucrose cushion and centrifuged at 35,000 × g for 20 min at 25 °C. The pellet was separated by SDS-PAGE and transferred to nitrocellulose in 25 mm Tris (pH 8.3), 192 mm glycine, and 20% methanol. The membrane was blocked in Tris-buffered saline that contained 5% nonfat dry milk or 5% bovine serum albumin and 0.5% Tween 20; then incubated with anti-dynein intermediate chain (clone 70.1) (Sigma), or anti-kinesin (clone IBII) (Sigma), or anti-detyrosinated α-tubulin (Glu-tubulin) (Millipore Corp, Billerica, MA), or anti-tyrosinated α-tubulin (Tyr-tubulin) (Millipore), or anti-GAPDH (Chemicon International, Temecula, CA), or anti-aPKCι (BD Biosciences), or anti-p50/dynamitin (Millipore); washed; further incubated with the appropriate horseradish peroxidase-conjugated secondary antibody; developed with enhanced chemiluminescence (ECL) (Pierce); and then quantified by densitometry using the ImageQuant program (GE Healthcare).
Indirect Immunofluorescence—HeLa cells were plated on coverslips (5 × 105 cells/75-mm dish) and then transiently mock-transfected or transfected with pCR3.1-Rab2 or with pcDNA4/HisMax-PKCι using Lipofectamine (Invitrogen) for 36 h at 37 °C in a 5% CO2 incubator. The coverslips were transferred to 0.1% Triton X-100, 80 mm Pipes (pH 6.9), 5 mm EGTA, 1 mm MgCl2, for 3 min to permeabilize the cells, fixed for 20 min with 4% formaldehyde, washed three times with PBS, and then blocked for 1 h in PBS, 5% normal goat serum. The cells were then incubated for 30 min with either anti-Tyr-tubulin (Millipore), or an affinity-purified Rab2 polyclonal antibody (35), or an affinity-purified polyclonal to p53/p58 (37), or anti-GAPDH (Cell Signaling, Danvers, MA), anti-aPKCι (GenScript Corp., Piscataway, NJ), or fluorescein isothiocyanate-conjugated anti-Xpress (Invitrogen); washed extensively with PBS; incubated with Alexa Fluor 488 chicken anti-mouse antibody and Alex Fluor 594 chicken anti-rabbit; washed extensively with PBS; mounted in Mowiol containing 1,4-diazabicyclo[2.2.2]octane (Sigma); and then viewed with a Zeiss AxioImager epifluorescence microscope (Carl Zeiss, Gottingen, Germany) and photographed with an AxioCamMRm camera (Zeiss Microimaging, Thornwood, NY) using AxioVision Z-stack software to capture 20 images of ∼3.6 μm (Zeiss Microimaging). The image threshold was obtained for both channels, and the extent of true co-localization in the boxed areas (yellow pixels in merged image) was analyzed using the Manders overlap co-localization coefficient (co-localization module, Zeiss Microimaging). A value of 1 is high co-localization, and a value of 0 is low co-localization.
MT Binding Assay—MTs were assembled from 1 μg of pure bovine brain tubulin (Cytoskeleton, Inc., Denver, CO) in 20 μl of Buffer B (100 mm Pipes (pH 6.8), 2 mm MgCl2, and 0.5 mm EGTA) containing 20 μm paclitaxel (Sigma) for 20 min at 37 °C. The MTs were then incubated with purified recombinant Rab2, His6-aPKCι, or His6-GAPDH (200 ng) in a total volume of 50 μl for 30 min at 37 °C. The reaction mixtures were layered onto 250 μl of a 20% sucrose cushion in Buffer B containing 20 μm paclitaxel, and then centrifuged for 30 min at 100,000 × g in a Sorvall M120 Discovery ultra-microcentrifuge using a S120-AT2 rotor. The resultant supernatant and pellet were separated by SDS-PAGE and transferred to nitrocellulose. The blot was probed with the appropriate antibody as indicated under “Results,” washed, further incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody, developed with ECL, and then quantified by densitometry, as above.
Construction of the Rab2 Amino-terminal Fragment/Deletion Mutant—The Rab2 amino-terminal domain was generated by PCR using the 5′ oligonucleotide primer, 5′-CACCATGGCGTACGCCTATCTC-3′, in tandem with the 3′ antisense oligonucleotide, 5′-AATAGTTATCATTCGAGCACCGAA-3′. The amplified product was subcloned into pET102/D-TOPO (C-term) (Invitrogen), and the sequence was verified by DNA sequence analysis. BL21 (DE3) pLysS cells (EMD Biosciences, La Jolla, CA) transformed with the clone were grown at 37 °C to 0.6 A600 and then induced with 0.4 mm isopropyl β-d-thiogalactopyranoside for 3 h at 37 °C. The liquid culture was centrifuged at 6,000 rpm for 30 min, and the pellet was resuspended in 50 mm Tris (pH 7.4), 1 mm dithiothreitol, 1 mm EDTA, 1% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride; sonicated; centrifuged at 22,000 × g for 30 min; and then the supernatant was applied to a 1-ml column of Ni2+-nitrilotriacetic acid-agarose (Qiagen, Valencia, CA) equilibrated in Buffer A (10 mm Hepes (pH 7.9), 5 mm MgCl2, 0.1 mm EDTA, 50 mm NaCl, and 0.8 mm imidazole). The column was washed with 10 volumes of Buffer A containing 25 mm imidazole. The tagged protein was eluted with Buffer A supplemented with 200 mm imidazole. An aliquot of the collected fractions (100 μl) was analyzed by SDS-PAGE and immunoblotted with an anti-His6 monoclonal antibody (Cell Signaling). His6-Rab2 fragment enriched fractions were pooled and concentrated, and the protein concentration was determined by Micro BCA protein assay reagent (Pierce). The Rab2 amino-terminal deletion mutant was generated, as outlined previously (9).
RESULTS
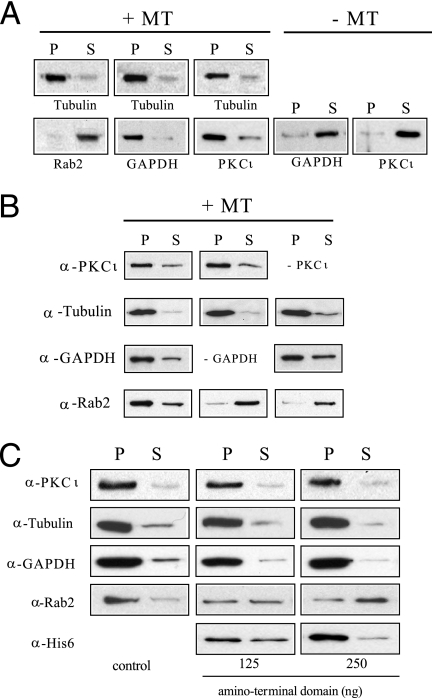
Rab2 Requires GAPDH and aPKCι to Associate with MTs—To learn if Rab2 binds directly to MTs, an in vitro MT binding assay was performed in which purified recombinant Rab2 was incubated with paclitaxel assembled MTs for 30 min at 37 °C. The binding reaction was centrifuged through a 20% sucrose cushion, and then the pellet containing MTs and associated protein and the supernatant containing unbound protein were analyzed by SDS-PAGE and Western blot. As shown in Fig. 1A, Rab2 was found almost exclusively in the high speed supernatant, which contained a negligible amount of tubulin. In contrast, when the MT binding assay was performed with purified aPKCι or with purified GAPDH, both proteins co-sedimented with MTs in the high speed pellet consistent with previous reports that showed GAPDH and aPKC interact directly with MTs (12, 13, 17, 38). Because GAPDH and aPKCι bind directly to the Rab2 amino terminus, we determined whether either protein could promote Rab2-MT association by supplementing the assay with either purified GAPDH or purified aPKCι (Fig. 1B). As observed previously, GAPDH and aPKCι independently bound to MTs, whereas Rab2 displayed no binding affinity for MTs and fractionated with the supernatant. However, Rab2 was found in the MT-enriched pellet when the MT binding assay was conducted in the presence of both GAPDH and aPKCι (Fig. 1B). Rab2 indirect association with MTs was prevented when the binding assay was supplemented with a recombinant protein fragment corresponding to the Rab2 amino terminus (residues 2-70) that directly interacts with GAPDH and aPKCι (Fig. 1C) (8, 9). The amount of Rab2 bound to MTs was reduced ∼50% in the presence of Rab2 (residues 2-70) (125 ng), and ∼70% of Rab2 was found in the high speed supernatant when co-incubated with 250 ng of the Rab2 fragment (Fig. 1C). Moreover, Rab2 (residues 2-70) was found in the GAPDH-aPKCι-MT pellet indicating that the recombinant fragment interfered with Rab2-MT binding by competition for GAPDH and aPKCι interaction. These combined results suggest that Rab2 requires and utilizes both GAPDH and aPKCι as adaptor/scaffolding proteins to indirectly associate with MTs.
FIGURE 1.
Rab2 requires GAPDH and PKCι to associate with MTs. A, pure bovine brain tubulin (1 μg) was preassembled as outlined under “Experimental Procedures” and then incubated with Rab2 (200 ng), aPKCι (200 ng), or GAPDH (200 ng) for 30 min at 37 °C. The reaction mixtures were layered onto a 20% sucrose cushion and centrifuged for 30 min at 100,000 × g. The resultant supernatant (S) and pellet (P) were separated by SDS-PAGE and transferred to nitrocellulose, and the blot was probed with anti-α/β tubulin, anti-PKCι, anti-GAPDH, or anti-Rab2. As expected based on previous reports, PKCι and GAPDH co-sedimented with MTs in the high speed pellet. In contrast, Rab2 was found almost exclusively in the high speed supernatant. The results shown are representative of three independent experiments. B, MTs were assembled from pure bovine brain tubulin as above, and then incubated with Rab2 (200 ng), with or without aPKCι, (200 ng) and with or without GAPDH (200 ng), for 30 min at 37 °C. The reaction mixtures were processed as above. The blot was probed with the indicated primary antibodies. Rab2 was only found in the MT-enriched pellet when both aPKCι and GAPDH were present in the binding reaction. The results shown are representative of three independent experiments. C, MT binding assay was performed as above with Rab2 (200 ng), aPKCι (200 ng), and purified GAPDH (200 ng) in the absence or presence Rab2 amino-terminal fragment. The amino-terminal recombinant fragment caused a dose-dependent inhibition of Rab2 association with MTs. The results shown are representative of four independent experiments.
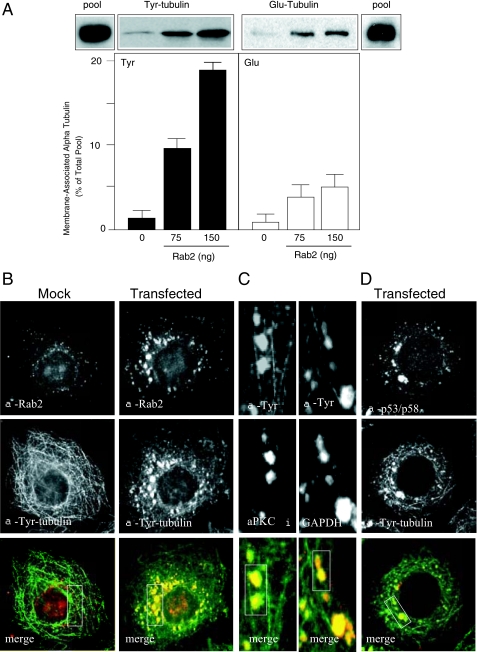
Rab2 via aPKCι Preferentially Recruits Tyrosinated α-Tubulin and Dynein to Membranes—Tubulin can undergo a variety of post-translational modifications, including removal of the tyrosine residue from the carboxyl terminus of α-tubulin and subsequent tyrosine re-addition (detyrosination/tyrosination cycle) that influences interaction with specific MAPs and motor proteins (25). It is notable that GAPDH has been reported to bind specifically to the carboxyl terminus of α-tubulin (13). Therefore, we analyzed the distribution of Rab2 recruited tyrosinated α-tubulin (Tyr-tubulin) versus detyrosinated α-tubulin (Glu-tubulin) to VTCs by performing a quantitative membrane binding assay (10, 39). Salt-washed HeLa cell membranes were incubated with rat liver cytosol and GTPγS in the absence or presence of increasing Rab2 concentrations. The reaction was then centrifuged through a 20% sucrose cushion, and the pellet containing membranes and associated proteins, including MTs, was subjected to SDS-PAGE and Western blot analysis. The relative level of Tyr- and Glu-tubulin recruited to membranes is reported as percent of total pool because of the use of two monoclonal antibodies with different binding affinities. As shown in Fig. 2A, Rab2 promoted a dose-dependent increase in membrane-bound Tyr-tubulin. From the total pool of cytosolic Tyr-tubulin added to the binding assay and therefore available for Rab2-dependent recruitment, ∼9-11% of Tyr-tubulin became membrane-associated after co-incubation with Rab2 (75 ng), and addition of a higher Rab2 concentration (150 ng) resulted in ∼17-20% increase in membrane-bound Tyr-tubulin. In contrast, ∼3-6% of Glu-Tyr was bound to Rab2-incubated membranes regardless of the Rab2 concentration.
FIGURE 2.
α-Tubulin recruited to Rab2-treated membranes is predominantly tyrosinated. A, quantitative membrane binding assay was performed as described under “Experimental Procedures.” Salt-washed HeLa microsomes were incubated with cytosol and GTPγS in the presence or absence of purified Rab2 (75 or 150 ng) for 12 min at 32 °C. The membrane pellet was subjected to SDS-PAGE and transferred to nitrocellulose, and the blot was probed with anti-Tyr-tubulin monoclonal antibody and with anti-Glu-tubulin polyclonal antibody. The pool indicates total Tyr- and Glu-tubulin present in the cytosol added to each binding reaction. Results are presented as percent of total pool for each tubulin subtype. A representative Western blot from one of five independent experiments is shown. HeLa cells were mock-transfected or transfected with Rab2 cDNA. Thirty six hours post-transfection, cells were permeabilized, fixed, and then labeled with anti-Rab2 and anti-Tyr-tubulin (B) or with anti-Tyr-tubulin and anti-p53/p58 (D), as described under “Experimental Procedures.” C, high magnification of Rab2 cDNA-transfected cells labeled with anti-Tyr-tubulin and anti-aPKCι or anti-GAPDH. Boxed areas (yellow indicates co-localization) were analyzed using the Manders overlap co-localization, as described under “Experimental Procedures” and reported under “Results.” Rab2, GAPDH, aPKCι, and p53/p58 co-distribute with Tyr-tubulin-labeled VTCs.
Because Rab2-treated membranes contained a significant amount of Tyr-tubulin, HeLa cells were transfected with Rab2 cDNA, and the distribution of Rab2 and Tyr-tubulin in vivo was analyzed by indirect immunofluorescence. Cells ectopically expressing Rab2 displayed prominent Rab2- and Tyr-tubulin-labeled pleomorphic elements (boxed area; Manders coefficient of 0.942) that were primarily juxtaposed to the nucleus as well as smaller Tyr-tubulin-labeled punctate structures dispersed throughout the cell periphery (Fig. 2B). These Tyr-tubulin/Rab2-containing entities did not stain with anti-Glu-tubulin antibody (data not shown) but did co-distribute with GAPDH (boxed area; Manders coefficient of 0.921) and aPKCι (boxed area; Manders coefficient of 0.925) (Fig. 2C). Importantly, the Tyr-tubulin/Rab2 structures co-labeled with anti-p53/p58 (boxed area; Manders coefficient of 0.928) demonstrating that the pleomorphic elements are VTCs and not tubulin aggregates (Fig. 2D). In that regard, we observed in Rab2-overexpressing cells VTCs associated with and aligned with long MT tracks (Fig. 2C).
Rab2 recruits GAPDH upstream of aPKCι, and aPKCι membrane association requires GAPDH. Interestingly, increasing amounts of GAPDH added to the binding assay did not significantly increase the level of membrane-associated Tyr-tubulin, indicating that GAPDH is not a limiting factor (data not shown). However, the ability of Rab2 to promote Tyr-tubulin membrane binding was eliminated when purified recombinant Rab2 N′Δ19, a mutant protein that fails to recruit aPKCι to membranes, was added to the assay (8) (Fig. 3A). This truncation in Rab2 reduces association with GAPDH but does not abolish Rab2-GAPDH interaction (9). To learn whether aPKCι played a specific role in Tyr-tubulin recruitment, the membrane binding assay was supplemented with purified recombinant aPKCι. In this case and unlike GAPDH, increasing amounts of membrane-associated aPKCι caused ∼5-8-fold increase in membrane-bound Tyr-tubulin and a minimal increase (∼1-1.5-fold) in the amount of membrane-bound Glu-tubulin (Fig. 3B). The putative role of aPKCι in Tyr-tubulin binding was further assessed in the binding reaction by adding anti-aPKCι. Fig. 3C shows that increasing amounts of anti-aPKCι reduced membrane association of aPKCι and Tyr-tubulin; however, anti-PKCι had no effect on Glu-tubulin recruitment. These combined biochemical results are highly suggestive that membrane-bound aPKCι influences dynamic MTs enriched in Tyr-tubulin to associate with VTCs.
FIGURE 3.
aPKCι is required for Tyr-tubulin membrane association. A, quantitative membrane binding assay was performed as described under “Experimental Procedures.” Salt-washed HeLa microsomes were incubated without or with 75 or 150 ng of purified recombinant Rab2 N′Δ19, cytosol, and GTPγS for 12 min at 32 °C, as described under “Experimental Procedures.” The membrane pellet was subjected to SDS-PAGE and transferred to nitrocellulose, and the blot was probed with anti-Tyr-tubulin. A representative Western blot from one of three independent experiments is shown. cont, control. B, salt-washed HeLa microsomes were incubated without or with 100 or 200 ng of purified recombinant aPKCι and incubated as above. The membrane pellet was processed as above, and the blot was probed with anti-Tyr-tubulin and with anti-Glu-tubulin. The pool indicates total Tyr- and Glu-tubulin present in the cytosol added to each binding reaction. Results are presented as percent of total pool for each tubulin subtype. A representative Western blot from one of three independent experiments is shown. C, salt-washed HeLa microsomes were incubated without or with 150 ng of purified recombinant Rab2, cytosol, and GTPγS in the presence or absence of increasing concentrations of anti-PKCι for 12 min at 32 °C. The membrane pellet was processed as above, and the blot was probed with the specific and indicated primary antibodies. A representative Western blot from one of three independent experiments is shown.
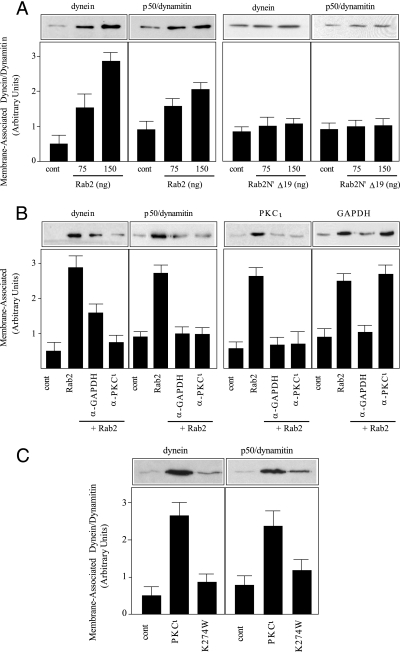
Rab proteins regulate MT motor protein recruitment, and MT motor proteins preferentially bind to either Glu or Tyr-MTs (22, 33, 34, 40, 41). Therefore, we examined whether Rab2 recruited a motor protein to membranes in the binding assay. Although kinesin has been reported to be present on the intermediate compartment (42-44), we did not detect any kinesin or any Rab2-recruited kinesin on the membranes (data not shown). In contrast, Rab2 promoted a dose-dependent increase in membrane-associated dynein as determined by probing the blot with anti-dynein intermediate chain (Fig. 4A). Similarly, Rab2 stimulated membrane association of p50/dynamitin, a component of the dynactin complex (Fig. 4A). However, addition of Rab2 N′Δ19 to the assay failed to increase dynein or p50/dynamitin binding to membranes suggesting that aPKCι was necessary for their downstream recruitment (Fig. 4A). To investigate the potential role of aPKCι in dynein/dynactin recruitment, anti-GAPDH or anti-aPKCι was added to the membrane binding assay. The anti-GAPDH reagent blocked membrane association of GAPDH, and consequently aPKCι and dynein/dynamitin, whereas anti-PKCι inhibited aPKCι and dynein/dynamitin membrane binding but had no effect on GAPDH recruitment (Fig. 4B). Because aPKCι-dependent GAPDH phosphorylation plays a role in MT binding to membranes, we determined whether aPKCι kinase activity was required for dynein/dynamitin recruitment by incubating membranes with kinase-dead PKCι (K274W) (36). As we observed for MTs, aPKCι kinase activity was necessary because supplementing the membrane binding assay with PKCι (K274W) failed to stimulate Rab2-dependent dynein/dynamitin recruitment (Fig. 4C).
FIGURE 4.
Rab2-recruited aPKCι is required for dynein binding to membranes. A, quantitative membrane binding assay was performed as described under “Experimental Procedures.” Salt-washed HeLa microsomes were incubated without or with 75 or 150 ng of purified recombinant Rab2 or purified recombinant Rab2N′Δ19, cytosol, and GTPγS for 12 min at 32 °C. The membrane pellet was subjected to SDS-PAGE and transferred to nitrocellulose, and the blot was probed with anti-dynein and with anti-p50/dynamitin. A representative Western blot from one of three independent experiments is shown. cont, control. B, salt-washed HeLa microsomes were incubated without or with 175 ng of purified recombinant Rab2, cytosol, and GTPγS in the presence or absence of anti-GAPDH (2.5 μg) or anti-PKCι (2.5 μg) for 12 min at 32 °C. The membrane pellet was processed as above, and the blot was probed with the specific and indicated primary antibodies. A representative Western blot from one of three independent experiments is shown. C, salt-washed HeLa microsomes were incubated without or with 150 ng of purified recombinant Rab2, cytosol, and GTPγS in the presence or absence of purified recombinant aPKCι (200 ng) or purified recombinant aPKCι (Lys-274) (200 ng) for 12 min at 32 °C. The membrane pellet was processed as above, and the blot was probed with the specific and indicated primary antibodies. A representative Western blot from one of three independent experiments is shown.
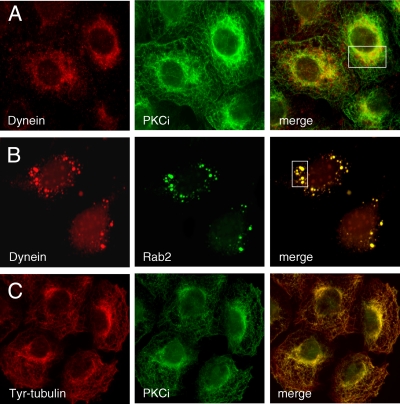
This apparent biochemical relationship between aPKCι and dynein was evaluated in vivo by indirect immunofluorescence microscopy. In interphase HeLa cells overexpressing aPKCι, the anti-dynein antibody predominantly labeled perinuclear pleomorphic structures that co-stained with anti-aPKCι (boxed area; Manders coefficient of 0.959) as well as small vesicles scattered throughout the cytosol (Fig. 5A). In addition to the juxtanuclear position, aPKCι displayed a MT-like distribution that was evident in cells co-labeled for aPKCι and Tyr-MTs (Fig. 5C). As expected based on the biochemical results, dynein was also found to co-distribute with Rab2 on large vesicular elements (boxed area; Manders coefficient of 0.951) that were concentrated near the Golgi region in HeLa cells overexpressing Rab2 (Fig. 5B).
FIGURE 5.
Rab2 and aPKCι co-distribute with dynein. HeLa cells were transfected with either Rab2 cDNA or PKCι cDNA, as described under “Experimental Procedures.” aPKCι overexpressing cells were permeabilized, fixed, and then co-stained with anti-dynein (intermediate chain) and fluorescein isothiocyanate-anti-Xpress (A) or fluorescein isothiocyanate anti-Xpress and anti-Tyr-tubulin (C). B, Rab2 overexpressing cells processed as above were co-labeled with anti-dynein (intermediate chain) and anti-Rab2. Boxed areas (yellow indicates co-localization) were analyzed using the Manders overlap co-localization, as described under “Experimental Procedures” and reported under “Results.” Both Rab2 and aPKCι co-distribute with dynein in perinuclear pleomorphic entities.
DISCUSSION
Microtubules undergo dynamic alterations in organization and distribution during various cellular events, including mitosis and cell migration. Although MT assembly does not appear to be directly regulated by Rab proteins, these small GTPases provide a molecular link between vesicle cargo, motor proteins, and the cytoskeleton (22, 30). Indeed, by using an in vitro MT binding assay, we found that Rab2 can only associate with MTs when both GAPDH and aPKCι are present despite Rab2 directly interacting with GAPDH and aPKCι and GAPDH and aPKCι binding directly and independently to MTs. A Rab2 amino-terminal recombinant fragment corresponding to the first 70 residues can disrupt the indirect interaction of Rab2 with MTs. Because GAPDH and aPKCι associate with Rab2 via residues 1-50, it was not surprising that the recombinant fragment competitively inhibited Rab2-GAPDH-aPKCι-MT association in the MT binding assay. The Rab2 amino-terminal domain like Rab2 promotes GAPDH and aPKCι recruitment to membranes (8, 45). However, the peptide fragment does not promote vesicle formation indicating that the intact protein is required for full Rab2 activity at the VTC (7).
Our finding that GAPDH promotes Rab2 interaction with MTs is reminiscent of the role of GAPDH in facilitating MT interaction with the EF-hand Ca2+-binding protein p22, which like Rab2 is required for membrane traffic (46, 47). GAPDH was the first glycolytic enzyme found associated with tubulin and binds to the carboxyl terminus of α-tubulin (12, 13). MT assembly is not influenced by GAPDH; however, the enzyme is known to modulate the cytoskeleton by promoting MT bundling and cross-linking (12, 14, 38). This alteration in the spatial organization of the intracellular milieu could have profound consequences on organelle position and cargo transport by selectively bundling parallel or anti-parallel MTs and thereby providing a directional cue. Indeed, we observed VTCs containing Rab2-GAPDH-aPKCι associated with and aligned with MTs enriched in Tyr-tubulin in HeLa cells overexpressing Rab2. Additionally/alternatively, MT bundles can function as supportive entities that provide structural organization within the cell (15). This potential role of MT bundles at the VTC is also consistent with the finding of Ben-Tekaya et al. that the endoplasmic reticulum-Golgi intermediate compartment is a stationary compartment and not a collection of mobile carriers (48). In either case, many proteins reported to bundle or to cross-link MTs are immunologically related to MAPs or associate with MAPs (15). For example, it was recently reported that GAPDH binds to the heavy chain of MAP1b in adult brain (49). Likewise, GAPDH was shown to co-immunoprecipitate with the MT-binding protein Tau located in neurofibrillary tangles (50). Unlike brain tissue, we did not detect in the rat liver cytosol used in our assays Tau, MAP1b, or any immunologically related proteins to Tau and MAP1b recruited to membranes by Rab2 (data not shown). The role of GAPDH in association with MAPs is unknown; however, the suggestion that MAP-GAPDH interaction could ultimately provide ATP for cytoskeleton regulation (49) is contrary to our results that showed GAPDH glycolytic activity is not required for ER to Golgi complex transport (9).
The results reported here are consistent with the previous findings that aPKCι interacts with MTs (17, 18). Although aPKCι can bind to MTs in vitro independently of GAPDH, the presence of Src-dependent tyrosine-phosphorylated GAPDH on the VTC as well as Src phosphorylation of a PKCι is required for aPKCι association with those membranes indicating multiple levels of upstream regulation (10, 39). We have proposed that Rab2-Src-aPKCι-GAPDH are components of a signaling complex that defines and regulates the site of vesicle budding from VTCs in association with MT-directed vesicle movement to the acceptor compartment (39). This interpretation is compatible with other studies indicating that aPKC influences MT dynamics. For example, aPKC has been reported to control adherens junction symmetry by regulating MT organization (20). Additionally, aPKC regulates the synaptic skeleton in Drosophila by facilitating association of the MAP1B-related protein Futsch to MTs (19). Furthermore, aPKC acts in concert with Par3 and Par6 where it localizes to cilia and is required for MT organization during ciliogenesis (51). Interestingly, aPKCι like GAPDH has been found in Tau-positive neurofibrillary inclusions (52). Moreover, Tau overexpression decreases Glu-tubulin levels and causes a concomitant increase in Tyr-tubulin (53). It may not be coincidental that HeLa cells overexpressing Rab2 contain VTCs enriched in Tyr-tubulin.
α-Tubulin is synthesized with a carboxyl-terminal tyrosine. The residue is removed by a tubulin tyrosine carboxypeptidase and enzymatically re-added to the carboxyl-terminal glutamate by tubulin tyrosine ligase in what constitutes a cycle of detyrosination/tyrosination (54). Tubulin tyrosine carboxypeptidase acts preferentially on assembled MTs, and phosphorylation may regulate tubulin tyrosine carboxypeptidase-tubulin interaction suggesting a potential role for protein kinase(s) (55, 56). Although numerous in vivo and in vitro studies have been performed to address the role of α-tubulin tyrosination/detyrosination, the specific function has not been elucidated (54). Because Tyr-MTs are more dynamic with a t½ of 10 min compared with Glu-MTs that have a t½ of 1 h, it has been proposed that Glu-tubulin imparts MT stability (57, 58). However, recent studies indicate that the detyrosination/tyrosination cycle differentially affects recruitment of MT-binding proteins and molecular motors to MTs, which as a consequence influences MT stability and motility. In that regard, kinesin-1 preferentially binds to Glu-MTs, whereas p150 glued/dynactin and +TIPs prefer Tyr-MTs (28, 59, 60).
Dynactin is a multisubunit protein complex containing p50/dynamitin that binds directly to and is required for cytoplasmic dynein motile function and for membrane association (61). Live cell imaging studies have demonstrated that overexpression of p50/dynamitin blocks ER to Golgi transport (62). There are two forms of cytoplasmic dynein, cytoplasmic dynein 1 and cytoplasmic dynein 2. Cytoplasmic dynein 1 is found in all MT-containing cells and has been shown to co-localize with p53/p58 and β-COP in cells incubated at 15 °C to accumulate VTCs (43, 63). We found that Rab2 recruited dynein and p50/dynamitin to membranes in the quantitative membrane binding assay. Additionally, HeLa cells overexpressing Rab2 displayed large perinuclear pleomorphic structures that co-labeled with Rab2 and dynein. The Rab2 downstream effector aPKCι appears to be a limiting factor for dynein/dynactin association with membranes because interfering with aPKCι membrane binding and aPKCι kinase activity concomitantly decrease membrane-bound dynein and p50/dynamitin. Harris and Peifer (20) have also found that aPKC regulates dynein association with the adherens junction in a Drosophila aPKC mutant. This relationship between aPKCι and dynein was further evidenced in HeLa cells overexpressing PKCι that displayed extensive co-distribution of the kinase with dynein in the Golgi region. The specific recruitment of dynein by Rab2 versus the motor protein kinesin would preferentially promote Tyr-MTs binding to subdomains of the VTC engaged in protein sorting and recycling. Indeed, we found that membranes treated with Rab2 contained more Tyr-tubulin (∼3-fold) than Glu-tubulin.
Rab2 joins the list of other Rab proteins reported to utilize dynein, including Rab4 (40) and Rab6 (33, 41). Interestingly, the phylogenetic tree of the Ras superfamily shows that Rab2 is most closely related to Rab4 (64), and both Rab4 and Rab6 are involved in recycling/retrograde transport (65, 66). Although we have found dynein on Rab2-generated vesicles (data not shown), it is possible that dynein motor function is inactive and recycles back to the ER on Rab2 vesicles for use in COPII vesicle transport (26). Alternatively, because dynein is a minus-end directed motor, it could be that Rab2 retrograde-directed vesicles use Tyr-MTs derived from the ER as tracks for retrieval to the ER. Studies are in progress to delineate between these two possibilities.
This work was supported, in whole or in part, by National Institutes of Health Grant GM068813 (to E. J. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VTC, vesicular tubular cluster; ER, endoplasmic reticulum; aPKCι, atypical protein kinase C iota; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MAPs, microtubule associated proteins; MTs, microtubules; Tyr-tubulin, tyrosinated α-tubulin; Glu-tubulin, detyrosinated tubulin; GTPγS, guanosine 5′-3-O-(thio)triphosphate; PBS, phosphate-buffered saline; Pipes, 1,4-piperazinediethanesulfonic acid.
References
- 1.Tisdale, E. J., Bourne, J. R., Khosravi-Far, R., Der, C. J., and Balch, W. E. (1992) J. Cell Biol. 119 749-761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisdale, E. J., and Balch, W. E. (1996) J. Biol. Chem. 271 29372-29379 [DOI] [PubMed] [Google Scholar]
- 3.Balch, W. E., McCaffery, J. M., Plutner, H., and Farquhar, M. G. (1994) Cell 76 841-852 [DOI] [PubMed] [Google Scholar]
- 4.Altan-Bonnet, N., Sougrat, R., and Lippincott-Schwartz, J. (2004) Curr. Opin. Cell Biol. 16 364-372 [DOI] [PubMed] [Google Scholar]
- 5.Lee, M. C. S., Miller, E. A., Goldberg, J., Orci, L., and Schekman, R. (2004) Annu. Rev. Cell Dev. Biol. 20 87-123 [DOI] [PubMed] [Google Scholar]
- 6.Appenzeller-Herzog, C., and Hauri, H. P. (2006) J. Cell Sci. 119 2173-2183 [DOI] [PubMed] [Google Scholar]
- 7.Tisdale, E. J. (1999) Mol. Biol. Cell 10 1837-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tisdale, E. J. (2003) J. Biol. Chem. 278 52524-52530 [DOI] [PubMed] [Google Scholar]
- 9.Tisdale, E. J., Kelly, C., and Artalejo, C. R. (2004) J. Biol. Chem. 279 54046-54052 [DOI] [PubMed] [Google Scholar]
- 10.Tisdale, E. J., and Artalejo, C. R. (2007) Traffic 8 733-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisdale, E. J. (2002) J. Biol. Chem. 277 3334-3341 [DOI] [PubMed] [Google Scholar]
- 12.Kumagai, H., and Sakai, H. (1983) J. Biochem. (Tokyo) 93 1259-1269 [DOI] [PubMed] [Google Scholar]
- 13.Volker, K. W., and Knull, H. R. (1997) Arch. Biochem. Biophys. 338 237-243 [DOI] [PubMed] [Google Scholar]
- 14.Somers, M., Engelborghs, Y., and Baert, J. (1990) Eur. J. Biochem. 193 437-444 [DOI] [PubMed] [Google Scholar]
- 15.MacRae, T. H. (1992) Biochim. Biophys. Acta 1160 145-155 [DOI] [PubMed] [Google Scholar]
- 16.Glaser, P., Han, X., and Gross, R. W. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 14104-14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Rocha, M., Avila, J., and Lozano, J. (1997) Exp. Cell Res. 230 1-8 [DOI] [PubMed] [Google Scholar]
- 18.Seibenhener, M. L., Roehm, J., White, W., Neidigh, K., Vandenplas, M., and Wooten, M. W. (1999) Mol. Cell Biol. Res. Commun. 2 28-31 [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Canada, C., Ashley, J., Moeckel-Cole, S., Drier, E., Yin, J., and Budnik, V. (2004) Neuron 42 567-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, T., and Peifer, M. (2007) Dev. Cell 12 727-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etienne-Manneville, S., and Hall, A. (2003) Curr. Opin. Cell Biol. 15 67-72 [DOI] [PubMed] [Google Scholar]
- 22.Caviston, J., and Holzbaur, E. (2006) Trends Cell Biol. 16 530-537 [DOI] [PubMed] [Google Scholar]
- 23.Wade, R. H. (2007) Methods Mol. Med. 137 1-16 [DOI] [PubMed] [Google Scholar]
- 24.Verhey, K., and Gaertig, J. (2007) Cell Cycle 6 2152-2160 [DOI] [PubMed] [Google Scholar]
- 25.Hammond, J., Cai, D., and Verhey, K. (2007) Curr. Opin. Cell Biol. 19 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson, P., Forster, R., Palmer, K., Pepperkok, R., and Stephens, D. (2004) Nat. Cell Biol. 7 48-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honnappa, S., Okhrimenko, O., Jaussi, R., Jawhari, H., Jeselarov, I., Winkler, F., and Steinmetz, M. (2006) Mol. Cell 23 663-671 [DOI] [PubMed] [Google Scholar]
- 28.Peris, L., Thery, M., Faure, J., Saoudi, Y., Lafanechere, L., Chilton, J., Gordon-Weeks, P., Galijart, N., Bornens, M., Wordeman, L., Wehland, J., Andrieux, A., and Job, D. (2007) J. Cell Biol. 174 839-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisbrich, A., Honnappa, S., Jaussi, R., Okhrimenko, O., Frey, D., Jelesarov, I., Akhmanova, A., and Steinmetz, M. (2007) Nat. Struct. Mol. Biol. 14 959-967 [DOI] [PubMed] [Google Scholar]
- 30.Ross, J., Ali, M. Y., and Warshaw, D. (2008) Curr. Opin. Cell Biol. 20 41-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross, S., Vershinin, M., and Shubeita, G. (2007) Curr. Biol. 17 R478-R486 [DOI] [PubMed] [Google Scholar]
- 32.Echard, A., Jollivet, F., Martinez, O., Lacapere, J. J., Rousselet, A., Janoueix-Lerosey, I., and Goud, B. (1998) Science 279 580-585 [DOI] [PubMed] [Google Scholar]
- 33.Short, B., Preisinger, C., Schaletzky, J., Kopajtich, R., and Barr, F. (2002) Curr. Biol. 12 1792-1795 [DOI] [PubMed] [Google Scholar]
- 34.Hoepfner, S., Severin, F., Cabezas, A., Habermann, B., Runge, A., Gillooly, D., Stenmark, H., and Zerial, M. (2005) Cell 121 437-450 [DOI] [PubMed] [Google Scholar]
- 35.Tisdale, E. J., and Jackson, M. R. (1998) J. Biol. Chem. 273 17269-17277 [DOI] [PubMed] [Google Scholar]
- 36.Tisdale, E. J. (2000) Traffic 1 702-712 [DOI] [PubMed] [Google Scholar]
- 37.Tisdale, E. J., Plutner, H., Matteson, J., and Balch, W. E. (1997) J. Cell Biol. 137 581-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volker, K. W., Reinitz, C. A., and Knull, H. R. (1995) Comp. Biochem. Physiol. B 112 503-514 [DOI] [PubMed] [Google Scholar]
- 39.Tisdale, E. J., and Artalejo, C. R. (2006) J. Biol. Chem. 281 8436-8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bielli, A., Thornqvist, P. O., Hendrick, A., Finn, R., Fitzgerald, K., and McCaffrey, M. (2001) Biochem. Biophys. Res. Commun. 281 1141-1153 [DOI] [PubMed] [Google Scholar]
- 41.Wanschers, B., van de Vorstenbosch, R., Wijers, M., Wieringa, B., King, S., and Fransen, J. (2007) Cell Motil. Cytoskeleton 65 183-196 [DOI] [PubMed] [Google Scholar]
- 42.Lippincott-Schwartz, J., Cole, N., Marotta, A., Conrad, P., and Bloom, G. S. (1995) J. Cell Biol. 128 293-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roghi, C., and Allan, V. J. (1999) J. Cell Sci. 112 4673-4685 [DOI] [PubMed] [Google Scholar]
- 44.Stauber, T., Simpson, J., Pepperkok, R., and Vernos, I. (2006) Curr. Biol. 16 2245-2251 [DOI] [PubMed] [Google Scholar]
- 45.Tisdale, E. J. (2001) J. Biol. Chem. 276 2480-2486 [DOI] [PubMed] [Google Scholar]
- 46.Andrade, J., Pearce, S., Zhao, H., and Barroso, M. (2004) Biochem. J. 384 327-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barroso, M., Bernd, K., DeWitt, N., Chang, A., Mills, K., and Sztul, E. (1996) J. Biol. Chem. 271 10183-10187 [DOI] [PubMed] [Google Scholar]
- 48.Ben-Tekaya, H., Miura, K., Pepperkok, R., and Hauri, H. P. (2004) J. Cell Sci. 118 357-367 [DOI] [PubMed] [Google Scholar]
- 49.Cueille, N., Tallichet Blanc, C., Riederer, I., and Riederer, B. (2007) J. Proteome Res. 6 2640-2647 [DOI] [PubMed] [Google Scholar]
- 50.Wang, Q., Woltjer, R., Pan, C., Montine, K., Zhang, J., and Montine, T. (2005) FASEB J. 19 869-871 [DOI] [PubMed] [Google Scholar]
- 51.Fan, S., Hurd, T., Liu, C.-J., Straight, S., Weimbs, T., Hurd, E. A., and Margolis, B. (2004) Curr. Biol. 14 1451-1461 [DOI] [PubMed] [Google Scholar]
- 52.Shao, C., Crary, J., Rao, C., Sacktor, T., and Mirra, S. (2006) J. Neuropathol. Exp. Neurol. 65 327-335 [DOI] [PubMed] [Google Scholar]
- 53.Yoshiyama, Y., Zhang, B., Bruce, J., Trojanowski, J., and Lee, V. (2003) J. Neurosci. 23 10622-10671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idriss, H. (2000) Cell Motil. Cytoskeleton 200 173-184 [DOI] [PubMed] [Google Scholar]
- 55.Arce, C., and Barra, H. (1985) Biochem. J. 226 311-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sironi, J., Barra, H., and Arce, C. (1997) Mol. Cell. Biochem. 170 9-16 [DOI] [PubMed] [Google Scholar]
- 57.Kreis, T. (1987) EMBO J. 6 2597-2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster, D., Gundersen, G., Bulinski, J., and Borisy, G. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 9040-9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao, G., and Gundersen, G. (1998) J. Biol. Chem. 273 9797-9803 [DOI] [PubMed] [Google Scholar]
- 60.Lin, S. X., Gundersen, G., and Maxfield, F. (2002) Mol. Biol. Cell 13 96-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroer, T. (2004) Annu. Rev. Cell Dev. Biol., 20 759-779 [DOI] [PubMed] [Google Scholar]
- 62.Presley, J., Cole, N., Schroer, T., Hirschberg, K., Zaal, K., and Lippincott-Schwartz, J. (1997) Nature 389 81-85 [DOI] [PubMed] [Google Scholar]
- 63.Hook, P., and Vallee, R. (2006) J. Cell Sci. 119 4369-4371 [DOI] [PubMed] [Google Scholar]
- 64.Pereira-Leal, J., and Seabra, M. (2000) J. Mol. Biol. 301 1077-1087 [DOI] [PubMed] [Google Scholar]
- 65.McCaffrey, M., Bielli, A., Cantalupo, G., Mora, S., Roberti, V., Santillo, M., Drummond, F., and Bucci, C. (2001) FEBS Lett. 495 21-30 [DOI] [PubMed] [Google Scholar]
- 66.White, J., Johannes, L., Mallard, F., Girod, A., Grill, S., Reinsch, S., Keller, P., Tzschaschel, B., Echard, A., Goud, B., and Stelzer, E. H. (1999) J. Cell Biol. 147 743-760 [DOI] [PMC free article] [PubMed] [Google Scholar]