Abstract
Objective To investigate the association between treatment induced change in high density lipoprotein cholesterol and total death, coronary heart disease death, and coronary heart disease events (coronary heart disease death and non-fatal myocardial infarction) adjusted for changes in low density lipoprotein cholesterol and drug class in randomised trials of lipid modifying interventions.
Design Systematic review and meta-regression analysis of randomised controlled trials.
Data sources Medline, Embase, Central, CINAHL, and AMED to October 2006 supplemented by contact with experts in the field.
Study selection In teams of two, reviewers independently determined eligibility of randomised trials that tested lipid modifying interventions to reduce cardiovascular risk, reported high density lipoprotein cholesterol and mortality or myocardial infarctions separately for treatment groups, and treated and followed participants for at least six months.
Data extraction and synthesis Using standardised, pre-piloted forms, reviewers independently extracted relevant information from each article. The change in lipid concentrations for each trial and the weighted risk ratios for clinical outcomes were calculated.
Results The meta-regression analysis included 108 randomised trials involving 299 310 participants at risk of cardiovascular events. All analyses that adjusted for changes in low density lipoprotein cholesterol showed no association between treatment induced change in high density lipoprotein cholesterol and risk ratios for coronary heart disease deaths, coronary heart disease events, or total deaths. With all trials included, change in high density lipoprotein cholesterol explained almost no variability (<1%) in any of the outcomes. The change in the quotient of low density lipoprotein cholesterol and high density lipoprotein cholesterol did not explain more of the variability in any of the outcomes than did the change in low density lipoprotein cholesterol alone. For a 10 mg/dl (0.26 mmol/l) reduction in low density lipoprotein cholesterol, the relative risk reduction was 7.2% (95% confidence interval 3.1% to 11%; P=0.001) for coronary heart disease deaths, 7.1% (4.5% to 9.8%; P<0.001) for coronary heart disease events, and 4.4% (1.6% to 7.2%; P=0.002) for total deaths, when adjusted for change in high density lipoprotein cholesterol and drug class.
Conclusions Available data suggest that simply increasing the amount of circulating high density lipoprotein cholesterol does not reduce the risk of coronary heart disease events, coronary heart disease deaths, or total deaths. The results support reduction in low density lipoprotein cholesterol as the primary goal for lipid modifying interventions.
Introduction
Large cohort studies have identified high density lipoprotein cholesterol as a strong, independent, inverse predictor of risk of coronary heart disease.1 2 3 4 Although the inverse relation seems not to apply to particular subgroups of patients with genetic variations, such as ABCA1 or cholesteryl ester transfer protein mutations leading to abnormal low or high levels of high density lipoprotein cholesterol,5 6 the National Cholesterol Education Program has recognised high density lipoprotein cholesterol as an independent cardiovascular risk factor and recommended screening measurements of high density lipoprotein cholesterol for all adults.7 8 9
The association between high density lipoprotein cholesterol concentrations and coronary heart disease in observational studies does not, however, establish the extent to which changes in high density lipoprotein cholesterol will alter the risk of coronary heart disease events. Many large randomised trials and meta-analyses led to the identification of low density lipoprotein cholesterol as the principal target for lipid modifying interventions.8 9 Evidence that raising high density lipoprotein cholesterol will reduce cardiovascular adverse outcomes remains controversial.
Clinical trials of the high density lipoprotein raising agent niacin have shown a reduction in coronary events, but these trials either did not measure change in high density lipoprotein cholesterol or failed to include analyses adjusted for changes in low density lipoprotein cholesterol.10 11 12 13 14 Sub-studies of two trials using the fibrate gemfibrozil suggested that an increase in high density lipoprotein cholesterol reduces the risk of coronary heart disease.15 16 In the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), a multivariable analysis adjusting for several coronary heart disease risk factors and on-treatment low density lipoprotein cholesterol and triglycerides suggested an independent association of increased high density lipoprotein cholesterol with reduction in coronary heart disease death and non-fatal myocardial infarction. However, the VA-HIT investigators also found that on-treatment lipid concentrations as variables in a multivariable model explained only a small amount of the beneficial effect of gemfibrozil.15 New approaches to increase high density lipoprotein cholesterol by the cholesteryl ester transfer protein inhibitor torcetrapib or by infusion of reconstituted high density lipoprotein failed to show beneficial effects.17 18 19 20 As part of a systematic review of lipid trials published up to 2001, an analysis focusing on 19 statin trials failed to establish a statistically significant association between changes in high density lipoprotein cholesterol and relative risk reductions for patient important outcomes.21
We used meta-regression techniques in an updated, more comprehensive systematic review of randomised trials to explore an independent link between changes in high density lipoprotein cholesterol, not limited to statins but covering all lipid modifying treatment, and coronary heart disease related morbidity and mortality.
Methods
Data sources and searches
We included studies if they compared any lipid modifying agent or diet with placebo or usual care or compared a more intensive with a less intensive lipid modifying treatment; targeted reduction in cardiovascular risk; had a randomised control design; reported mortality or myocardial infarctions separately for treatment groups; and followed patients for at least six months. We excluded studies that failed to report either change from baseline or follow-up concentrations of high density lipoprotein cholesterol and low density lipoprotein cholesterol and studies for which critics have raised serious questions about the integrity of the data.22 23
We built our search for relevant studies on the sensitive search strategies used in previous systematic reviews supplemented with relevant keywords and medical subject headings.21 24 25 26 27 28 To update this evidence, MB and NB (an experienced librarian) searched Medline, Embase, and Central (all from January 2003 to October 2006) in addition to CINAHL and AMED (both from their inception to October 2006). We used Cochrane sensitive search strategies for identifying randomised trials and made no restriction as to language. The detailed search strategy is available from the authors. We reviewed reference lists of eligible articles, recently published editorials, and reviews on the topic and consulted with experts.
Study selection and quality assessment
Investigators trained in research methods worked in pairs and independently reviewed potentially eligible titles and abstracts. If either reviewer believed that the study might be eligible, we obtained the full report. Two investigators then independently assessed the eligibility of each article by using a pilot tested, standardised form with written instructions. We did calibration exercises to enhance consistency among review teams before using the forms for eligibility assessment and data extraction. Any disagreement was resolved by consensus or third party arbitration (MB). We used Cohen’s κ to measure agreement beyond chance between reviewers.29 Two reviewers independently assessed the methodological quality of each eligible study by using the following criteria: concealment of allocation; blinding of patients, caregivers, or clinical outcome assessors; adherence to the intention to treat principle; stopping early for benefit; and the proportion of patients lost to follow-up.30
Data extraction and end points
Two investigators independently extracted all relevant information on baseline characteristics of trials, patient populations, interventions, and outcomes from each eligible article by using standardised, pre-piloted data extraction forms. We recorded all available baseline and follow-up concentrations of total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and triglycerides. In five trials that failed to report exact lipid concentrations, we abstracted data from graphs. Clinical end points were total deaths, coronary heart disease deaths, and coronary heart disease events (combined outcome of non-fatal myocardial infarction and coronary heart disease death). For coronary heart disease death, we extracted data by using the following hierarchy: coronary heart disease death, fatal myocardial infarction and sudden death, fatal myocardial infarction, fatal cardiac events, fatal cardiovascular events.
Data synthesis and analysis
We report means and standard deviations of lipid concentrations as milligrams per decilitre; we used the method of Hozo to convert data reported as median and range,31 and we followed the recommendations of the Cochrane methods group for data reported as median and 25th and 75th centiles.32 We calculated change in lipid concentrations for each trial as the difference between the mean change in the lipid concentration from baseline to the average follow-up concentrations in the intervention and control groups. We pooled treatment effects across studies by calculating inverse variance weighted average risk ratios and associated 95% confidence intervals for specified outcomes by using a random effects model.
We used inverse variance weighted meta-regression analysis to investigate the association between differences in the change in high density lipoprotein cholesterol and low density lipoprotein cholesterol concentrations between treatment and control groups and the risk ratios of clinical outcomes of interest.33 34 To take into account non-lipid effects of specific drugs (such as potential pro-thrombotic effects of hormone therapy), we included a categorical variable of drug class in the meta-regression model and did a meta-regression analysis stratified by drug class. We used R2 to measure the proportion of the variability in the log risk ratio of an outcome explained by the statistical model. We analysed residuals to check model assumptions. To determine whether the potential relation between treatment induced changes in high density lipoprotein cholesterol and risk ratios for coronary heart disease events varied across drug classes, we tested for interactions between change in high density lipoprotein cholesterol and different classes of interventions by means of an F test. We found little evidence for interaction (P=0.73). That is, our analysis provided no support for the hypothesis that increase in high density lipoprotein cholesterol had a greater effect on patient important outcomes with some drugs than with others. Therefore, we omitted interaction terms to simplify the final model. Analyses with percentage change in lipid subfractions yielded similar results to analyses with absolute change (mg/dl) and are therefore not reported.
In pre-specified sensitivity analyses, we focused on a more homogeneous sample of trials that used interventions known to raise high density lipoprotein cholesterol concentrations (rather than reducing triglycerides) and therefore excluded trials using n-3 fatty acids, low fat diets, or probucol, as well as trials focusing on patients with renal failure. In addition, we excluded trials with agents that are associated with harmful effects such as torcetrapib or hormones. We did further pre-specified sensitivity analyses excluding trials with one year or less of follow-up and excluding trials with two years or less of follow-up, as lipid effects may take more than a year to fully translate into clinical effects.35 36 In response to reviewers’ comments, we added a sensitivity analysis focusing on trials with interventions specifically chosen to raise high density lipoprotein cholesterol (fibrates and niacin combinations) and another focusing on trials that reported intention to treat results. Finally, we investigated the association of treatment induced change in triglycerides concentrations with clinical outcomes by using the same approach as for high density lipoprotein cholesterol. We used SAS version 9.1 for analyses. We set the threshold for statistical significance at P<0.05.
Results
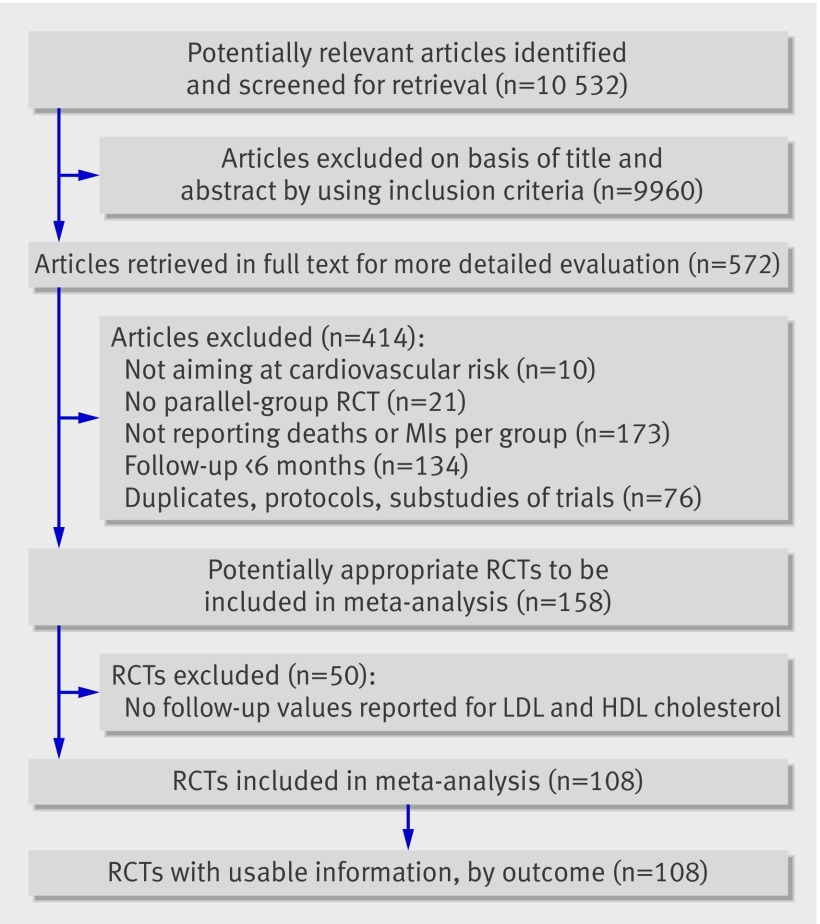
Of 158 eligible randomised controlled trials, 50 did not report change or follow-up values for both high density lipoprotein cholesterol and low density lipoprotein cholesterol and were therefore excluded, leaving 108 trials for analysis (figure). Three of these 108 trials had multiple treatment arms, so we used the control group for comparison against all treatment arms. In total, 146 890 participants were included in the intervention groups and 152 420 in the control groups. The web appendix summarises the methodological quality assessment of included trials and characteristics of patient populations. On the basis of pharmacological characteristics, we classified trials according to the following classes of intervention37: statins (54 trials comparing statins with placebo or usual carew1-w54 and eight trials comparing more intensive with less intensive statin treatmentw55-w62); fibrates (nine trialsw63-w71); resins (three trialsw72-w74); niacin combinations with a statin, fibrate, or resin (six trialsA1;A75-A79); n-3 fatty acids (nine trialsw80-w88); acyl-CoA:cholesterol acyltransferase inhibitors (two trialsw92 w93); probucol (two trialsw21 w94); glitazones (two trialsw95 w96); hormones (nine trialsw97-w105); torcetrapib (two trialsw106 w107); low fat diets and surgery (five trialsw74 w89-w91 w108). Agreement between reviewers for study eligibility was very high (κ range 0.84-0.94).
Flowchart of trials. HDL=high density lipoprotein; LDL=low density lipoprotein; MI=myocardial infarction; RCT=randomised controlled trial
Lipid modifying effects
Table 1 summarises the baseline concentrations and changes in lipid subfractions for the different classes of intervention. The average weighted mean baseline low density lipoprotein cholesterol concentration of all included participants was 140 (SD 23; range 84-279) mg/dl (3.62 mmol/l), and the high density lipoprotein cholesterol concentration was 47 (7.4; 32-62) mg/dl (1.22 mmol/l). The weighted mean change in low density lipoprotein cholesterol was −23 (SD 19) mg/dl (−0.59 mmol/l), and the weighted mean change in high density lipoprotein cholesterol was 1.7 (3.1) mg/dl (0.04 mmol/l). Almost all classes of intervention reduced low density lipoprotein cholesterol except for n-3 fatty acids and glitazones. High density lipoprotein cholesterol was raised by most classes of intervention except for n-3 fatty acids, low-fat diets, acyl-CoA:cholesterol acyltransferase inhibitors, and probucol. In addition, high dose statin treatment (defined as 80 mg daily simvastatin or atorvastatin) slightly reduced high density lipoprotein cholesterol compared with less intensive statin treatment (weighted mean change −0.23 (SD 0.83) mg/dl),w56-w59 w61 w62 whereas statins overall raised it moderately (weighted mean change 1.6 (1.5) mg/dl).
Table 1.
Effects of different lipid modifying interventions on lipid subfractions. Values are weighted mean (SD) unless stated otherwise
| Trials | No. of trials | No of randomised participants | Median (interquartile range) follow-up (months), | Total cholesterol (mg/dl) | LDL cholesterol (mg/dl) | HDL cholesterol (mg/dl) | Triglycerides (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | |||||||
| All trials | 111* | 299 310 | 34 (24-54) | 222 (23) | −27 (22) | 140 (23) | −23 (19) | 47.3 (7.4) | 1.7 (3.1) | 155 (19) | −15 (18) | |||
| Statins | 62 | 157 151 | 32 (24-51) | 221 (25) | −43 (15) | 142 (24) | −38 (13) | 44.7 (5.5) | 1.6 (1.5) | 156 (18) | −21 (9) | |||
| Fibrates | 9 | 22 370 | 60 (55-60) | 213 (32) | −15 (7) | 138 (29) | −8.9 (6.7) | 41.1 (4.9) | 2.6 (2.3) | 162 (16) | −44 (14) | |||
| Resins | 3 | 4 005 | 60 (39-89) | 280 (4) | −23 (7) | 206 (6) | −25 (8) | 44.2 (1.2) | 2.7 (0.5) | 155 (6) | 6.1 (1) | |||
| Combinations with niacin | 6 | 779 | 27 (24-30) | 231 (65) | −41 (28) | 156 (57) | −42 (28) | 39.9 (5.4) | 12 (3.0) | 166 (26) | −48 (30) | |||
| n-3 fatty acids | 9 | 13 768 | 24 (12-27) | 216 (14) | 1.1 (2.2) | 142 (13) | 7.6 (1.9) | 41.8 (1.9) | −0.1 (0.3) | 166 (11) | −12 (15) | |||
| Diet/surgery | 5 | 62 645 | 78 (39-97) | 228 (7) | −4.0 (6) | 139 (10) | −6.4 (8.4) | 55.5 (7.2) | −0.1 (0.4) | 152 (23) | 0.8 (5) | |||
| ACAT inhibitors | 2 | 717 | 12 (6-18) | 179 | −23 | 106 | −2.1 | 42.5 | −0.3 | 156 | −10 | |||
| Probucol | 2 | 481 | 16 (7-24) | 242 | −31 | 160 | −19 | 48.2 | −12.3 | 171 | 1.1 | |||
| Glitazones | 2 | 9 589 | 42 (36-48) | 204 | NA | 116 | 3.6 | 44.5 | 3.1 | 162 | −15 | |||
| Hormones | 9 | 25 710 | 38 (24-49) | 226 (6) | −2.4 (1.4) | 132 (9) | −13 (5) | 53.1 (2.3) | 4.3 (3.1) | 148 (17) | 26 (11) | |||
| Torcetrapib (+ statin) | 2 | 2 095 | 24 (24-24) | 182 | 5.1 | 107 | −21 | 48.5 | 27.7 | 112 | −6.2 | |||
ACAT=acyl-CoA:cholesterol acyltransferase; HDL=high density lipoprotein; LDL=low density lipoprotein; NA=not available.
*Includes three studies with three trial arms; excludes one study that did not report baseline values (only change during follow-up).
Meta-regression analysis for clinical outcomes
Change in low density lipoprotein cholesterol was associated with and explained a statistically significant degree of variability in the log risk ratio for coronary heart disease events, coronary heart disease death, and total death in univariable and multivariable meta-regression analysis adjusted for change in high density lipoprotein cholesterol and different drug classes (table 2). For example, the risk ratio for coronary heart disease events (death or non-fatal myocardial infarction) was reduced, on average, by 7.1% (95% confidence interval 4.5% to 9.8%; P <0.001) per 10 mg/dl (0.26 mmol/l) reduction in low density lipoprotein cholesterol in a multivariable model. Change in low density lipoprotein cholesterol explained 32% of the variability in the log risk ratio for coronary heart disease events (see R2 for univariable model with low density lipoprotein cholesterol in table 2).
Table 2.
Meta-regression models investigating association of change in HDL cholesterol, LDL cholesterol, or both with log risk ratios of clinical outcomes
| Regression model and predictor | Change in risk per 10 mg/dl increase in lipid subfraction—% (95% CI) | P value | R2* |
|---|---|---|---|
| CHD events (CHD death and non-fatal MI) (n=95)† | |||
| Univariable: | |||
| Change in LDL | 4.9 (3.4 to 6.5) | <0.001 | 0.32 |
| Change in HDL | −8.2 (−24.7 to 8.1) | 0.32 | 0.01 |
| Bivariable: | |||
| Change in LDL | 5.1 (3.6 to 6.7) | <0.001 | 0.33 |
| Change in HDL | 6.4 (−7.8 to 20.4) | 0.37 | |
| Multivariable‡: | |||
| Change in LDL | 7.1 (4.5 to 9.8) | <0.001 | 0.46 |
| Change in HDL | 16.0 (−4.2 to 36.9) | 0.12 | |
| Total death (n=107)† | |||
| Univariable: | |||
| Change in LDL | 2.8 (1.4 to 4.3) | <0.001 | 0.12 |
| Change in HDL | 5.5 (−8.5 to 19.2) | 0.44 | 0.01 |
| Bivariable: | |||
| Change in LDL | 3.1 (1.7 to 4.6) | <0.001 | 0.15 |
| Change in HDL | 12.1 (−1.1 to 25.2) | 0.07 | |
| Multivariable‡: | |||
| Change in LDL | 4.4 (1.6 to 7.2) | 0.002 | 0.28 |
| Change in HDL | 11.0 (−6.5 to 28.1) | 0.21 | |
| CHD death (n=94)† | |||
| Univariable: | |||
| Change in LDL | 4.5 (2.4 to 6.6) | <0.001 | 0.16 |
| Change in HDL | −0.2 (−24.0 to 23.1) | 0.99 | <0.01 |
| Bivariable: | |||
| Change in LDL | 4.8 (2.6 to 7.0) | <0.001 | 0.17 |
| Change in HDL | 11.3 (−10.8 to 32.9) | 0.31 | |
| Multivariable‡: | |||
| Change in LDL | 7.2 (3.1 to 11.3) | 0.001 | 0.33 |
| Change in HDL | 12.2 (−18.0 to 41.5) | 0.42 | |
CHD=coronary heart disease; HDL=high density lipoprotein; LDL=low density lipoprotein; MI=myocardial infarction.
*Proportion of total variability in log risk ratio of outcome explained by model.
†Absence of outcome events in intervention and control groups or absence of reporting this outcome event led to reduced sample of trials.
‡Models include adjustment for drug class in addition to variables of lipid subfractions.
We found no significant association of change in high density lipoprotein cholesterol with the log risk ratio in any model after adjustment for changes in low density lipoprotein cholesterol (see table 2 and results of analyses stratified by drug class in web table A). For example, the risk ratio for coronary heart disease events was increased, on average, by 16% (−4.2% to 36.9%; P=0.12) per 10 mg/dl increase in high density lipoprotein cholesterol according to our multivariable model in table 2. Change in high density lipoprotein cholesterol hardly explained any variability in any of the outcomes (see R2 results in table 2 and web table A). The change in the quotient of low density lipoprotein cholesterol and high density lipoprotein cholesterol explained 32%, 12%, and 15% of the variability in log risk ratios for coronary heart disease events, total death, and coronary heart disease death, which is no more than the change in low density lipoprotein cholesterol alone explained for these outcomes (see R2 results for univariable models with low density lipoprotein cholesterol in table 2).
Sensitivity analyses focusing on a more homogeneous sample of trials (interventions known to raise high density lipoprotein cholesterol and excluding trials using agents associated with harmful effects) revealed a significant association of change in high density lipoprotein cholesterol and the log risk ratio for coronary heart disease events in univariable analysis, with a 29% (51.7% to 6.6%; P=0.01) risk reduction for each 10 mg/dl increase in high density lipoprotein cholesterol (table 3). This association was, however, no longer detectable in models adjusted for changes in low density lipoprotein cholesterol (bivariable or multivariable), indicating that the apparent reduction in outcomes was due to the association of changes in high density lipoprotein cholesterol with changes in low density lipoprotein cholesterol. Change in low density lipoprotein cholesterol remained significantly associated with the log risk ratio for coronary heart disease events, explaining greater variability in trials that had longer follow-up (R2 of 0.41, 0.46, and 0.51 for trials with a follow-up of six months or more, more than one year, and more than two years). Two additional sensitivity analyses focusing on trials with interventions specifically chosen to raise high density lipoprotein cholesterol and on trials reporting intention to treat results did not show any significant association of change in high density lipoprotein cholesterol with risk of coronary heart disease events.
Table 3.
Sensitivity analyses for outcome of coronary heart disease death and non-fatal myocardial infarction with different samples of trials*
| Regression model and predictor | Change in risk per 10 mg/dl increase in lipid subfraction—% (95% CI) | P value | R2† |
|---|---|---|---|
| Trials using interventions known to raise HDL cholesterol, excluding trials using agents associated with harmful effects‡ | |||
| Follow-up ≥6 months (n=70) | |||
| Univariable: | |||
| Change in LDL | 5.5 (3.9 to 7.0) | <0.001 | 0.41 |
| Change in HDL | −28.9 (−51.7 to −6.6) | 0.01 | 0.09 |
| Bivariable: | |||
| Change in LDL | 5.4 (3.6 to 7.2) | <0.001 | 0.41 |
| Change in HDL | −2.2 (−22.1 to 17.4) | 0.83 | |
| Multivariable§: | |||
| Change in LDL | 6.9 (4.2 to 9.6) | <0.001 | 0.51 |
| Change in HDL | 15.2 (−8.2 to 38.1) | 0.20 | |
| Follow-up >1 year (n=59) | |||
| Univariable: | |||
| Change in LDL | 5.5 (4.0 to 7.1) | <0.001 | 0.46 |
| Change in HDL | −29.0 (−52.7 to −5.9) | 0.02 | 0.10 |
| Bivariable: | |||
| Change in LDL | 5.5 (3.7 to 7.2) | <0.001 | 0.46 |
| Change in HDL | −2.0 (−22.0 to 17.6) | 0.84 | |
| Multivariable§: | |||
| Change in LDL | 7.1 (4.5 to 9.8) | <0.001 | 0.58 |
| Change in HDL | 16.2 (−7.0 to 38.8) | 0.17 | |
| Follow-up >2 years (n=49) | |||
| Univariable: | |||
| Change in LDL | 5.6 (4.0 to 7.2) | <0.001 | 0.51 |
| Change in HDL | −27.5 (−53.6 to −2.0) | 0.04 | 0.09 |
| Bivariable: | |||
| Change in LDL | 5.7 (3.9 to 7.5) | <0.001 | 0.52 |
| Change in HDL | 3.1 (−17.9 to 23.7) | 0.77 | |
| Multivariable§: | |||
| Change in LDL | 7.5 (4.8 to 10.1) | <0.001 | 0.63 |
| Change in HDL | 22.4 (−1.4 to 45.7) | 0.06 | |
| Trials using interventions specifically chosen to raise HDL cholesterol (niacin combinations and fibrates) (n=13) | |||
| Univariable: | |||
| Change in LDL | 5.7 (−102 to 20.8) | 0.44 | 0.07 |
| Change in HDL | −10.1 (−61.8 to 39.2) | 0.55 | 0.04 |
| Bivariable: | |||
| Change in LDL | 5.0 (−16.1 to 24.7) | 0.62 | 0.06 |
| Change in HDL | −5.0 (−74.7 to 60.1) | 0.89 | |
| Multivariable§: | |||
| Change in LDL | −2.3 (−23.7 to 18.6) | 0.81 | 0.30 |
| Change in HDL | −6.3 (−70.6 to 54.2) | 0.82 | |
| Trials with intention to treat data (n=78) | |||
| Univariable: | |||
| Change in LDL | 4.9 (3.4 to 6.5) | <0.001 | 0.35 |
| Change in HDL | −11.7 (−30.4 to 6.7) | 0.21 | 0.02 |
| Bivariable: | |||
| Change in LDL | 5.0 (3.4 to 6.6) | <0.001 | 0.35 |
| Change in HDL | 2.6 (−13.2 to 18.1) | 0.74 | |
| Multivariable§: | |||
| Change in LDL | 7.1 (4.1 to 10.0) | <0.001 | 0.47 |
| Change in HDL | 11.1 (−13.6 to 35.2) | 0.37 | |
HDL=high density lipoprotein; LDL=low density lipoprotein.
*I2 associated with risk ratios for coronary heart disease events was 41.1% (95% uncertainty interval 24.5% to 54.0%) in all trials (n=95), 28.3% (3.4% to 46.8%) in trials using interventions known to raise HDL cholesterol and excluding trials using agents associated with harmful effects (n=70), and 13.3% (0% to 52.5%) in trials using interventions specifically chosen to raise HDL cholesterol (n=13).
†Proportion of total variability in log risk ratio of outcome explained by model.
‡Excluding trials using n-3 fatty acids, low fat diets, probucol, torcetrapib, and hormones and trials focusing on patients with renal failure.
§Models include adjustment for drug class in addition to variables of lipid subfractions.
Similarly, we found no association between change in triglycerides and risk of coronary heart disease events whenever the model included an adjustment for the change in low density lipoprotein cholesterol (data available from the authors). Change in low density lipoprotein cholesterol, however, remained a significant predictor in a multivariable model adjusting for change in high density lipoprotein cholesterol, change in triglycerides, and class of intervention, with a 7.4% (4.4% to 10.4%; P <0.001) relative risk reduction for coronary heart disease events.
Model residuals were normally distributed with constant variance (homoscedasticity). Including quadratic or exponential terms did not improve the fit of models. We found no evidence of collinearity.
Discussion
This systematic review and meta-regression analysis of 108 randomised controlled trials using lipid modifying interventions did not show an association between treatment mediated change in high density lipoprotein cholesterol and risk ratios for coronary heart disease events, coronary heart disease deaths, or total deaths whenever change in low density lipoprotein cholesterol was taken into account. We found a statistically significant, substantial association between change in low density lipoprotein cholesterol and risk ratios for coronary heart disease events, coronary heart disease deaths, or total deaths, adjusted for other lipid subfractions and drug class. Our results indicate a 7% relative risk reduction in coronary heart disease events for every 10 mg/dl (0.26 mmol/l) reduction in low density lipoprotein cholesterol, which is equivalent to a 10% relative reduction in coronary heart disease events for every 10% decrease in low density lipoprotein cholesterol; this is consistent with the magnitude of reduction reported in current National Cholesterol Education Program guidelines.8
Strengths and limitations
Strengths of this study include a comprehensive scope that included a wide range of patients at risk of cardiovascular events and a wide variety of lipid modifying interventions, maximising the power of our analysis. Our extensive literature search supplemented by contacting experts in the field to retrieve all relevant eligible trials minimised the potential for publication bias, but we cannot exclude it completely. We could not include 50 otherwise eligible trials that failed to measure or did not report follow-up values or change in low density lipoprotein cholesterol or high density lipoprotein cholesterol (for details see web appendix). Unidentified trials or trials that failed to report data on high density lipoprotein cholesterol or low density lipoprotein cholesterol reduced the power of our analysis but were unlikely to bias its results.
A meta-regression such as ours relies on variability across studies in both the differences between high density lipoprotein cholesterol in treatment and control groups and variability in magnitude of effect. Thus, the analysis is powerful to the extent that it includes studies in which differences in high density lipoprotein cholesterol are modest (such as with statins) or non-existent and studies in which differences are large (niacin). Our analysis would have been stronger if we had had access to data from additional studies in which differences in changes in high density lipoprotein cholesterol between treatment and control were large. Nevertheless, variability was sufficient that if a strong effect of high density lipoprotein cholesterol existed we would probably have detected it.
To limit the risk of data driven spurious associations and overfitting, we pre-specified a limited number of predictors for our statistical models.38 We verified the model assumptions of linearity, normality of residuals, homoscedasticity, and absence of collinearity. In addition, our results proved robust in several pre-specified sensitivity analyses and were consistent with other investigations that have examined similar data.8 Our systematic review is far more comprehensive than previous studies on this subject.21
Nevertheless, the relation described by a meta-regression is observational—that is, although the original studies may be randomised trials, a meta-regression across trials does not have the benefit of randomisation to support a causal interpretation and thus risks bias by confounding. Moreover, regression analysis typically ignores the effect of measurement errors in the independent variables. Although this may be problematic to the extent that older studies used less precise methods to determine lipid subfractions, our meta-regression involves mean values of the independent variables. The standard errors of these means are substantially smaller than the standard deviations of individual participants’ values, which should mitigate the problem.
Our classification of lipid modifying interventions may be argued to combine antilipidaemic agents and diets that have important pharmacological differences or mechanisms of action37—for example, in trials classified under niacin treatment, niacin could be combined with a statin, a fibrate, or a resin. Different interventions that alter high density lipoprotein cholesterol may have different impacts on cardiovascular risk. Interventions may lead to different types of high density lipoprotein cholesterol with differences in function (see below); they may raise high density lipoprotein cholesterol by reduced catabolism (which may be detrimental) or increased production (more likely to be beneficial). Our adjustment of the analysis by type of intervention (drug class) deals with this problem to a considerable extent but may not fully solve it.
Finally, meta-regression relies on aggregated data from studies rather than data from individual patients—that is, the relation with patient averages across trials may not be the same as the relation for patients within trials. This is known as the ecological fallacy.34 Therefore, ideally our results would be confirmed by an analysis of data from individual patients, with a large pooled dataset of trials testing lipid modifying interventions, such as data from the Cholesterol Treatment Trialists’ Collaborators.39
New views on high density lipoprotein cholesterol
Our findings contribute to accumulating evidence that simply increasing the amount of circulating high density lipoprotein cholesterol does not necessarily confer cardiovascular benefits.17 18 19 20 40 In the case of torcetrapib, one of the most potent high density lipoprotein raising agents to date, the failure to improve intracoronary atheroma burden in ultrasound studies and the excess mortality seen in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial may be explained by a molecule specific increase in blood pressure (perhaps owing to increased concentrations of aldosterone) or unforeseen interactions between torcetrapib and atorvastatin.17 18 20 41 42 Recent data suggest the former possibility43; if so, other cholesteryl ester transfer protein inhibitors may still hold promise.
An alternative hypothesis would suggest that inhibition of the cholesteryl ester transfer protein leads to production of dysfunctional high density lipoprotein cholesterol with pro-inflammatory and atherogenic properties.44 45 High density lipoprotein cholesterol particles vary substantially in size, density, composition, and functional properties. The varying functionality of different high density lipoprotein cholesterol subfractions most likely affects their relation to atherosclerosis.46 47 48 Lipid modifying agents and diets may affect the functionality of high density lipoprotein cholesterol. For example, recent data suggest that a low fat, high fibre diet, in combination with exercise, converts high density lipoprotein cholesterol from a pro-inflammatory to an anti-inflammatory state.49
Available measures of the function of high density lipoprotein cholesterol include indices of inflammation, oxidation, monocyte chemotaxis, nitric oxide production, endothelial function, and thrombosis, as well as tests assessing the reverse cholesterol transport effects of treatments.50 However, further development is necessary to satisfy the urgent need for a reliable and easily applicable assay of high density lipoprotein cholesterol function.
Implications for clinical practice and future research
The lack of association between treatment induced change in high density lipoprotein cholesterol and the risk of coronary heart disease events, coronary heart disease deaths, or total deaths raises questions about the rationale for development of therapeutic agents that increase high density lipoprotein cholesterol. Raising high density lipoprotein cholesterol without considering effects on high density lipoprotein function seem to have little promise for the prevention of cardiovascular events. Future research should prospectively consider the results of assays to measure high density lipoprotein function and then provide definitive evidence of pharmacological effects on patient important outcomes in long term randomised trials.50
Results from this study corroborate recommendations from current clinical guidelines (Adult Treatment Panel III, American Heart Association/National Heart, Lung and Blood Institute, and American Diabetes Association) that emphasise targeting primarily low density lipoprotein cholesterol in the prevention of cardiovascular morbidity and mortality.8 51 52 Recent updates of the National Cholesterol Education Program guidelines confirmed low levels of high density lipoprotein cholesterol (<40 mg/dl) as a major cardiovascular risk factor. Although the guidelines recommend that clinicians should consider combining a low density lipoprotein cholesterol lowering drug with a fibrate or niacin in patients with low high density lipoprotein cholesterol, they refrained from making recommendations about specific targets for raising high density lipoprotein cholesterol concentrations.8 9
What is already known on this topic
Current guidelines recognise high density lipoprotein cholesterol as an independent cardiovascular risk factor
The extent to which changes in high density lipoprotein cholesterol alter risk of coronary heart disease events remains controversial
What this study adds
No association exists between treatment induced change in high density lipoprotein cholesterol and risk ratios for coronary heart disease events, coronary heart disease deaths, or total deaths when changes in low density lipoprotein cholesterol are adjusted for
Whereas change in high density lipoprotein cholesterol failed to explain variability in risk ratios for coronary heart disease events across studies, change in low density lipoprotein cholesterol explained about one third of the variability
Scepticism about interventions to lower coronary heart disease risk by increasing the amount of circulating high density lipoprotein cholesterol without considering effects on its function is reinforced
We thank Christina Lacchetti, Jean Mackay, Kate Bak, and Sara Kaffashian, who helped with screening of titles and abstracts of potentially eligible publications.
Contributors: MB, SDW, COR, EJM, HCB, VMM, and GHG conceived, designed, and planned the study. MB, XW, AS, NV, and NB were responsible for the electronic search, data collection, full text retrieval, and data entry. MB, IFG, JJY, PJK, EAA, PW, BB, DB, AS, IW, SAdS, ZK, AJN, and EJM checked eligibility, assessed trial quality, and extracted data. QZ and SDW provided statistical expertise. COR, EJM, and GHG obtained funding. All authors discussed the results. MB and GHG drafted the manuscript; all the other authors critically revised the manuscript for important intellectual content. MB and GHG are the guarantors.
Funding: The study was supported by an unrestricted educational grant from Pfizer. MB is supported by a scholarship award of the Swiss National Foundation (PASMA—112951/1). JJY is supported by an Ontario Ministry of Health and Long-Term Care Career Scientist Award. The funding sources had no role in the study design; the collection, analysis, and interpretation of data; or the writing of the report.
Competing interests: COR is a salaried employee of Pfizer UK.
Ethics approval: Not needed.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2009;338:b92
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham study. Am J Med 1977;62:707-14. [DOI] [PubMed] [Google Scholar]
- 2.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk: the PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124(suppl):S11-20. [DOI] [PubMed] [Google Scholar]
- 3.DeGoma EM, Leeper NJ, Heidenreich PA. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J Am Coll Cardiol 2008;51:49-55. [DOI] [PubMed] [Google Scholar]
- 4.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301-10. [DOI] [PubMed] [Google Scholar]
- 5.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524-32. [DOI] [PubMed] [Google Scholar]
- 6.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb D, Tall AR. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest 1996;97:2917-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 1993;269:3015-23. [PubMed] [Google Scholar]
- 8.Adult Treatment Panel III. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol 2004;24:e149-61. [DOI] [PubMed] [Google Scholar]
- 10.Coronary Drug Project. Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360-81. [PubMed] [Google Scholar]
- 11.Carlson LA, Rosenhamer G. Reduction of mortality in the Stockholm ischaemic heart disease secondary prevention study by combined treatment with clofibrate and nicotinic acid. Acta Med Scand 1988;223:405-18. [DOI] [PubMed] [Google Scholar]
- 12.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583-92. [DOI] [PubMed] [Google Scholar]
- 13.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med 1990;323:1289-98. [DOI] [PubMed] [Google Scholar]
- 14.Whitney EJ, Krasuski RA, Personius BE, Michalek JE, Maranian AM, Kolasa MW, et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med 2005;142:95-104. [DOI] [PubMed] [Google Scholar]
- 15.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT—a randomized controlled trial. JAMA 2001;285:1585-91. [DOI] [PubMed] [Google Scholar]
- 16.Manninen V, Elo MO, Frick MH, Haapa K, Heinonen OP, Heinsalmi P, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki heart study. JAMA 1988;260:641-51. [PubMed] [Google Scholar]
- 17.Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol 2007;27:257-60. [DOI] [PubMed] [Google Scholar]
- 18.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304-16. [DOI] [PubMed] [Google Scholar]
- 19.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007;297:1675-82. [DOI] [PubMed] [Google Scholar]
- 20.Kastelein JJ, van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007;356:1620-30. [DOI] [PubMed] [Google Scholar]
- 21.Dean BB, Borenstein JE, Henning JM, Knight K, Merz CN. Can change in high-density lipoprotein cholesterol levels reduce cardiovascular risk? Am Heart J 2004;147:966-76. [DOI] [PubMed] [Google Scholar]
- 22.White C. Suspected research fraud: difficulties of getting at the truth. BMJ 2005;331:281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berthold HK, Unverdorben S, Degenhardt R, Bulitta M, Gouni-Berthold I. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. JAMA 2006;295:2262-9. [DOI] [PubMed] [Google Scholar]
- 24.Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med 2005;165:725-30. [DOI] [PubMed] [Google Scholar]
- 25.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004;(4):CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper L, Summerbell CD, Higgins JP, Thompson RL, Clements G, Capps N, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2001;(3):CD002137. [DOI] [PubMed] [Google Scholar]
- 27.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Pioglitazone for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;(4):CD006060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabriel SR, Carmona L, Roque M, Sanchez GL, Bonfill X. Hormone replacement therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2005;(2):CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993;2:121-45. [DOI] [PubMed] [Google Scholar]
- 30.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054-60. [DOI] [PubMed] [Google Scholar]
- 31.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deeks JJ, Higgins JPT, Altman DG, eds. Data extraction for continuous outcomes. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 8. www.cochrane.org/resources/handbook/Handbook4.2.6Sep2006.pdf.
- 33.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708. [DOI] [PubMed] [Google Scholar]
- 34.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. [DOI] [PubMed] [Google Scholar]
- 35.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9. [PubMed] [Google Scholar]
- 36.The Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-57. [DOI] [PubMed] [Google Scholar]
- 37.McAlister FA, Laupacis A, Wells GA, Sackett DL. Users’ guides to the medical literature: XIX. Applying clinical trial results B: guidelines for determining whether a drug is exerting (more than) a class effect. JAMA 1999;282:1371-7. [DOI] [PubMed] [Google Scholar]
- 38.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411-21. [DOI] [PubMed] [Google Scholar]
- 39.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. [DOI] [PubMed] [Google Scholar]
- 40.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA 2007;298:786-98. [DOI] [PubMed] [Google Scholar]
- 41.Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007;370:153-60. [DOI] [PubMed] [Google Scholar]
- 42.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109-22. [DOI] [PubMed] [Google Scholar]
- 43.Nicholls SJ, Brennan DM, Wolski K, Kalidindi SR, Moon K, Tuzcu EM, et al. Changes in levels of high density lipoprotein cholesterol predict the impact of torcetrapib on progression of coronary atherosclerosis: insights from ILLUSTRATE. Circulation 2007;116:II-127 (abstract 684). [Google Scholar]
- 44.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelmann AM. Mechanisms of disease: proatherogenic HDL—an evolving field. Nat Clin Pract Endocrinol Metab 2006;2:504-11. [DOI] [PubMed] [Google Scholar]
- 45.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 2004;114:529-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol 2005;46:1792-8. [DOI] [PubMed] [Google Scholar]
- 47.Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep 2006;8:405-11. [DOI] [PubMed] [Google Scholar]
- 48.Meyers CD, Kashyap ML. Pharmacologic elevation of high-density lipoproteins: recent insights on mechanism of action and atherosclerosis protection. Curr Opin Cardiol 2004;19:366-73. [DOI] [PubMed] [Google Scholar]
- 49.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol 2006;101:1727-32. [DOI] [PubMed] [Google Scholar]
- 50.DeGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol 2008;51:2199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735-52. [DOI] [PubMed] [Google Scholar]
- 52.Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care 2004;27(suppl 1):S68-71. [DOI] [PubMed] [Google Scholar]