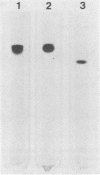
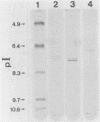
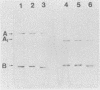
Abstract
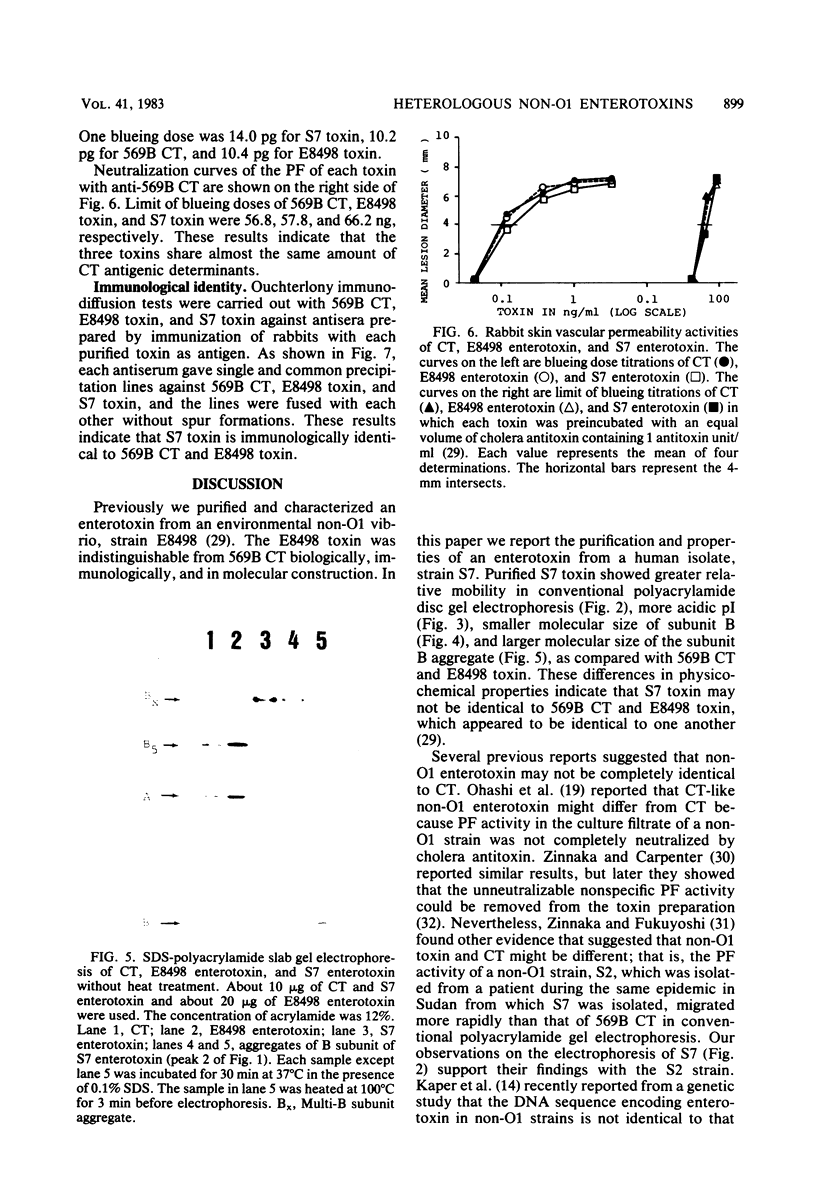
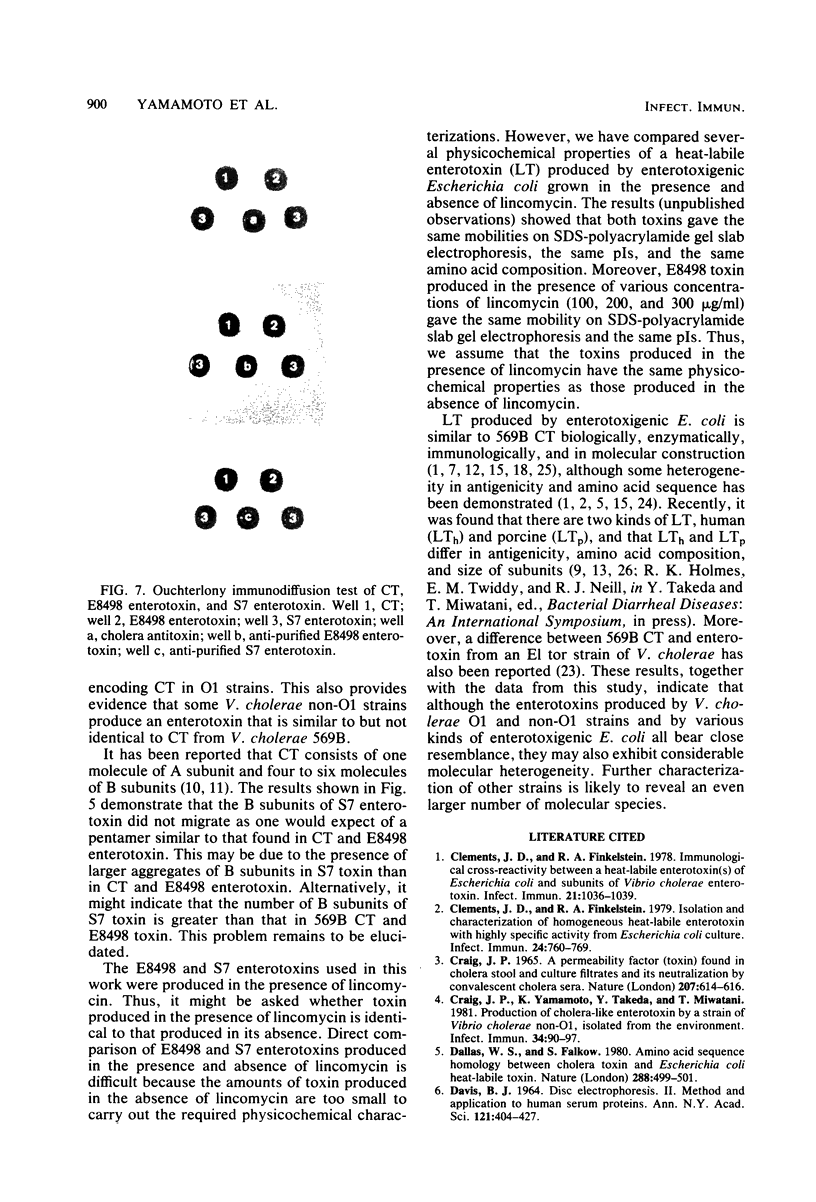
Cholera-like enterotoxin produced by a non-O1 strain of Vibrio cholerae, S7 (S7 enterotoxin), isolated from human diarrheal stool, was purified, and its physicochemical, biological, and immunological properties were compared with those of cholera enterotoxin from V. cholerae O1 569B (CT) and an enterotoxin produced by another non-O1 V. cholerae (E8498 enterotoxin) reported previously (Yamamoto et al., Infect. Immun. 39:1128-1135, 1983). The purified S7 enterotoxin had physicochemical properties different from those of CT and E8498 enterotoxin. S7 enterotoxin had greater relative mobility in conventional polyacrylamide gel disc electrophoresis and a lower isoelectric point, and its B subunit was smaller than those of CT and E8498 enterotoxin. The results of sodium dodecyl sulfate-polyacrylamide slab gel electrophoresis suggested that the size of the aggregate of the B subunits of S7 enterotoxin is larger than that of CT and E8498 enterotoxin. The biological and immunological properties of S7 enterotoxin were indistinguishable from those of CT and E8498 enterotoxin. These results indicate that non-O1 vibrios may produce more than one kind of cholera-like enterotoxin: one which is identical to CT (E8498 enterotoxin type) and another which is not identical to CT (S7 enterotoxin type).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Geary S. J., Marchlewicz B. A., Finkelstein R. A. Comparison of heat-labile enterotoxins from porcine and human strains of Escherichia coli. Infect Immun. 1982 Apr;36(1):215–220. doi: 10.1128/iai.36.1.215-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Tsuji T., Takeda Y., Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Purification and chemical characterization of the heat-labile enterotoxin produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):586–596. doi: 10.1128/iai.25.2.586-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Richardson S. H. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest. 1978 Aug;62(2):281–285. doi: 10.1172/JCI109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Saunders D. W., Schanbacher K. J., Bramucci M. G. Mapping of a gene in Vibrio cholerae that determines the antigenic structure of cholera toxin. Infect Immun. 1982 Dec;38(3):1109–1116. doi: 10.1128/iai.38.3.1109-1116.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Kavanaugh W. M., Dallas W. S., Falkow S., Konigsberg W. H., Schafer D. E. Sequence homologies between A subunits of Escherichia coli and Vibrio cholerae enterotoxins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):50–54. doi: 10.1073/pnas.78.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Honda T., Taga S., Miwatani T. In vitro formation of hybrid toxins between subunits of Escherichia coli heat-labile enterotoxin and those of cholera enterotoxin. Infect Immun. 1981 Nov;34(2):341–346. doi: 10.1128/iai.34.2.341-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Taga S., Honda T., Takeda Y., Miwatani T. Molecular heterogeneity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1982 Nov;38(2):444–448. doi: 10.1128/iai.38.2.444-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takeda Y., Miwatani T., Craig J. P. Purification and some properties of a non-o1 Vibrio cholerae enterotoxin that is identical to cholera enterotoxin. Infect Immun. 1983 Mar;39(3):1128–1135. doi: 10.1128/iai.39.3.1128-1135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]