Abstract
Purpose
To determine whether a low-fat diet high in vegetables, fruit, and fiber differentially affects prognosis in breast cancer survivors with hot flashes (HF) or without HF after treatment.
Patients and Methods
A secondary analysis was conducted on 2,967 breast cancer survivors, age 18 to 70 years, who were randomly assigned between 1995 and 2000 in a multicenter, controlled trial of a dietary intervention to prevent additional breast cancer events and observed through June 1, 2006. We compared the dietary intervention group with a group who received five-a-day dietary guidelines.
Results
Independent of HF status, a substantial between-group difference among those who did and did not receive dietary guidelines was achieved and maintained at 4 years in intake of vegetable/fruit servings per day (54% higher; 10 v 6.5 servings/d, respectively), fiber (31% higher; 25.5 v 19.4 g/d, respectively), and percent energy from fat (14% lower; 26.9% v 31.3%, respectively). Adjusting for tumor characteristics and antiestrogen treatment, HF-negative women assigned to the intervention had 31% fewer events than HF-negative women assigned to the comparison group (hazard ratio [HR] = 0.69; 95% CI, 0.51 to 0.93; P = .02). The intervention did not affect prognosis in the women with baseline HFs. Furthermore, compared with HF-negative women assigned to the comparison group, HF-positive women had significantly fewer events in both the intervention (HR = 0.77; 95% CI, 0.59 to 1.00; P = .05) and comparison groups (HR = 0.65; 95% CI, 0.49 to 0.85; P = .002).
Conclusion
A diet with higher vegetable, fruit, and fiber and lower fat intakes than the five-a-day diet may reduce risk of additional events in HF-negative breast cancer survivors. This suggestive finding needs confirmation in a trial in which it is the primary hypothesis.
INTRODUCTION
Self-report of hot flashes (HFs) after treatment for early-stage breast cancer has been associated with an approximately 25% to 30% decreased risk for additional breast cancer events, independent of the type of antiestrogen therapy.1,2 HFs have been associated with lower estrogen levels during the menopausal transition in some,3,4 but not all,5 studies. Discontinuation of menopausal hormone therapy (MHT), chemotherapy-induced amenorrhea, and antiestrogen therapies such as tamoxifen or an aromatase inhibitor contribute to the development of HFs in women undergoing breast cancer therapy.6 However, HFs are reported by women without menses, women who continue to menstruate while on chemotherapy, and women whose menses resume after temporary amenorrhea,7 suggesting that low circulating estrogen concentrations do not fully explain HF status.
Changes in dietary pattern to either decrease energy from fat or increase fiber intake can alter the enterohepatic recirculation of estrogens, leading to lower circulating estrogen concentrations.8,9 Binding of fiber to unconjugated estrogens in the gut impedes reabsorption of estrogen.10,11 A 20% difference in circulating estrogen levels has been reported for postmenopausal Mexican American women in the highest versus lowest quartile of fiber intake.12 Pooled data from 13 dietary intervention studies with reductions in dietary fat intake (many with increases in dietary fiber intake) suggest that circulating estradiol could be decreased by 18% to 27%.13 Conceivably, the increased risk of additional breast cancer events observed among women who do not report HFs after treatment may be reduced by lifestyle interventions that lower circulating estrogen concentrations.
The Women's Healthy Eating and Living (WHEL) Study tested whether a dietary pattern high in vegetables, fruit, and fiber and low in fat might reduce additional breast cancer events in women with early-stage breast cancer.14 Such a dietary pattern has previously been shown to lower circulating estradiol concentrations.15 Furthermore, women with higher circulating estradiol concentrations at baseline were less likely to report HFs and also more likely to have a secondary cancer event within a 7.3-year follow-up period.15 The primary analyses of the WHEL Study did not demonstrate an event-free survival advantage in following the study's dietary pattern.14 On the basis of findings of improved disease-free survival in women with HFs1 and significantly higher circulating estradiol levels in women without HFs,15 we performed secondary analyses to test the hypothesis that the dietary intervention had a differential impact on prognosis depending on women's baseline HF status. We hypothesized that the protective dietary effect might be limited to the subgroup of patients with potentially higher circulating estradiol levels and worse prognosis (ie, women without HFs at baseline).
PATIENTS AND METHODS
Patients
Between 1995 and 2000, the WHEL Study randomly assigned women within 4 years of diagnosis of stage I (≥ 1 cm), II, or IIIA breast cancer16 to a dietary intervention or comparison group at seven clinical sites.17 We observed 96% of participants for an average of 7.3 years (range, 6 to 11 years).14 Institutional review boards at each clinical site approved the protocol; all participants provided written informed consent. Analyses focused on the 2,967 women (96% of all enrolled) whose baseline HF severity report in the prior 4 weeks (scored 0 [none] to 3 [severe]) was obtained using the Women's Health Initiative symptom inventory.4 Women were classified as having any HFs (score = 1 to 3; HF positive) or no HFs (HF negative) because reporting any HFs, irrespective of severity, was associated with disease-free survival.1 This analysis included women who were premenopausal at baseline because 30% of such women reported HFs. At baseline, participants had a mean age of 53 years; 54.2% were college graduates; 85.3% were white; 95% had had stage I or II breast cancer; 57.5% were node negative; 61.6% had had estrogen receptor (ER)–positive and progesterone receptor (PR)–positive tumors; 61.5% had received radiation therapy; 69.9% had received adjuvant chemotherapy; and 67.3% had received antiestrogen therapy.14
Study Groups
Details of the dietary intervention have been reported previously.16,18 Briefly, an intensive telephone counseling intervention based on social cognitive theory promoted a daily dietary intake of five vegetable servings, an additional 16 oz of vegetable juice, three fruit servings, 30 g of fiber, and 15% to 20% of energy from fat for the intervention group.18,19 The comparison group received printed materials (but no counseling) promoting the five-a-day guidelines20 of daily intakes of five servings of fruit and vegetables, more than 20 g of fiber, and less than 30% of energy intake from fat.
Dietary Assessment
Dietary intake was assessed with four 24-hour telephone dietary recalls using the Nutrition Data System software (NDS-R 1994-2006; University of Minnesota, Minneapolis, MN), conducted on random days over a 3-week period, stratified for weekend and weekdays. For this analysis, we consider dietary assessment conducted at baseline (before random assignment), 1 year, and 4 years. Fasting blood samples were collected to validate self-reported intake of vegetables and fruits.21,22
Outcomes
Primary study end points were additional breast cancer events (local/regional recurrence or distant metastasis or new primary breast cancer) and death from any cause. Carcinoma in situ was not included as an outcome. All such additional breast cancer events reported in semiannual telephone interviews were confirmed by medical record review. Outcome ascertainment also included a search of the National Death Index.
Other Data
Reproductive history (including prior MHT use and gynecologic surgical history, eg, oophorectomy) was self-reported at baseline. At each clinic visit, height and weight were measured to calculate body mass index (BMI).23 Women were considered postmenopausal if they were amenorrheic for more than 12 months and perimenopausal if they reported irregular menstrual cycles in the past 12 months.24 Cancer characteristics and treatments were obtained from medical records. Depressive symptoms were measured with the eight-item Center for Epidemiologic Studies Depression Scale.25,26 Health-related quality of life was assessed using the Medical Outcomes Study 36-Item Short Form Health Survey.27,28
Data Analyses
Breast cancer event rates were estimated by study group and baseline HF status. Kaplan-Meier plots of breast cancer event-free survival were generated. The breast cancer event-free interval was defined as the time from date of enrollment to the occurrence of an additional breast cancer event. Follow-up time was censored at the time of a woman's death (if not from breast cancer), at the last documented contact date, or at the study completion date (June 1, 2006). χ2 and t tests were used to test associations of measured variables by study group and baseline HF status. We estimated between-group differences in dietary variables using a conservative imputation analysis, described previously,21 that assumed that nonrespondents were following the comparison group dietary pattern. Tests for significant dietary differences by baseline HF status, time, and study group were examined using mixed models including group × time and group × time × HF interactions.
To develop parsimonious multiple regression models, we tested bivariate associations with breast cancer events and included any variables that were significant at the P = .1 level. We computed likelihood ratio tests using a Cox model that controlled for these variables and included baseline HF status and study group as main effects and a term for the interaction between them. To examine whether other factors modified any observed interaction between study group and HF status, further analyses included three-way interaction terms between study group, HF status, and individual variables. Finally, we conducted a sensitivity analysis of covariates that were omitted from the final Cox model.
RESULTS
Study Outcomes by Baseline HF Status and Study Group
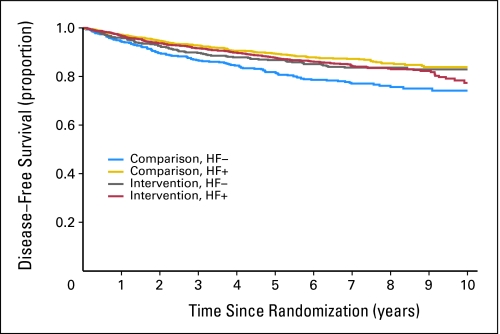
Participants were equally allocated across random assignment arms within the HF-positive and HF-negative subgroups. Among the 2,067 HF-positive women (69.7%), 1,029 (49.8%) were randomly assigned to the intervention group, and 1,038 (50.2%) were assigned to the comparison group. Among the 900 women in the HF-negative group, 447 (50.3%) were randomly assigned to the intervention group, and 453 (49.7%) were assigned to the comparison group (χ2 test of HF by group association, P = .99). Kaplan-Meier curves demonstrated that HF-negative women in the comparison group had significantly worse disease-free survival than the other three groups (Fig 1; likelihood ratio test for group × HF interaction, P = .002). Five-year disease-free survival rates by group were very similar for three of the four subgroups (88% for HF-positive women in intervention group; 89% for HF-positive women in comparison group, and 87% for HF-negative women in intervention group) but lower for the subgroup of HF-negative women in the comparison group (82%).
Fig 1.
Kaplan-Meier curve of disease-free survival by study group and hot flash (HF) status. Comparison without HF (HF−; n = 453), unadjusted hazard ratio (HR) = 1.00; intervention HF− (n = 447), unadjusted HR = 0.67; P = .01. Comparison with HF (HF+; n = 1038), unadjusted HR = 0.56; P < .001; intervention HF+ (n = 1,029), unadjusted HR = 0.68; P = .002.
Within the HF-negative subgroup, women in the intervention group had a significantly lower rate of additional breast cancer events than women in the comparison group (intervention, n = 72, 16.1%; comparison, n = 107, 23.6%; log-rank test, P < .01) with a major between-group difference in distant recurrences (Table 1). This between-group difference was also observed in deaths from any cause (comparison = 14.1% v intervention = 9.4%; log-rank test, P = .03). Among the HF-positive women, no significant between-group difference was observed in additional breast cancer events (intervention, n = 170, 16.5%; comparison, n = 143, 13.8%; log-rank test, P = .10) or in all-cause mortality (comparison = 8.6% v intervention = 10.3%; log-rank test, P = .20).
Table 1.
Additional Breast Cancer Events Over 7.3 Years of Follow-Up by Baseline Hot Flash Status and Study Group
| Event | Baseline Hot Flashes
|
No Baseline Hot Flashes
|
||||||
|---|---|---|---|---|---|---|---|---|
| Comparison
|
Intervention
|
Comparison
|
Intervention
|
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Local/regional recurrence | 16 | 1.5 | 27 | 2.6 | 20 | 4.4 | 15 | 3.3 |
| Distant recurrence | 111 | 10.7 | 119 | 11.6 | 72 | 15.9 | 42 | 9.4 |
| New primary | 16 | 1.5 | 24 | 2.3 | 15 | 3.3 | 15 | 3.4 |
| No evidence of recurrence | 895 | 86.2 | 859 | 83.5 | 346 | 76.4 | 375 | 83.9 |
| Total | 1,038 | 100 | 1,029 | 100 | 453 | 100 | 447 | 100 |
NOTE. Likelihood ratio test of group by hot flash status interaction, P = .002.
Univariate Differences by Study Group Among the HF-Negative and HF-Positive Subgroups
As expected, differences were observed between the HF-positive and HF-negative subgroups. Women in the HF-negative subgroup were significantly younger, more likely to be premenopausal and node negative, and less likely to have had bilateral oophorectomy; fewer had had ER-positive/PR-positive tumors, taken MHT, or received chemotherapy or antiestrogen therapy; and they reported a better quality of life (Table 2). Random assignment achieved reasonably comparable groups for key covariates by baseline HF category (Table 2). However, history of antiestrogen therapy was marginally higher in the intervention versus comparison group within both subgroups (HF negative: 56.5% v 49.7%, respectively; P = .05; HF positive: 76.8% v 73.3%, respectively; P = .0.07). The oophorectomy rate was higher in the intervention versus comparison group, particularly in the HF-negative subgroup (HF negative: 11.7% v 6.4%, respectively; P = .01; HF positive: 16.4% v 13.6%, respectively; P = .09). Report of prior MHT use also differed by study group in the HF-negative subgroup (intervention = 42.6% v comparison = 33.9%; P = .01), as did hormone receptor status in the HF-positive subgroup (P = .01). BMI did not vary across HF subgroups (P = .94) or by intervention and comparison group for either HF-positive (P = .47) or HF-negative women (P = .22).
Table 2.
Variables Associated With Random Assignment Group or Reported HF Status at Baseline
| Variable | HF Negative at Baseline
|
HF Positive at Baseline
|
P (HF positive vHF negative) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison
|
Intervention
|
P* | Comparison
|
Intervention
|
P* | ||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||||
| Age, years | .35 | .80 | < .001 | ||||||||
| < 45 | 131 | 28.9 | 112 | 25.1 | 137 | 13.2 | 125 | 12.2 | |||
| 45-54 | 132 | 29.1 | 132 | 29.5 | 478 | 46.1 | 491 | 47.7 | |||
| 55-59 | 61 | 13.5 | 54 | 12.1 | 204 | 19.7 | 193 | 18.8 | |||
| ≥ 60 | 129 | 28.5 | 149 | 33.3 | 219 | 21.1 | 220 | 21.4 | |||
| No. of positive lymph nodes | .58 | .63 | .01 | ||||||||
| 0 | 288 | 63.6 | 269 | 60.2 | 573 | 55.2 | 578 | 56.2 | |||
| 1-3 | 110 | 24.3 | 119 | 26.6 | 320 | 30.8 | 298 | 29 | |||
| > 3 | 55 | 12.1 | 59 | 13.2 | 145 | 14 | 152 | 14.8 | |||
| Grade | .26 | .47 | .04 | ||||||||
| Poor | 183 | 40.4 | 167 | 37.4 | 351 | 33.8 | 364 | 35.4 | |||
| Moderate | 170 | 37.5 | 170 | 38 | 423 | 40.8 | 422 | 41 | |||
| Well | 54 | 11.9 | 72 | 16.1 | 184 | 17.7 | 157 | 15.3 | |||
| Unspecified | 46 | 10.2 | 38 | 8.5 | 80 | 7.7 | 86 | 8.4 | |||
| Hormone receptor status | .63 | .01 | < .001 | ||||||||
| ER positive/PR positive | 239 | 54.9 | 252 | 57.7 | 680 | 66.7 | 666 | 66.1 | |||
| ER positive/PR negative | 54 | 12.4 | 56 | 12.8 | 105 | 10.3 | 132 | 13.1 | |||
| ER negative/PR positive | 16 | 3.7 | 19 | 4.3 | 59 | 5.8 | 32 | 3.2 | |||
| ER negative/PR negative | 126 | 29 | 110 | 25.2 | 175 | 17.2 | 177 | 17.6 | |||
| Tumor size, cm | .52 | .60 | .77 | ||||||||
| ≤ 2 | 268 | 59.2 | 254 | 56.8 | 614 | 59.3 | 596 | 58 | |||
| > 2 | 185 | 40.8 | 193 | 43.2 | 422 | 40.7 | 431 | 42 | |||
| Chemotherapy | .99 | .12 | < .001 | ||||||||
| No | 162 | 35.8 | 159 | 35.6 | 305 | 29.4 | 270 | 26.2 | |||
| Yes | 290 | 64.2 | 288 | 64.4 | 732 | 70.6 | 759 | 73.8 | |||
| Antiestrogen therapy | .05 | .07 | < .001 | ||||||||
| No | 225 | 50.3 | 190 | 43.5 | 274 | 26.7 | 237 | 23.2 | |||
| Yes | 222 | 49.7 | 247 | 56.5 | 751 | 73.3 | 784 | 76.8 | |||
| Previous menopausal hormone therapy | .01 | .75 | < .001 | ||||||||
| No | 297 | 66.1 | 256 | 57.4 | 507 | 48.9 | 494 | 48.1 | |||
| Yes | 152 | 33.9 | 190 | 42.6 | 529 | 51.1 | 532 | 51.9 | |||
| Oophorectomy | .01 | .09 | <.001 | ||||||||
| No | 424 | 93.6 | 393 | 88.3 | 892 | 86.4 | 857 | 83.6 | |||
| Yes | 29 | 6.4 | 52 | 11.7 | 141 | 13.6 | 168 | 16.4 | |||
| Menopausal status | .21 | .84 | < .001 | ||||||||
| Premenopausal | 128 | 28.3 | 103 | 23.1 | 51 | 4.9 | 46 | 4.5 | |||
| Perimenopausal | 44 | 9.7 | 47 | 10.5 | 95 | 9.2 | 90 | 8.8 | |||
| Postmenopausal | 281 | 62 | 296 | 66.4 | 891 | 85.9 | 891 | 86.8 | |||
| BMI, kg/m2 | .22 | .47 | .94 | ||||||||
| < 25 | 191 | 42 | 191 | 43 | 461 | 44 | 431 | 42 | |||
| 25-29.9 | 152 | 34 | 129 | 29 | 309 | 30 | 327 | 32 | |||
| ≥ 30 | 110 | 24 | 127 | 28 | 268 | 26 | 271 | 26 | |||
| Quality of life (SF-36 scale) | .31 | .12 | < .001 | ||||||||
| Mean | 77.5 | 78.1 | 74.3 | 75.6 | |||||||
| SD | 14.6 | 15.2 | 17.3 | 16.3 | |||||||
| Depression (CESD-log scale) | .31 | .69 | < .001 | ||||||||
| Mean | 0.04 | 0.04 | 0.07 | 0.06 | |||||||
| SD | 0.1 | 0.1 | 0.2 | 0.2 | |||||||
Abbreviations: HF, hot flash; ER, estrogen receptor; PR, progesterone receptor; BMI, body mass index; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey; SD, standard deviation; CESD, Center for Epidemiologic Studies Depression Scale.
P values are for comparison within random assignment arm based on χ2 tests for categorical variables or t tests for continuous variables.
Dietary Change Achieved by Intervention
Regardless of baseline HF status, the intervention group changed their diets significantly; compared with baseline, at 1 year, daily vegetable servings approximately doubled, fiber consumption increased 33%, fruit servings increased 20%, and energy from fat was reduced by 20% (Table 3), whereas intakes in the comparison group remained relatively unchanged. At 4 years, the intervention group consumed approximately 65% more vegetables than the comparison group, 25% more fruit, 30% more fiber, and 13% less energy from fat.21 Between-group differences in dietary intake were statistically significant at 1 and 4 years (P < .001). For each dietary component (vegetables, fruit, fiber, and energy from fat) as the dependent variable, the group × time interaction was statistically significant (P < .001), but the HF × group × time interaction was not statistically significant.
Table 3.
Mean Dietary Intake Over Time by Random Assignment Group and HF Status
| Dietary Intake by Random Assignment Group | Baseline
|
1 Year*
|
4 Years*
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF Negative†
|
HF Positive‡
|
HF Negative†
|
HF Positive‡
|
HF Negative†
|
HF Positive‡
|
|||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Total vegetable servings per day | ||||||||||||
| Intervention | 3.7 | 0.08 | 3.9 | 0.06 | 7.8 | 0.15 | 7.8 | 0.10 | 6.2 | 0.14 | 6.5 | 0.10 |
| Comparison | 3.8 | 0.09 | 3.8 | 0.06 | 3.9 | 0.09 | 3.8 | 0.06 | 3.6 | 0.08 | 3.7 | 0.06 |
| Total fruit servings per day | ||||||||||||
| Intervention | 3.4 | 0.10 | 3.5 | 0.07 | 4.0 | 0.09 | 4.3 | 0.06 | 3.5 | 0.10 | 3.7 | 0.06 |
| Comparison | 3.3 | 0.10 | 3.5 | 0.06 | 3.3 | 0.09 | 3.4 | 0.06 | 2.8 | 0.09 | 2.8 | 0.06 |
| Fiber, g/d | ||||||||||||
| Intervention | 20.8 | 0.37 | 21.2 | 0.26 | 29.2 | 0.47 | 29.2 | 0.26 | 24.5 | 0.40 | 25.7 | 0.31 |
| Comparison | 21.2 | 0.40 | 21.2 | 0.25 | 21.4 | 0.40 | 21.2 | 0.26 | 19.3 | 0.30 | 19.4 | 0.20 |
| Fat, % energy | ||||||||||||
| Intervention | 28.4 | 0.34 | 28.6 | 0.22 | 22.9 | 0.33 | 22.5 | 0.22 | 27.5 | 0.40 | 26.6 | 0.22 |
| Comparison | 28.9 | 0.34 | 28.6 | 0.22 | 28.5 | 0.33 | 28.3 | 0.22 | 31.2 | 0.40 | 31.4 | 0.20 |
NOTE. No significant three-way interactions between HF status, group, and time were observed (ie, the differences in intake by study group did not differ by HF status or duration of follow-up).
Abbreviation: HF, hot flash.
Differences between intervention and comparison group at this time period, P < .001 mixed models.
HF negative indicates no HFs reported at baseline.
HF positive indicates HFs reported at baseline.
Predictors of Additional Breast Cancer Events
As expected, larger tumor size, positive nodes, higher grade/proliferation markers, and being premenopausal were significantly associated with worse breast cancer event-free survival in the final multiple regression model. After controlling for these variables in the Cox model, HF-negative intervention women were 31% less likely to have an additional breast cancer event than HF-negative comparison women (hazard ratio [HR] = 0.69; 95% CI, 0.51 to 0.93; P = .02; Table 4). Both the intervention (HR = 0.77; 95% CI, 0.59 to 1.00; P = .05) and comparison groups (HR = 0.65; 95% CI, 0.49 to 0.85; P = .002) in the HF-positive subgroup had a significantly better prognosis than the HF-negative comparison group (HF × group interaction, P = .005).
Table 4.
Final Multiple Regression Model* of Predictors of Additional Breast Cancer Events
| Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Menopausal status at baseline | |||
| Postmenopausal | 1.00 | ||
| Perimenopausal | 1.10 | 0.80 to 1.52 | .55 |
| Premenopausal | 1.75 | 1.33 to 2.29 | < .001 |
| Tumor size, cm | |||
| ≤ 2 | 1.00 | ||
| > 2 | 1.71 | 1.40 to 2.07 | < .001 |
| No. of positive lymph nodes | |||
| 0 | 1.00 | ||
| 1-3 | 1.53 | 1.22 to 1.90 | < .001 |
| > 3 | 3.24 | 2.58 to 4.09 | < .001 |
| Tumor grade (differentiation) | |||
| Well | 1.00 | ||
| Moderate | 1.49 | 1.05 to 2.12 | .03 |
| Poor | 1.74 | 1.21 to 2.49 | .003 |
| Unspecified | 1.62 | 1.05 to 2.51 | .03 |
| Hormone receptor status | |||
| ER positive/PR positive | 1.00 | ||
| ER positive/PR negative | 1.22 | 0.93 to 1.61 | .15 |
| ER negative/PR positive | 1.47 | 0.98 to 2.20 | .07 |
| ER negative/PR negative | 1.01 | 0.76 to 1.33 | .96 |
| Antiestrogen therapy | |||
| No | 1.00 | ||
| Yes | 0.84 | 0.66 to 1.06 | .14 |
| HF × group interaction† | |||
| HF negative,‡ comparison group | 1.00 | ||
| HF negative,‡ intervention group | 0.69 | 0.51 to 0.93 | .02 |
| HF positive,§ comparison group | 0.65 | 0.49 to 0.85 | .002 |
| HF positive,§ intervention group | 0.77 | 0.59 to 1.00 | .05 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HF, hot flash.
Adjusted for all variables listed and for clinical site and quality of life.
Likelihood ratio test from group × HF status interaction, P = .005.
HF negative indicates no hot flashes reported at baseline.
HF positive indicates hot flashes reported at baseline.
Sensitivity Analyses
Additional stratified multiple regression analyses were conducted to test the robustness of this association (Table 5). The trends reported earlier were also observed for the following subgroups: postmenopausal women, antiestrogen users, nonusers of MHT, women with ER-positive/PR-positive tumors, and nonoophorectomized women. However, although the dietary intervention effect was observed in postmenopausal women (HR = 0.53, P = .003), no such effect of the intervention was observed for premenopausal women in the HF-negative subgroup (HR = 1.16; 95% CI, 0.68 to 1.97; P = .60). The protective trends for the intervention diet were in the same direction but not statistically significant for perimenopausal women, all subgroups of ER/PR status, nonusers of antiestrogen therapy, and users of prior MHT.
Table 5.
Multiple Regression* HRs for Additional Breast Cancer Events Stratified by Key Tumor or Treatment Variables
| Variable and Group† | HR | 95% CI | P |
|---|---|---|---|
| All | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.69 | 0.51 to 0.93 | .02 |
| HF positive, comparison group | 0.65 | 0.49 to 0.85 | .002 |
| HF positive, intervention group | 0.77 | 0.59 to 1.00 | .05 |
| Received antiestrogen | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.54 | 0.34 to 0.84 | .007 |
| HF positive, comparison group | 0.57 | 0.40 to 0.82 | .002 |
| HF positive, intervention group | 0.65 | 0.46 to 0.92 | .01 |
| Did not receive antiestrogen | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.79 | 0.52 to 1.21 | .28 |
| HF positive, comparison group | 0.67 | 0.43 to 1.03 | .07 |
| HF positive, intervention group | 0.83 | 0.55 to 1.27 | .4 |
| Received prior menopausal hormone therapy | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.77 | 0.44 to 1.34 | .36 |
| HF positive, comparison group | 0.80 | 0.51 to 1.25 | .32 |
| HF positive, intervention group | 0.91 | 0.59 to 1.42 | .68 |
| Did not receive prior menopausal hormone therapy | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.70 | 0.48 to 1.01 | .06 |
| HF positive, comparison group | 0.60 | 0.42 to 0.86 | .005 |
| HF positive, intervention group | 0.73 | 0.52 to 1.02 | .07 |
| ER positive/PR positive | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.65 | 0.42 to 1.00 | .05 |
| HF positive, comparison group | 0.60 | 0.42 to 0.87 | .007 |
| HF positive, intervention group | 0.65 | 0.45 to 0.93 | .02 |
| ER positive/PR negative | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.48 | 0.20 to 1.13 | .09 |
| HF positive, comparison group | 0.51 | 0.24 to 1.12 | .09 |
| HF positive, intervention group | 0.66 | 0.33 to 1.30 | .23 |
| ER negative/PR positive | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.81 | 0.16 to 4.01 | .80 |
| HF positive, comparison group | 0.56 | 0.14 to 2.23 | .42 |
| HF positive, intervention group | 1.00 | 0.23 to 4.25 | 1.0 |
| ER negative/PR negative | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.82 | 0.47 to 1.44 | .48 |
| HF positive, comparison group | 0.66 | 0,38 to 1.15 | .14 |
| HF positive, intervention group | 0.95 | 0.56 to 1.60 | .84 |
| Premenopausal | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 1.16 | 0.68 to 1.97 | .60 |
| HF positive, comparison group | 0.53 | 0.22 to 1.25 | .15 |
| HF positive, intervention group | 0.70 | 0.31 to 1.57 | .39 |
| Perimenopausal | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.52 | 0.17 to 1.62 | .26 |
| HF positive, comparison group | 0.65 | 0.26 to 1.59 | .34 |
| HF positive, intervention group | 0.60 | 0.25 to 1.47 | .26 |
| Postmenopausal | |||
| HF negative, comparison group | 1.0 | ||
| HF negative, intervention group | 0.53 | 0.35 to 0.81 | .003 |
| HF positive, comparison group | 0.61 | 0.45 to 0.84 | .003 |
| HF positive, intervention group | 0.72 | 0.53 to 0.98 | .04 |
| Had bilateral oophorectomy | |||
| HF negative, comparison group | 1.00 | ||
| HF negative, intervention group | 2.49 | 0.48 to 13.01 | .28 |
| HF positive, comparison group | 3.50 | 0.79 to 15.47 | .10 |
| HF positive, intervention group | 2.23 | 0.50 to 9.95 | .29 |
| Did not have bilateral oophorectomy | |||
| HF negative, comparison group | 1.00 | ||
| HF negative, intervention group | 0.64 | 0.46 to 0.88 | .006 |
| HF positive, comparison group | 0.59 | 0.44 to 0.78 | .0002 |
| HF positive, intervention group | 0.75 | 0.57 to 0.99 | .04 |
Abbreviations: HR, hazard ratio; HF, hot flash; ER, estrogen receptor; PR, progesterone receptor.
Adjusted for all variables listed as well as tumor size, grade, number of lymph nodes, clinical site and quality of life. (Note: tumor grade was omitted from the ER−/PR+ model, and menopause status was omitted from the “had bilateral oophorectomy” model due to small cell counts).
bHF− = no hot flashes reported at baseline.
cHF+ = hot flashes reported at baseline.
Furthermore, additional separate Cox models were run that included covariates omitted by the modeling criteria, excluded women who were premenopausal or ≤ 45 years old, and excluded 205 women who had stopped taking antiestrogen therapy before enrollment. None of these models yielded different results. When previously omitted covariates (eg, stage, tumor type, history of chemotherapy or radiation therapy, oophorectomy, race, smoking status, age, prior MHT use, and BMI) were included, the HRs changed less than 10% for study group, HF status, and group × HF interaction terms. Additional analyses tested three-way interaction terms in the Cox models, considering HF × study group with hormone receptor status, menopausal status, and use of antiestrogen therapy, none of which were statistically significant (all likelihood ratio tests for interactions, P > .3). When we reran the Cox model excluding women who were premenopausal or ≤ 45 years old at baseline, the HR for HF-negative women in the intervention group was 0.49 (95% CI, 0.32 to 0.75; P = .001; reference = HF-negative women in comparison group), indicating a strong diet effect among HF-negative peri-/postmenopausal women. Exclusion of the 205 women who had stopped antiestrogen therapy before entering the study also did not alter the results.
DISCUSSION
Although the WHEL Study found no overall benefit to early-stage breast cancer survivors who were assigned to adopt the dietary intervention pattern that was high in vegetables, fruit, and fiber and low in fat compared with survivors who were assigned to the five-a-day group, this dietary intervention was associated with reduced risk of second breast cancer events among women who reported no HFs at baseline. These women had a 31% lower event rate than HF-negative women in the comparison group over 7.3 years of follow-up; among HF-negative postmenopausal women, the intervention effect was even stronger, with a 47% reduction in risk compared with HF-negative women assigned to the comparison group. Compared with HF-negative women in the comparison group, women with baseline HFs had a lower risk of additional breast cancer events, regardless of whether they were randomly assigned to the dietary intervention group or to the comparison group.
The poorer prognosis among HF-negative women and the protective effect of the WHEL intervention diet in this subgroup may be related to circulating estrogen concentrations. In our study, circulating estrogen concentrations were significantly associated both with HFs and with study outcomes.15 Furthermore, early work suggests that this dietary pattern may lower bioavailable estradiol, at least in the short term.29 Such an effect is expected from the between-group differences in both dietary fiber and energy from fat that were achieved and maintained in the study. Measurement of changes in circulating estrogen concentrations over the duration of the study will enable us to address this issue more fully in the future.
Although lower circulating estrogen concentrations may be at least partially responsible for the favorable impact of the intervention in the HF-negative group, other studies have reported that fewer women taking the aromatase inhibitor anastrozole had HFs than those taking tamoxifen, despite having lower circulating levels of estradiol;30 consistent with our findings, women without HFs had a poorer prognosis.2 Polymorphisms of genes involved in estrogen synthesis and metabolism are also linked to HFs,31,32 and polymorphism phenotypes of CYP2D6 have been hypothesized to metabolize tamoxifen differentially to its active form (eg, endoxifen), thus leading to differential success of antiestrogen therapy and also differential occurrence of HFs. We are currently measuring polymorphisms of CYP2D6 and endoxifen levels to test this hypothesis further. However, in the WHEL Study, baseline HFs were associated with more favorable disease outcomes, regardless of whether the woman was on antiestrogen therapy (ie, tamoxifen). Thus, the explanations of the etiology of HFs, their apparent relationship to breast cancer outcome, and the interaction with dietary intake are likely to be much more complex than that provided by simple estrogen concentrations.33–38
It is possible that the observed protective effect of the dietary intervention among HF-negative women was an artifact resulting from the intervention group having a higher proportion of women who had either used antiestrogens or had an oophorectomy or were postmenopausal or perimenopausal (86% in the intervention group v 78% in the comparison group, P = .003). Imbalances in antiestrogen use and bilateral oophorectomy between study groups were observed in both the HF-negative and HF-positive subgroups but were statistically significant only in the HF-negative subgroup and could, by themselves, have resulted in a small reduction in breast cancer–related events. Similar imbalances between intervention and comparison groups were observed in the entire WHEL sample but did not result in any overall difference in event rates.14 Notably, in the present analyses, the selective protective effect of the WHEL dietary intervention in the HF-negative subgroup persisted when we controlled for these imbalances in the multiple regression Cox model and when the study population was limited to the following groups: women who received antiestrogen therapy; women who did not receive antiestrogen therapy; women who did not have bilateral oophorectomy; and postmenopausal women. Nonetheless, because we did not stratify on these important variables in our original random assignment scheme, our findings need to be interpreted with caution.
In this secondary analysis of the WHEL Study, a diet high in vegetables, fruit, and fiber with reduced fat seemed to remove the excess risk of additional breast cancer events after treatment for early-stage breast cancer in women without HFs, previously reported for both the WHEL comparison group1 and in the Arimidex, Tamoxifen Alone or in Combination study.2 This dietary effect was robustly observed across a series of sensitivity analyses. It was not modified by hormone receptor status or use of antiestrogen therapy and was not observed in participants who reported HFs. These results suggest that a major change in dietary pattern may be beneficial for some breast cancer survivors, although these findings need to be confirmed in a trial that has as its primary objective the determination of the differential effect of the diet in breast cancer survivors with and without HFs.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ellen B. Gold, John P. Pierce, Loki Natarajan, Gail A. Laughlin, Cheryl L. Rock, Joanne E. Mortimer
Financial support: Ellen B. Gold, John P. Pierce, Marcia L. Stefanick, Bette J. Caan, Cynthia A. Thomson, Njeri Karanja, Vicky Jones, Richard A. Hajek
Administrative support: John P. Pierce, Shirley W. Flatt, Lisa Madlensky, Sheila Kealey, Linda Wasserman, Barbara A. Parker
Provision of study materials or patients: Ellen B. Gold, John P. Pierce, Marcia L. Stefanick, Bette J. Caan, Linda Wasserman, Cynthia A. Thomson, Barbara A. Parker, Njeri Karanja, Vicky Jones, Richard A. Hajek
Collection and assembly of data: Shirley W. Flatt
Data analysis and interpretation: Ellen B. Gold, John P. Pierce, Loki Natarajan, Bette J. Caan, Shirley W. Flatt, Jennifer A. Emond, Nazmus Saquib, Lisa Madlensky, Linda Wasserman, Cynthia A. Thomson, Cheryl L. Rock, Barbara A. Parker, Minya Pu
Manuscript writing: Ellen B. Gold, John P. Pierce, Loki Natarajan, Gail A. Laughlin, Bette J. Caan, Cynthia A. Thomson
Final approval of manuscript: Ellen B. Gold, John P. Pierce, Loki Natarajan, Shirley W. Flatt, Sheila Kealey, Joanne E. Mortimer
Acknowledgments
We thank Wael Al-Delaimy, MD, PhD, for constructive comments on the article; Chris Hayes, MA, Patricia Hallstrom, and Hollie Ward, BA, for administrative support and assistance in article preparation; and the dietary counselors and Women's Healthy Eating and Living Study participants.
Appendix
The Women's Healthy Eating and Living (WHEL) study group included the following members and institutions. WHEL Study Coordinating Center: University of California, San Diego (UCSD), Cancer Prevention and Control Program, Moores UCSD Cancer Center, San Diego, CA (John P. Pierce, PhD; Susan Faerber, BA; Barbara A. Parker, MD; Loki Natarajan, PhD, Cheryl L. Rock, PhD, RD; Vicky A. Newman, MS, RD; Shirley W. Flatt, MS; Sheila Kealey, MPH; Linda Wasserman, MD, PhD; Wayne A. Bardwell, PhD; Lisa Madlensky, PhD; Wael Al-Delaimy, MD, PhD).
WHEL Study clinical sites: Center for Health Research-Portland, Portland, OR (Njeri Karanja, PhD; Mark U. Rarick, MD); Kaiser Permanente Northern California, Oakland, CA (Bette J. Caan, DrPH; Lou Fehrenbacher, MD); Stanford Prevention Research Center, Stanford University, Stanford, CA (Marcia L. Stefanick, PhD; Robert Carlson, MD); University of Arizona, Tucson and Phoenix, AZ (Cynthia Thomson, PhD, RD; James Warneke, MD; Cheryl Ritenbaugh, PhD, MPH); University of California, Davis, Davis, CA (Ellen B. Gold, PhD; Sidney Scudder, MD); UCSD, Moores UCSD Cancer Center, San Diego, CA (Kathryn A. Hollenbach, PhD; Vicky Jones, MD); The University of Texas M.D. Anderson Cancer Center, Houston, TX (Lovell A. Jones, PhD; Richard Hajek, PhD; Richard Theriault, DO).
published online ahead of print at www.jco.org on December 15, 2008.
Supported by the Walton Family Foundation and National Cancer Institute Grant No. CA 69375. Some of the data were collected from General Clinical Research Centers supported by National Institutes of Health Grant Nos. M01-RR00070, M01-RR00079, and M01-RR00827.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003787
REFERENCES
- 1.Mortimer JE, Flatt SW, Parker BA, et al: Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 108:421-426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J: Hot flushes and the risk of recurrence: Retrospective, exploratory results from the ATAC trial. 30th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 13-16, 2007. (poster 2069)
- 3.Wilbur J, Miller AM, Montgomery A, et al: Sociodemographic characteristics, biological factors, and symptom reporting in midlife women. Menopause 5:43-51, 1998 [PubMed] [Google Scholar]
- 4.Matthews KA, Wing RR, Kuller LH, et al: Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med 154:2349-2355, 1994 [PubMed] [Google Scholar]
- 5.Randolph JF Jr, Sowers M, Bondarenko I, et al: The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 90:6106-6112, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Mom CH, Buijs C, Willemse PH, et al: Hot flushes in breast cancer patients. Crit Rev Oncol Hematol 57:63-77, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Swain SM, Land SR, Ritter MW, et al: Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat [epub ahead of print on February 27, 2008] [DOI] [PMC free article] [PubMed]
- 8.Kendall A, Folkerd EJ, Dowsett M: Influences on circulating oestrogens in postmenopausal women: Relationship with breast cancer. J Steroid Biochem Mol Biol 103:99-109, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Goldin BR, Woods MN, Spiegelman DL, et al: The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer 74:1125-1131, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Whitten C, Shultz T: Binding of steroid hormone in vitro by water-soluble dietary fiber. FASEB J 1:A862, 1988. (abstr) [Google Scholar]
- 11.Shultz TD, Howie BJ: In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer 8:141-147, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Monroe KR, Murphy SP, Henderson BE, et al: Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The multiethnic cohort study. Nutr Cancer 58:127-135, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Wu AH, Pike MC, Stram DO: Meta-analysis: Dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst 91:529-534, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Pierce JP, Natarajan L, Caan BJ, et al: Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women's Healthy Eating and Living (WHEL) randomized trial. JAMA 298:289-298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rock CL, Flatt SW, Laughlin GA, et al: Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomarkers Prev 17:614-620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer: Manual for Staging of Cancer (ed 6). New York, NY, Springer-Verlag, 2002
- 17.Pierce JP, Faerber S, Wright FA, et al: A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials 23:728-756, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Pierce JP, Newman VA, Flatt SW, et al: Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr 134:452-458, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Newman VA, Thomson CA, Rock CL, et al: Achieving substantial changes in eating behavior among women previously treated for breast cancer: An overview of the intervention. J Am Diet Assoc 105:382-391, 2005 [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture/Department of Health and Human Services: Dietary Guidelines for Americans. Home Health and Garden Bulletin No. 232. Washington, DC, U.S. Department of Health and Human Services, 1995
- 21.Pierce JP, Newman VA, Natarajan L, et al: Telephone counseling helps maintain long-term adherence to a high-vegetable dietary pattern. J Nutr 137:2291-2296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce JP, Natarajan L, Sun S, et al: Increases in plasma carotenoid concentrations in response to a major dietary change in the Women's Healthy Eating and Living Study. Cancer Epidemiol Biomarkers Prev 15:1886-1892, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Saquib N, Flatt SW, Natarajan L, et al: Weight gain and recovery of pre-cancer weight after breast cancer treatments: Evidence from the Women's Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat 105:177-186, 2007 [DOI] [PubMed] [Google Scholar]
- 24.WHO: Research on the Menopause in the 1990s. Geneva, Switzerland, WHO, 1996
- 25.Burnam MA, Wells KB, Leake B, et al: Development of a brief screening instrument for detecting depressive disorders. Med Care 26:775-789, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Tuunainen A, Langer RD, Klauber MR, et al: Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res 103:261-270, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ware JE Jr, Sherbourne CD: The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 30:473-483, 1992 [PubMed] [Google Scholar]
- 28.Bardwell WA, Major JM, Rock CL, et al: Health-related quality of life in women previously treated for early-stage breast cancer. Psychooncology 13:595-604, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock CL, Flatt SW, Thomson CA, et al: Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol 22:2379-2387, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Dixon JM, Renshaw L, Young O, et al: Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol 26:1671-1676, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Crandall CJ, Crawford SL, Gold EB: Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med 119:S52-S60, 2006. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 32.Woods NF, Mitchell ES, Tao Y, et al: Polymorphisms in the estrogen synthesis and metabolism pathways and symptoms during the menopausal transition: Observations from the Seattle Midlife Women's Health Study. Menopause 13:902-910, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Stone SC, Mickal A, Rye PH: Postmenopausal symptomatology, maturation index, and plasma estrogen levels. Obstet Gynecol 45:625-627, 1975 [DOI] [PubMed] [Google Scholar]
- 34.Hutton JD, Jacobs HS, Murray MA, et al: Relation between plasma oestrone and oestradiol and climacteric symptoms. Lancet 1:678-681, 1978 [DOI] [PubMed] [Google Scholar]
- 35.Studd JW, Chakravarti S, Collins WP: Plasma hormone profiles after the menopause and bilateral oophorectomy. Postgrad Med J 54:25-30, 1978. (suppl 2) [PubMed] [Google Scholar]
- 36.James CE, Breeson AJ, Kovacs G, et al: The symptomatology of the climacteric in relation to hormonal and cytological factors. Br J Obstet Gynaecol 91:56-62, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Abe T, Furuhashi N, Yamaya Y, et al: Correlation between climacteric symptoms and serum levels of estradiol, progesterone, follicle-stimulating hormone, and luteinizing hormone. Am J Obstet Gynecol 129:65-67, 1977 [DOI] [PubMed] [Google Scholar]
- 38.Freedman RR, Norton D, Woodward S, et al: Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab 80:2354-2358, 1995 [DOI] [PubMed] [Google Scholar]