Abstract
Purpose
To investigate whether retinyl palmitate (RP) alone or plus beta-carotene (BC) would be as effective and less toxic than low-dose 13-cis retinoic acid (13cRA) in treating oral premalignant lesions (OPLs) and reducing the risk of oral cancer.
Patients and Methods
Initially, patients were randomly assigned to receive low-dose 13cRA or BC plus RP for 3 years (plus 2 years follow-up). After other randomized trials established an adverse effect of BC on lung cancer incidence/mortality, BC was dropped (patients randomly assigned to 13cRA or RP alone). The primary end point was OPL clinical response at 3 months.
Results
We randomly assigned 162 eligible patients. The 3-month clinical response rate of the combined BC plus RP and RP alone arm (32.5%) was not statistically equivalent to that of 13cRA (48.1%). The clinical response rate of RP alone (20.0%) was significantly lower than that of BC plus RP (42.9%; P = .03). Similar oral cancer–free survival rates were observed across all arms. There was no significant association between 3-month OPL response and subsequent oral cancer development (P = .11). Grades 2 and higher adverse events were more common in the 13cRA than other groups (P < .0001).
Conclusion
This large chemoprevention trial did not establish the equivalence of RP plus BC or RP alone with low-dose 13cRA in reducing the long-term risk of oral cancer. At present, 13cRA, BC plus RP, and RP alone cannot be recommended for chemoprevention, and new, better agents are needed in this setting. Our results did not establish short-term OPL response as a surrogate end point for oral cancer–free survival.
INTRODUCTION
Clinical oral premalignant lesions (OPLs) progress to carcinoma at a rate of approximately 30% over 8 years.1 The major standard approaches for managing OPLs are surgical resection and observation with biopsies indicated for clinical change or progression. However, surgery involves morbidity and high recurrence rates and does not address the development of multifocal neoplastic lesions in the entire epithelial field at risk.2 These limitations underscore the need for systemic agents for oral cancer prevention in the setting of OPLs.
A strong rationale supported the present systemic chemoprevention trial involving 13-cis retinoic acid (13cRA), beta-carotene (BC), and vitamin A (or retinol) in the form of retinyl palmitate (RP).2–4 Two prior randomized studies in oral leukoplakia patients have shown that vitamin A alone,5 BC alone,6 or vitamin A plus BC6 produced significantly more objective responses than did placebo, with negligible toxicity. Our group conducted two randomized trials of 13cRA in OPL patients leading up to the present trial. The first showed 3-month response rates of 67% for high-dose 13cRA (1 to 2 mg/kg/d) versus 10% for placebo (P = .0002),7 but intolerable toxicity and rapid OPL recurrence after 13cRA discontinuation. In the second trial (3 months of high-dose 13cRA [1.5 mg/kg/d] followed by random assignment of stable or responding patients to a 9-month maintenance phase of low-dose 13cRA [0.5 mg/kg/d] v BC),8 low-dose 13cRA prevented progression significantly better than did BC and improved the durability of responses (compared with the first trial), but still with substantial toxicity.9
These prior trials established the activity of low-dose 13cRA8 and suggested that an even lower dose would be needed for tolerable treatment lasting longer than 1 year and a follow-up trial should omit placebo, which could hinder accrual in light of the well-known clinical activity of 13cRA against OPLs.7,8 This trial was designed to investigate whether BC plus RP would be an effective and less-toxic alternative to lower-dose 13cRA (0.5 mg/kg/d for 1 year followed by 0.25 mg/kg/d for 2 years).
PATIENTS AND METHODS
This prospective, randomized, two-arm, open-label trial was conducted at a single institution, The University of Texas M. D. Anderson Cancer, with the approval of the institutional review board. The trial was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient Eligibility
Eligibility criteria were as follows: age ≥ 18 years; clinical and histologic evidence of measurable or assessable OPLs (leukoplakia and/or erythroplakia); histologic examination showing dysplasia or extensive leukoplakia with hyperplasia and symptoms (eg, pain), cosmetic cases (eg, leukoplakia of the lips), or high-risk location (ie, soft palate, floor of mouth, ventral tongue, and alveolar ridge); bilirubin lower than 2 mg/dL, creatinine lower than 1.5 mg/dL, WBC count higher than 3,000/mm3, and platelets higher than 100,000/mm3; Karnofsky performance status ≥ 80%; and signed informed consent form.
Exclusion criteria were female patients of child-bearing potential, unless practicing adequate contraception; acute intercurrent illnesses, infection, or a surgical procedure within the past 4 weeks unless fully recovered; history of cancer, excepting nonmelanoma skin cancer, within the preceding 2 years; use of retinoid or carotenoid supplements within the preceding 3 months; fasting triglyceride level before study entry ≥ 2.5 times the upper limit of normal.
Treatment Plan
After verification of eligibility, patients underwent complete history (including details of alcohol and tobacco consumption), physical examination with description, mapping and bidimensional measurements of the lesion(s), lesion photography, CBC and serum biochemistry tests, and a cotinine level measurement. Patients were stratified by dysplasia versus hyperplasia and randomly assigned to 13cRA (0.5 mg/kg/d orally for 1 year followed by 0.25 mg/kg/d orally for 2 years) or BC (50 mg/d orally) plus RP (25,000 U/d orally) for 3 years and later (by protocol revision) to 13cRA or RP alone (25,000 U/d orally; shown to be more active than BC10). After 3 years of treatment and an additional 2 years of follow-up on protocol, patients were observed whenever possible. Evaluations included regular clinic visits for toxicity assessments (according to the National Cancer Institute Common Toxicity Criteria modified to include retinoid-specific toxicities8), color photography and bi-dimensional lesion measurements and biopsies (at months 3, 12, 36, and 60), blood chemistry tests, and compliance measurements (by capsule counts). In the event of ≥ grade 2 toxicity, drug was held until the toxicity subsided to ≤ grade 1 and restarted at 50% of the dose; if ≥ grade 3 toxicity recurred, drug was held until toxicity subsided to grade 1 and restarted at the reduced (50%) dose. Treatment could be prematurely discontinued for clinical or histologic progression (either to carcinoma in situ or invasive carcinoma), unacceptable toxicity, loss to follow-up, intercurrent illness, or death.
Statistical Considerations
The primary end point was OPL clinical response at 3 months. Clinical responses were defined as follows: complete response—disappearance of all measurable and assessable lesions for at least 1 month; partial response—≥ 50% reduction of all measurable and assessable lesions for at least 1 month; progressive disease—appearance of new lesions or enlargement ≥ 25% of existent lesions; no change—stabilization of all existing lesions, with no new lesions developing, no progressive disease, or less than partial response. Clinical response was assessed by a dental oncologist (J.W.M.) who was blinded to the treatment assignment.
Secondary end points included toxicity, improvement in histology, and oral cancer free–survival (time from random assignment until diagnosis of any squamous cell carcinoma of the oral cavity). Improvement in histology was defined as regression of hyperplasia or dysplasia or downgrading of dysplastic lesions.
This study was originally designed as an equivalence (noninferiority) trial to compare the efficacy of 13cRA with that of BC plus RP. The original accrual goal was 124 patients to achieve a 72% power for detecting equivalent (within a range of 20%) 3-month clinical response rates with a two-sided 10% significance level assuming a 60% response rate in both arms based on our prior study.7 The trial was redesigned, after substantial accrual, to discontinue BC because of its adverse effect on lung cancer incidence and mortality in the Alpha-Tocopherol and Beta-Carotene (ATBC) study11 and Beta-Carotene and Retinol Efficacy trial (CARET).12 Assuming nonstatistically different efficacy of BC plus RP from RP alone, we planned to compare the two arms combined with the 13cRA arm. If statistically different, the BC plus RP and RP alone arms would be compared with 13cRA separately. Based on the 46% pooled-arms response rate at the time (no data unblinding), the revised sample size was 154 randomized and assessable patients (77 in the 13cRA arm and 77 in the BC plus RP and RP alone arms combined) to provide 80% power for detecting equivalence with a 20% equivalence range (true response rate difference of < 20% in either direction) and two-sided 10% significance level. If the upper limit of the 90% CI (two sided) for the response rate difference did not exceed 20%, equivalence would be established with 5% one-sided or 10% two-sided significance level.13–15 Analysis of the primary end point was based on randomized, eligible, and assessable patients; analysis of oral cancer free–survival was performed in the entire intent-to-treat population (ie, all randomly assigned patients). Time to cancer development between responders and nonresponders was compared in a landmark analysis using a starting point of month 3 after random assignment (the time of response evaluation) rather than month 0 (time of random assignment).
Continuous variables were compared by the Wilcoxon rank sum test and Wilcoxon signed-rank test for data obtained from independent and paired samples, respectively. Categoric variables were analyzed by χ2 test and Fisher's exact test whenever appropriate. Time-to-event end points were analyzed by the Kaplan-Meier method, and differences between treatment arms were assessed with the use of log-rank test. All P values are two sided.
RESULTS
Patient Characteristics
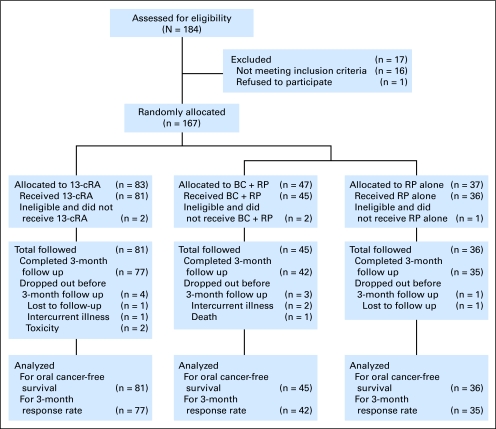
From August 1992 to March 2001, we randomly assigned a total of 167 patients, of whom 162 were eligible to continue on study; five patients were removed from study because of the use of high-dose BC (n = 2), a history of invasive squamous cell carcinoma (n = 1), intercurrent illness and drop-out (n = 1), and a misclassified oral lesion (n = 1). Eighty-one patients were assigned to the 13cRA arm, 45 to the BC plus RP arm and 36 to the RP arm (Fig 1). Patient characteristics are outlined in Table 1. There were no statistically significant differences in medical demographic variables among the three arms. Baseline cotinine levels (a marker of tobacco exposure) were available for 124 of 162 patients. Cotinine levels were below 20 ng/mL in 100% of never smokers, 92% of former smokers, and 8.5% of current smokers (n = 29, 48, and 47, respectively), indicating a high correlation between self-reported smoking status at baseline and expected levels. This correlation was maintained during the follow-up period—99%, 90%, and 9% of the samples from never, former, and current-smokers, respectively, had cotinine levels lower than 20 ng/mL. During follow-up, two former smokers in each treatment arm reported resuming smoking; and seven, two, and one patients in the 13cRA, BC plus RP, and RP arms, respectively, quit smoking (P = .52, Fisher's exact test), among whom two in the 13cRA arm and one in the RP arm resumed smoking again.
Fig 1.
Flow diagram of patients screened, enrolled, observed, and analyzed. 13cRA, 13-cis retinoic acid; BC + RP, beta-carotene plus retinyl palmitate; RP, retinyl palmitate.
Table 1.
Patient Characteristics
| Characteristic | All Patients | 13cRA |
BC + RP |
RP |
P | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Sex | .53 | |||||||
| Female | 77 | 36 | 44.4 | 21 | 46.7 | 20 | 55.6 | |
| Male | 85 | 45 | 55.6 | 24 | 53.3 | 16 | 44.4 | |
| Race | .14 | |||||||
| Asian | 6 | 2 | 2.5 | 1 | 2.2 | 3 | 8.3 | |
| Black | 4 | 2 | 2.5 | 0 | 0.0 | 2 | 5.6 | |
| Hispanic | 7 | 1 | 1.2 | 4 | 8.9 | 2 | 5.6 | |
| White | 145 | 76 | 93.8 | 40 | 88.9 | 29 | 80.5 | |
| Histology at baseline | .63 | |||||||
| Dysplasia | 53 | 26 | 32.1 | 13 | 28.9 | 14 | 38.9 | |
| Hyperplasia | 109 | 55 | 67.9 | 32 | 71.1 | 22 | 61.1 | |
| Mean age, years | 56.0 | 56.9 | 55.0 | 55.4 | .68 | |||
| SD | 13.5 | 14.0 | 12.2 | 14.4 | ||||
| Median | 56 | 58 | 58 | 54 | ||||
| Range | 23-90 | 27-81 | 24-79 | 23-90 | ||||
| Smoking status | .71 | |||||||
| Current | 56 | 31 | 38.3 | 15 | 33.3 | 10 | 27.8 | |
| Former | 65 | 32 | 39.5 | 19 | 42.2 | 14 | 38.9 | |
| Never | 41 | 18 | 22.2 | 11 | 24.5 | 12 | 33.3 | |
| Alcohol intake status | .07 | |||||||
| Current | 93 | 49 | 60.5 | 28 | 62.2 | 16 | 44.4 | |
| Former | 19 | 10 | 12.3 | 7 | 15.6 | 2 | 5.6 | |
| Never | 50 | 22 | 27.2 | 10 | 22.2 | 18 | 50.0 | |
| Total | 162 | 81 | 45 | 36 | ||||
Abbreviations: 13cRA, 13-cis retinoic acid; BC, beta-carotene; RP, retinyl palmitate; SD, standard deviation.
Interim Analysis
We performed an interim analysis in 1994 to assess whether BC plus RP was associated with harmful effects reported for the ATBC study.11 There were no safety concerns for BC plus RP, and the trial continued; BC was discontinued later (1996) when results of CARET12 were published. The related trial design revisions were approved by the M. D. Anderson institutional review board, trial data monitoring committee, and National Cancer Institute.
Treatment Characteristics and OPL Response
The median times on treatment for the 13cRA, BC plus RP, and RP arms were 1.1, 1.7, and 2.1 years, respectively. Median follow-up time for the censored observations was 7.5 years—7.7, 9.9, and 5.9 years for 13cRA, BC plus RP, and RP, respectively. Differences in these median times were due to longest overall enrollment in the BC plus RP arm (no new patients after 1996) and shortest overall enrollment in the RP arm (beginning in 1996). The median follow-up time for the combined arm of BC plus RP and RP alone was 7.2 years, which was comparable with median follow-up of the 13cRA arm (7.7 years). At the time of this analysis, all randomly assigned patients still being observed had completed at least 5 years of participation in the trial. The reasons for discontinuing treatment were disease progression (n = 86), completing 3 years of treatment (n = 18), lost to follow-up (n = 16), intercurrent illness (n = 11), toxicity (n = 6, all in the 13cRA arm) and requested withdrawal (n = 18, 13 of which were in the 13cRA arm), death (n = 2, unrelated to the study drugs), and other reasons (n = 5).
Rates of compliance (taking ≥ 85% of the prescribed medication) were similar in the three arms: 74.1% (13cRA), 72.7% (BC plus RP), and 77.8% (RP; P = .87, χ2 test). Dose reductions were required for 12 patients on 13cRA, one receiving BC plus RP, and three receiving RP.
The 3-month rates of clinical OPL response (complete plus partial) for the 13cRA, BC plus RP, and RP arms were 48.1%, 42.9%, and 20.0%, respectively. The rate of the combined BC plus RP and RP alone arm (32.5%) was not statistically equivalent to that of the 13cRA arm (P = .29). The two-sided 90% CIs for the difference in 3-month clinical response rate were −2.6% to 28.6% comparing 13cRA with the combined BC plus RP and RP arm. The upper CI limits exceed the prespecified equivalence margin of 20%. Therefore, the hypothesis of equivalence in clinical response rates was not established. The 3-month clinical response rate of the RP alone arm was significantly lower than that of the RP plus BC arm (P = .03). Histologic responses were similar between all arms of the study (Table 2).
Table 2.
Clinical and Histologic Responses at 3 Months
| Response | 13cRA |
BC + RP |
RP |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Clinical | ||||||
| CR | 4 | 4.9 | 2 | 4.4 | 1 | 2.8 |
| PR | 33 | 40.8 | 16 | 35.6 | 6 | 16.7 |
| No change | 36 | 44.5 | 19 | 40.0 | 26 | 66.6 |
| Progressive disease | 4 | 4.9 | 5 | 13.3 | 2 | 11.1 |
| Unassessable | 4 | 4.9 | 3 | 6.7 | 1 | 2.8 |
| Overall (CR + PR)/No.* | 37/77 | 48.1 | 18/42 | 42.9 | 7/35† | 20 |
| Histological | ||||||
| Improvement from baseline | 18 | 30 | 9 | 23.7 | 7 | 22.6 |
| No improvement from baseline | 42 | 70 | 29 | 76.3 | 24 | 77.4 |
Abbreviations: 13cRA, 13-cis retinoic acid; BC, beta-carotene; RP, retinyl palmitate; CR, complete response; PR, partial response; No., number of assessable patients.
The test of equivalence was not significant for the clinical response rates of the 13cRA arm and combined BC + RP and RP alone arm (P = .29).
The clinical response rate of the RP arm was significantly lower than that of the BC + RP arm (P = .03).
In a univariate analysis, there was no statistically significant association between a clinical objective response and sex, race, and baseline histology and smoking and alcohol consumption.
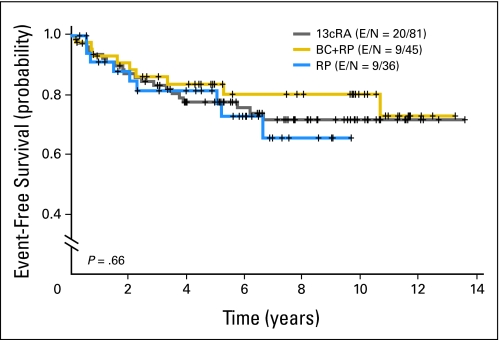
Oral Cancer–Free Survival
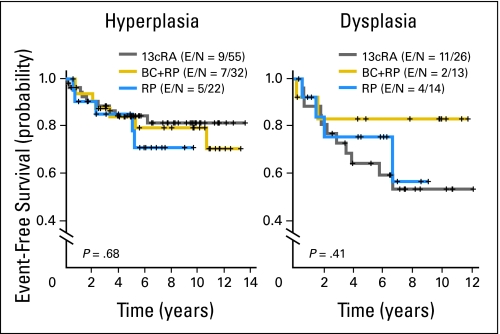
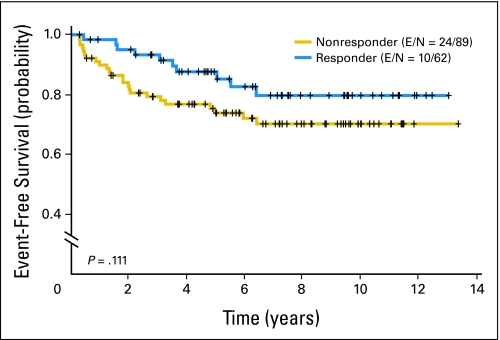
The 5-year oral cancer–free survival rates of the three arms were not significantly different—78% (13cRA), 84% (BC plus RP), and 82% (RP; P = .66 for the overall comparison; Fig 2). Patients with dysplasia had a trend toward a decreased oral cancer–free survival versus patients with hyperplasia—hazard ratio of 1.82 (v hyperplasia; 95% CI, 0.96 to 3.46; P = .07). In subgroup analyses, there were no significant differences in oral cancer–free survival among dysplasia or hyperplasia patients in the three treatment arms (Fig 3). There also was no statistically significant association between clinical responders (complete or partial) at 3 months and longer oral cancer–free survival—hazard ratio of 0.55 (v nonresponders; 95% CI, 0.27 to 1.16; P = .11, log-rank test; Fig 4).
Fig 2.
Oral cancer–free survival in the overall population according to treatment arm. 13cRA, 13-cis retinoic acid; BC + RP, beta-carotene plus retinyl palmitate; RP, retinyl palmitate; E, number of events; N, total number of patients per arm.
Fig 3.
Oral cancer–free survival by treatment in patients with hyperplasia or dysplasia. 13cRA, 13-cis retinoic acid; BC, beta-carotene; RP, retinyl palmitate; E, number of events; N, total number of patients per arm.
Fig 4.
Oral cancer-free survival according to the 3-month clinical response (all treatment arms combined). Landmark analysis was performed by resetting the time 0 to 3 months after random assignment, which corresponds to the time of response evaluation. Note that three nonresponders had oral cancer developed within 3 months and were removed from the analysis. E, number of events; N, total number of patients per arm.
Accounting for the differences in median follow-up times among the three arms, additional analyses censored follow-ups at 5 years and showed no statistically significant differences in cancer-free survival between the three arms or between the hyperplasia or dysplasia subgroups of the three arms. These analyses also showed no statistically significant association between OPL response and longer cancer-free survival (P = .072).
Toxicity
Overall rates of grades 2, 3, and 4 toxicities were 22%, 8%, and lower than 1%, respectively. Cheilitis, conjunctivitis, and skin reaction were significantly more common in the 13cRA arm compared with the combined BC plus RP and RP alone arm (P < .0001; P = .0003; and P < .0001, respectively; Fisher's exact test), and were mostly grade 1 (Table 3). Grade 2 or higher toxicities were also significantly higher in the 13cRA arm with the rates of 53% (13cRA), 9% (BC plus RP), and 6% (RP; P < .0001). No patients in the BC plus RP or RP alone arm and six patients in the 13cRA arm required discontinuation of treatment because of toxicity.
Table 3.
Worst Toxicities Per Patient for the Three Arms
| Toxicity | No. (by grade) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13cRA (n = 81) |
BC + RP (n = 45) |
RP (n = 36) |
||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Arthralgia, myalgia | 6 | 5 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cheilitis | 56 | 17 | 3 | 0 | 23 | 0 | 0 | 0 | 12 | 0 | 0 | 0 |
| Conjunctivitis | 29 | 8 | 5 | 0 | 5 | 2 | 0 | 0 | 11 | 1 | 0 | 0 |
| Fatigue | 6 | 1 | 2 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| GI symptoms | 5 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 1 | 0 | 0 |
| Headache | 4 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Nail changes | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Skin reaction | 57 | 12 | 3 | 0 | 22 | 1 | 0 | 0 | 20 | 0 | 0 | 0 |
| Triglyceride | 17 | 2 | 0 | 0 | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Other | 12 | 7 | 0 | 1* | 2 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Total | 36 | 30 | 12 | 1 | 28 | 3 | 1 | 0 | 24 | 2 | 0 | 0 |
| % | 45 | 37 | 15 | 1 | 62 | 7 | 2 | 0 | 67 | 6 | 0 | 0 |
Abbreviations: 13cRA, 13-cis retinoic acid; BC, beta-carotene; RP, retinyl palmitate.
Abnormal liver function test, which resolved within 3 months after stopping the treatment.
DISCUSSION
To our knowledge, this trial is the largest and longest-term prospective, randomized trial conducted in OPL patients to date and the first to include a prespecified secondary analysis of long-term oral cancer incidence. With respect to clinical OPL response, we did not establish the equivalence of the combined arm of BC plus RP and RP alone with low-dose 13cRA. Furthermore, RP alone was significantly inferior to BC plus RP.
In 1994, the large-scale ATBC study11 reported that BC, either alone or with alpha tocopherol, resulted in unexpected significantly higher incidence and mortality of lung cancer (v non-BC arms) in 29,133 chronic male smokers. This prompted an unplanned interim analysis that did not reveal any safety concerns, and so accrual continued to both arms. Despite a lack of safety concerns in a second unplanned interim analysis, we modified the protocol in 1996 after CARET12 showed an 18% increase in lung cancer mortality in the BC plus RP arm (v placebo), higher than the increased mortality with BC in the ATBC study. All subsequent patients were randomly assigned to either 13cRA or RP alone (25,000 U/d orally for 3 years) and those in the BC plus RP arm were instructed to discontinue BC and continue RP alone (25,000 U/d orally) for the remainder of their 3-year treatment.
Smoking has been recognized as an important risk factor for both initial head and neck cancer and second primary tumor development. Moreover, we observed that 13cRA was harmful for current smokers but beneficial for never-smokers in a large, multicenter, randomized lung chemoprevention trial.16 The smoking by 13cRA interaction was not evident, however, in a similarly designed chemoprevention trial in the head and neck.17 Self-reported tobacco exposure correlated well with cotinine levels at baseline and throughout this study. No differences in smoking-status changes among treatment arms could account for differences in response and cancer development in the this trial.
Although we screened for and excluded patients who used retinoids and/or carotenoids within 3 months of study entry, we did not systematically assess pre-3 month use of high doses of these agents, which could have influenced response in this trial. Only two of 184 screened patients, however, were excluded for using such agents (high-dose BC in both cases) within the 3-month window, suggesting a low frequency of prestudy exposure to retinoids and carotenoids.
Without a placebo arm, this study cannot determine the influence of low-dose 13cRA (or the other arms) on oral cancer risk. However, low-dose 13cRA did not reduce second cancers (v placebo) in more than 1,190 definitively treated head and neck cancer patients of another study.17 Toxicity and lack of efficacy present major obstacles to future study of 13cRA in any head and neck chemoprevention setting. The potential efficacy of BC plus RP in reducing oral cancer risk also will not likely be assessed further because of the harmful effects of BC on lung cancer incidence and mortality in smokers.11,12
In conclusion, the major findings of this study are that low-dose 13cRA was not well tolerated for long-term oral cancer prevention and better-tolerated RP alone was ineffective. Furthermore, we found no statistically significant association between OPL response and longer oral cancer–free survival. Current oral cancer prevention research aims to identify high (molecularly marked)-risk OPL patients18,19 in whom cancer end point trials are possible and to assess molecularly targeted drugs. Indeed, a cancer end point (phase III) trial of an epidermal growth factor receptor tyrosine kinase inhibitor in OPL patients at a high oral cancer risk marked by loss of heterozygosity at certain chromosomal sites20 is ongoing; a prespecified secondary study will analyze the potential relationship of the surrogate end point (OPL response) with the primary end point (oral cancer).
Footnotes
Supported by Grants No. P01 CA052051 and P30 CA016672 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (The University of Texas M. D. Anderson Cancer Center support grant).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Vassiliki A. Papadimitrakopoulou, J. Jack Lee, William N. William Jr, Jack W. Martin, Edward S. Kim, Fadlo R. Khuri, Dong M. Shin, Lei Feng, Waun Ki Hong, Scott M. Lippman
Collection and assembly of data: Margaret Thomas
Data analysis and interpretation: Vassiliki A. Papadimitrakopoulou, Fadlo R. Khuri, Dong M. Shin, Lei Feng
Manuscript writing: Vassiliki A. Papadimitrakopoulou, J. Jack Lee, William N. William Jr, Jack W. Martin, Edward S. Kim
Final approval of manuscript: Vassiliki A. Papadimitrakopoulou
REFERENCES
- 1.Silverman S, Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: A follow-up study of 257 patients. Cancer. 1984;53:563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Vokes EE, Weichselbaum RR, Lippman SM, et al. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 3.Mayne ST, Lippman SM. Cancer Prevention: Diet and Chemopreventive: Section 4: Retinoids, Carotenoids, and Micronutrients. In: Vincent T, DeVita J, Hellman S, et al., editors. Cancer: Principles & Practice of Oncology. ed 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 521–536. [Google Scholar]
- 4.Lotan R, Xu XC, Lippman SM, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 5.Stich HF, Hornby AP, Mathew B, et al. Response of oral leukoplakias to the administration of vitamin A. Cancer Lett. 1988;40:93–101. doi: 10.1016/0304-3835(88)90266-2. [DOI] [PubMed] [Google Scholar]
- 6.Stich HF, Rosin MP, Hornby AP, et al. Remission of oral leukoplakias and micronuclei in tobacco/betel quid chewers treated with beta-carotene and with beta-carotene plus vitamin A. Int J Cancer. 1988;42:195–199. doi: 10.1002/ijc.2910420209. [DOI] [PubMed] [Google Scholar]
- 7.Hong WK, Endicott J, Itri LM, et al. 13-cis retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 9.Papadimitrakopoulou VA, Hong WK, Lee JS, et al. Low-dose isotretinoin versus beta-carotene to prevent oral carcinogenesis: Long-term follow-up. J Natl Cancer Inst. 1997;89:257–258. doi: 10.1093/jnci/89.3.257. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Mathew B, Varghese C, et al. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: An assessment. Oral Oncol. 1997;33:231–236. doi: 10.1016/s0964-1955(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 11.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 12.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 13.Makuch R, Simon R. Sample size requirements for evaluating a conservative therapy. Cancer Treat Rep. 1978;62:1037–1040. [PubMed] [Google Scholar]
- 14.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3:345–353. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 15.Blackwelder WC, Chang MA. Sample size graphs for “proving the null hypothesis”. Control Clin Trials. 1984;5:97–105. doi: 10.1016/0197-2456(84)90116-8. [DOI] [PubMed] [Google Scholar]
- 16.Lippman SM, Lee JJ, Karp DD, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 17.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: Ten years of translational research. Clin Cancer Res. 2000;6:1702–1710. [PubMed] [Google Scholar]
- 19.Lippman SM, Lee JJ. Reducing the “risk” of chemoprevention: Defining and targeting high risk–2005 AACR Cancer Res and Prevention Foundation Award Lecture. Cancer Res. 2006;66:2893–2903. doi: 10.1158/0008-5472.CAN-05-4573. [DOI] [PubMed] [Google Scholar]
- 20.Lippman SM, Heymach JV. The convergent development of molecular-targeted drugs for cancer treatment and prevention. Clin Cancer Res. 2007;13:4035–4041. doi: 10.1158/1078-0432.CCR-07-0063. [DOI] [PubMed] [Google Scholar]