Abstract
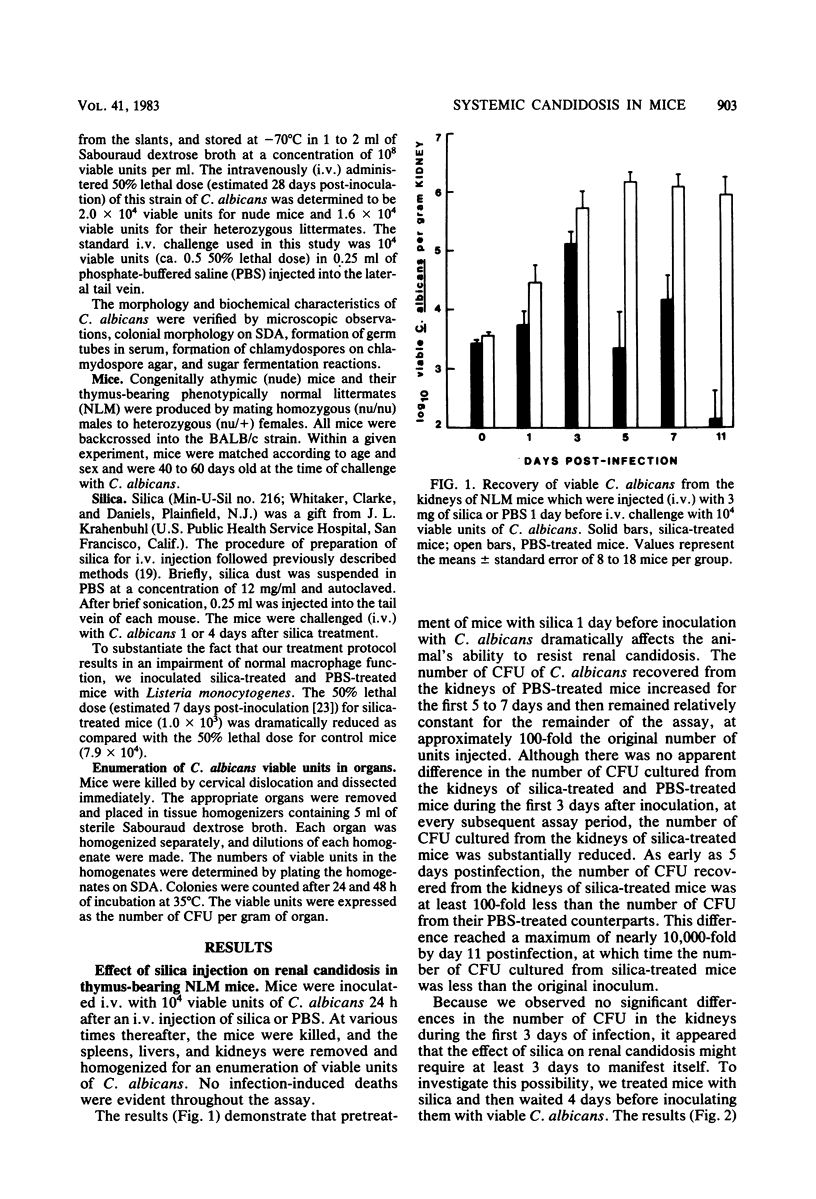
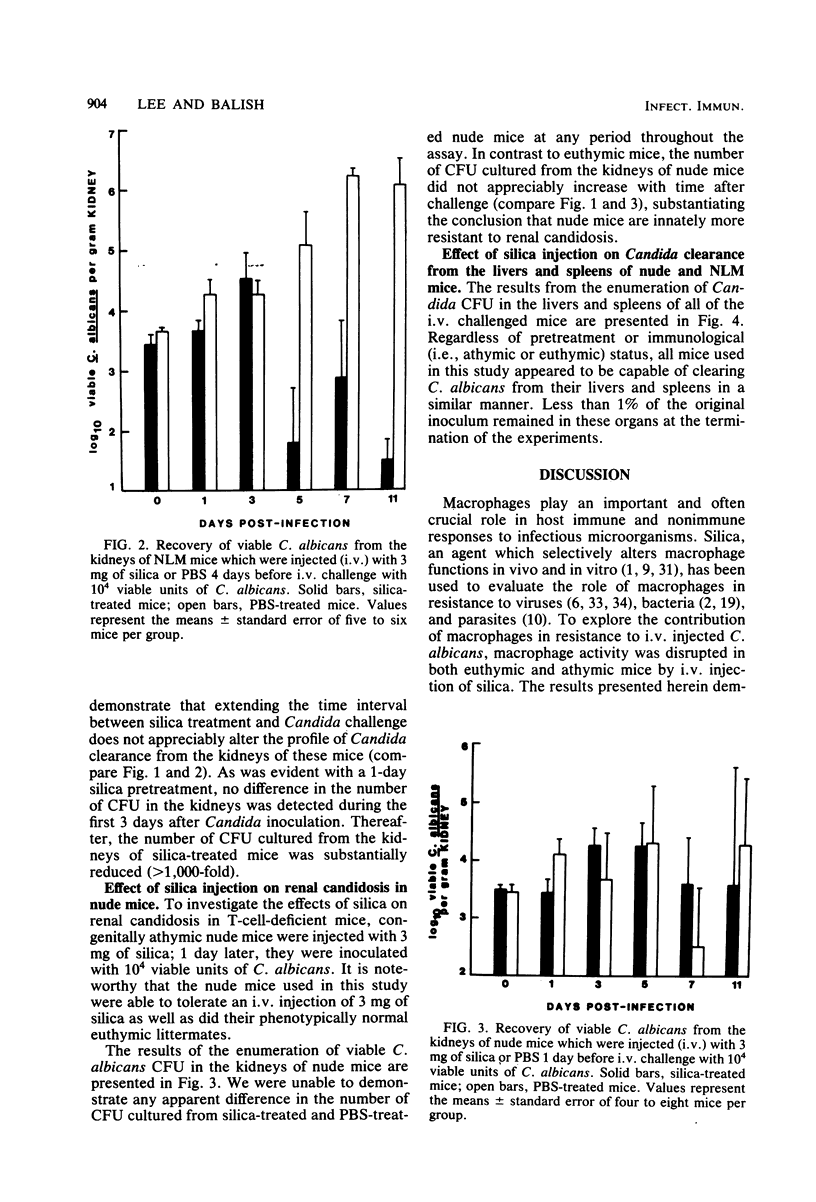
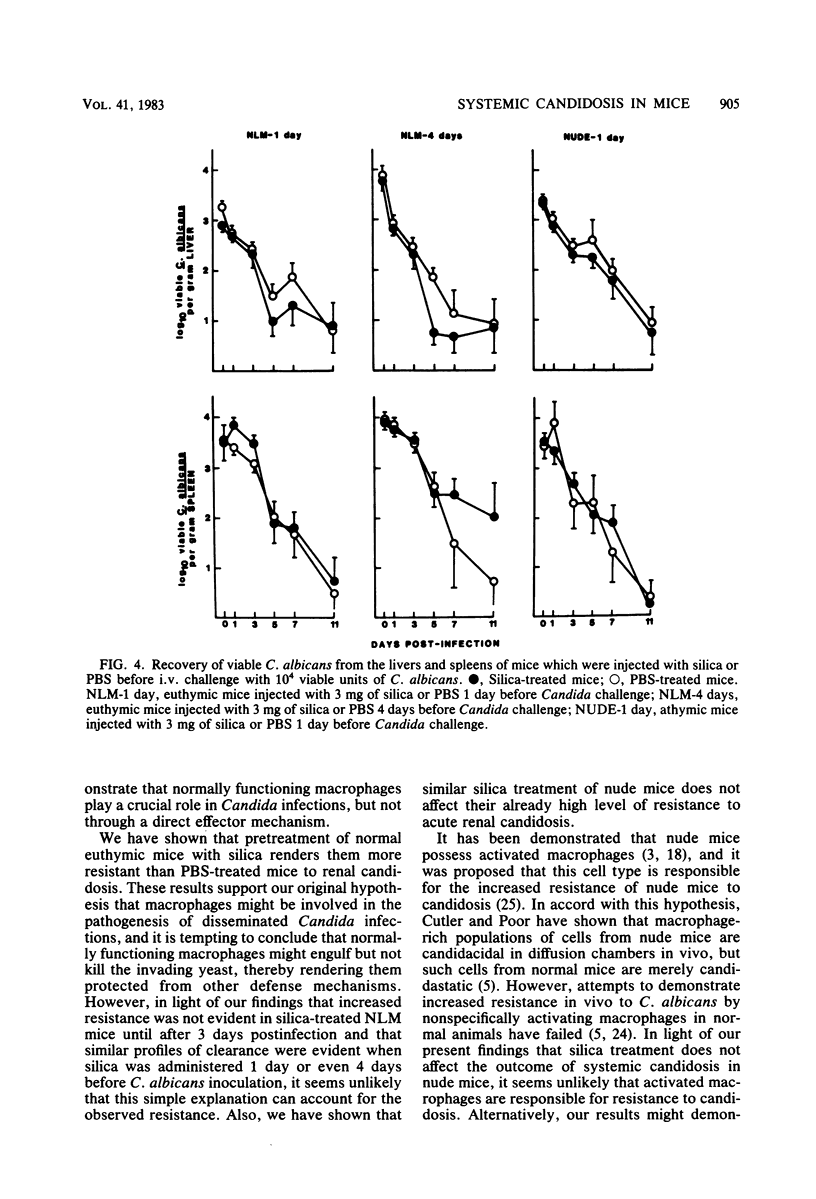
Intravenous silica injections were used to assess the role of macrophages in the resistance of BALB/c nude and euthymic mice to systemic candidosis. CFU of Candida albicans in the kidneys, livers, and spleens of saline- or silica-treated mice were enumerated at various times after inoculation with 10(4) viable yeast cells. The number of C. albicans organisms recovered from the kidneys of silica-treated euthymic mice was similar to the number recovered from saline-treated controls during the first 3 days of infection; however, at every assay period thereafter, the number of organisms recovered from the kidneys of silica-treated mice was dramatically reduced (100- to 1,000-fold). Conversely, silica-treated nude mice were no more susceptible to systemic candidosis than were saline-injected nude mice. Silica treatment did not alter the ability of treated or control mice to clear C. albicans from the liver and spleen. These results demonstrate that macrophages play an important role in susceptibility to Candida infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Cutler J. E., Poor A. H. Effect of mouse phagocytes on Candida albicans in in vivo chambers. Infect Immun. 1981 Mar;31(3):1110–1116. doi: 10.1128/iai.31.3.1110-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Impaired chemotactic responsiveness of macrophages from gnotobiotic rats. Infect Immun. 1978 Feb;19(2):553–561. doi: 10.1128/iai.19.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Knecht E., Budzko D. B., Pizzimenti M. C. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974 May;112(5):1839–1844. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Lee K. W., Balish E. Effect of T-cells and intestinal bacteria on resistance of mice to candidosis. J Reticuloendothel Soc. 1982 Mar;31(3):233–240. [PubMed] [Google Scholar]
- Lee K. W., Balish E. Resistance of germfree mice to systemic candidosis. J Reticuloendothel Soc. 1981 Mar;29(3):241–248. [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. Effects of intravenous silica on immune and non-immune functions of the murine host. J Immunol. 1975 Jul;115(1):41–48. [PubMed] [Google Scholar]
- Lew F. T., Yukiyama Y., Waks H. S., Osler A. G. Activation of the alternative (properdin) pathway by divalent cations. J Immunol. 1975 Sep;115(3):884–888. [PubMed] [Google Scholar]
- Miller S. D., Zarkower A. Alterations of murine immunologic responses after silica dust inhalation. J Immunol. 1974 Nov;113(5):1533–1543. [PubMed] [Google Scholar]
- Miller S. D., Zarkower A. Silica-induced alterations of murine lymphocyte immunocompetence and suppression of B lymphocyte immunocompetence: a possible mechanism. J Reticuloendothel Soc. 1976 Jan;19(1):47–61. [PubMed] [Google Scholar]
- Mørland B., Smievoll A. I., Midtvedt T. Comparison of peritoneal macrophages from germfree and conventional mice. Infect Immun. 1979 Dec;26(3):1129–1136. doi: 10.1128/iai.26.3.1129-1136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Pearsall N. N., Weiser R. S. The macrophage in allograft immunity. II. Passive transfer with immune macrophages. J Reticuloendothel Soc. 1968 Apr;5(2):121–133. [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Rogers T. J., Balish E. The role of activated macrophages in resistance to experimental renal candidiasis. J Reticuloendothel Soc. 1977 Oct;22(4):309–318. [PubMed] [Google Scholar]
- Scheuchenzuber W. J., Zarkower A., Eskew M. L. Immunological alterations in the mouse following injection or inhalation of silica and olivine dusts. Environ Res. 1982 Apr;27(2):398–411. doi: 10.1016/0013-9351(82)90095-0. [DOI] [PubMed] [Google Scholar]
- Sen P., Smith J. K., Buse M., Hsieh H. C., Lavenhar M. A., Lintz D., Louria D. B. Modification of an experimental mouse Candida infection by human dialyzable leukocyte extract. Sabouraudia. 1982 Jun;20(2):85–93. [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Unanue E. R., Kiely J. M., Calderon J. The modulation of lymphocyte functions by molecules secreted by macrophages. II. Conditions leading to increased secretion. J Exp Med. 1976 Jul 1;144(1):155–166. doi: 10.1084/jem.144.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Snoek M., Den Otter W. Effects of silica on macrophages and lymphocytes. J Reticuloendothel Soc. 1977 Dec;22(6):523–531. [PubMed] [Google Scholar]
- Vogel S. N., English K. E., O'Brien A. D. Silica enhancement of murine endotoxin sensitivity. Infect Immun. 1982 Nov;38(2):681–685. doi: 10.1128/iai.38.2.681-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkower A., Scheuchenzuber W. J., Burns C. A. Effects of silica dust inhalation on the susceptibility of mice to influenza infection. Arch Environ Health. 1979 Sep-Oct;34(5):372–376. doi: 10.1080/00039896.1979.10667434. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


