Abstract
FtsZ is a tubulin homolog and the major cytoskeletal protein in bacterial cell division. It assembles into the Z ring, which contains FtsZ and a dozen other division proteins, and constricts to divide the cell. We have constructed a membrane-targeted FtsZ (FtsZ-mts) by splicing an amphipathic helix to its C terminus. When mixed with lipid vesicles, FtsZ-mts was incorporated into the interior of some tubular vesicles. There it formed multiple Z rings that could move laterally in both directions along the length of the liposome and coalesce into brighter Z rings. Brighter Z rings produced visible constrictions in the liposome, suggesting that FtsZ itself can assemble the Z ring and generate a force. No other proteins were needed for assembly and force generation.
FtsZ is the primary cell division protein in almost all bacterial and archaeal species. In vitro, FtsZ assembles into short, one=-stranded protofilaments, averaging 30 subunits and 125 nm in length (1). In the bacterial cell, these are further assembled into a long, thin filamentous structure attached to the inner bacterial membrane. Normally this filament forms a single Z ring at the center of the cell but sometimes the ring separates into a close-pitched helix (2, 3) Conventional electron microscopy (EM) has failed to resolve any structure of the Z ring in vivo, but a recent study with the use of cryo-EM tomography has resolved the arrangement of protofilaments in the Z ring of Caulobacter crescentus (4). These images showed individual protofilaments scattered in a narrow band around the circumference of the cell. Figure 1A depicts a model of how these protofilaments can be arranged to make the Z ring, consistent with the cryo-EM and earlier indirect analyses (1, 5). This model raises two fundamental questions: (i) How are protofilaments connected to each other to make the very long and thin ring or helix? (ii) How are the protofilaments attached to the membrane? The first question still has no answer, but the second question was recently answered by Pichoff and Lutkenhaus (6), who demonstrated that FtsZ is tethered to the membrane by FtsA. Specifically, the C-terminal peptide of FtsZ binds FtsA, and the C terminus of FtsA forms an amphipathic helix that inserts into the membrane (Fig. 1B).
Fig. 1.
(A) Model of the Z ring. The Z ring is constructed from overlapping short protofilaments and averages 3 to 9 protofilaments in thickness, depending on the bacterial strain. (B) FtsZ is normally tethered to the membrane by FtsA. The Cterminal peptide (orange) of FtsZ binds FtsA, and FtsA binds the membrane by its amphipathic helix (purple). (C) In FtsZ-mts, the FtsA-binding peptide is replaced with yellow fluorescent protein (YFP) and an amphipathic helix.
In addition to FtsZ and FtsA, the Z ring contains nearly a dozen other proteins that are essential for cell division. These proteins appear to function in later steps, especially in remodeling the peptidoglycan wall. Z ring assembly requires only FtsZ and either FtsA or ZipA (6, 7). We have suggested earlier that FtsZ, in addition to providing the cytoskeletal framework, may also generate the constriction force (8). However, it has remained an open question whether force requires interactions with other proteins.
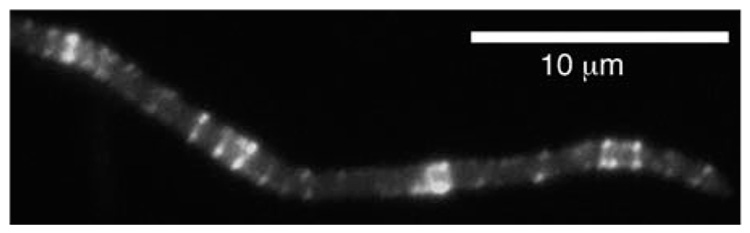
In the present study, we asked whether FtsZ could form a Z ring without FtsA if we provided a direct tether to the membrane. To test this possibility, we removed the FtsZ peptide that binds FtsA and ZipA and replaced it with an amphipathic helix, producing membrane-targeted FtsZ (FtsZ-mts) (Fig. 1C). We then expressed FtsZ-mts in the Escherichia coli strain JKD7-1(pKD3), in which the native FtsZ can be suppressed by growth at 42°C (9, 10). The depletion of FtsZ blocked cell division and caused the cells to grow into long filaments. In these filamentous cells, the FtsZ-mts formed abundant Z rings and helical structures, which are very similar to native Z rings (Fig. 2).We concluded that Z ring assembly does not require FtsA, but only the membrane-targeting amphipathic helix.
Fig. 2.
FtsZ-mts was expressed in E. coli depleted of wild-type FtsZ, which forms long filaments because division is blocked. FtsZ-mts formed numerous Z rings and tight-pitch helices.
Having achieved the reconstitution of the Z rings in E. coli, we next attempted reconstitution in a liposome system in vitro. We made large multilamellar vesicles (11), which were initially mostly spherical, with a tendency to aggregate into irregular clumps. We then mixed the liposomes with FtsZ and guanosine 5′-triphosphate (GTP) (GTP is necessary for polymerization), put a drop on a slide, and placed a coverslip on it. Initially, the FtsZ-mts was on the outside of the vesicles, but during these manipulations tubular liposomes formed and some of these incorporated FtsZ-mts inside. We do not know how this process occurs.
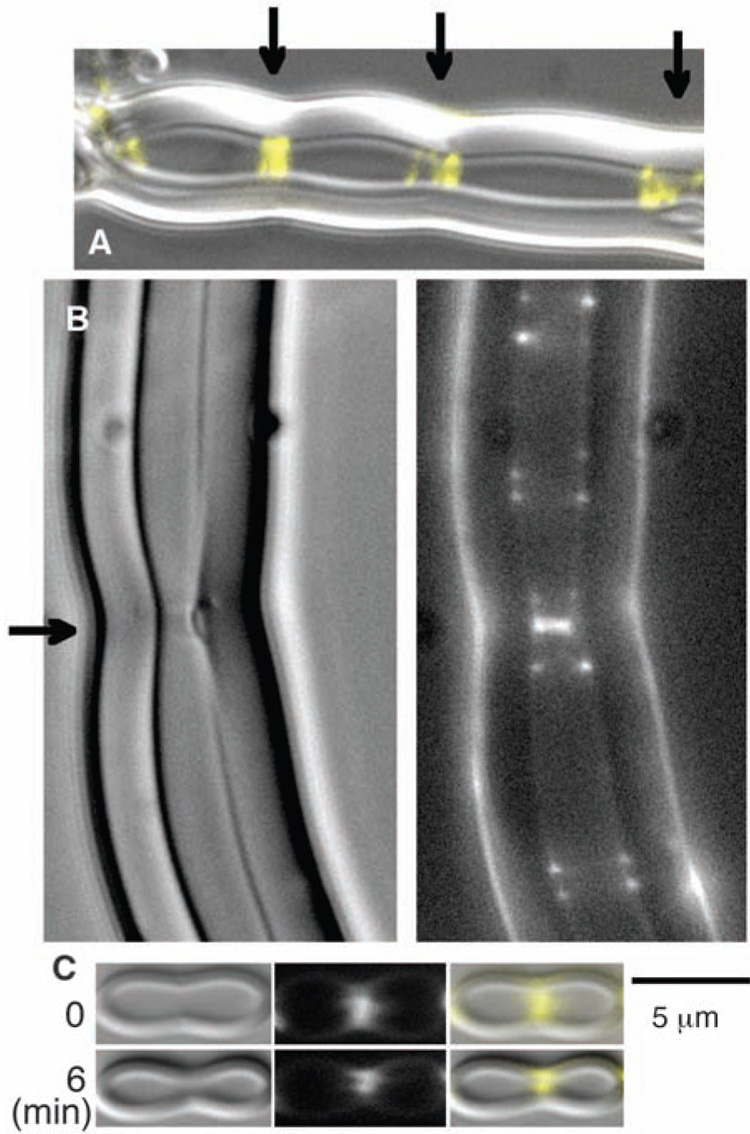
Inside the liposomes, the FtsZ-mts spontaneously assembled into multiple Z rings, almost all of them being closed rings perpendicular to the axis of the tube (Fig. 3). The liposomes frequently showed indentations or constrictions at the site of the brighter Z rings. The constrictions usually distorted the entire wall of the thick, multilamellar liposome (Fig. 3A) but sometimes appeared to detach the inner layers (Fig. 3B). In some cases, a constriction already present in our first image constricted further a few minutes later (Fig. 3C). We have not observed any convincing cases of constrictions proceeding all the way to liposome division. The limited constriction is perhaps due to the rigidity of the thick walls of the multilamellar liposomes; we have not yet succeeded in producing tubular liposomes with thinner walls.
Fig. 3.
FtsZ-mts and GTP were mixed with liposomes. Although the FtsZ-mts was initially outside the liposomes, some tubular, multilamellar liposomes were formed that enclosed FtsZ-mts and GTP. The FtsZ assembled into Z rings in these tubular liposomes. (A) A liposome with three bright Z rings, each centered on a constriction. The fluorescent FtsZ is shown in yellow, superimposed on the differential interference contrast image of the liposome. Arrows indicate Z rings. (B) The bright Z ring near the middle (indicated by the arrow) is forming a visible constriction and on the right side appears to have detached some inner layers of the multilamellar wall. (C) A liposome is shown here with a visible constriction at the Z ring when first observed, 5 to 10 min after making the specimen. Six minutes later the constriction has narrowed markedly. See movie S1 for a 10-min series showing Z rings coalescing into brighter ones, which generate constrictions.
Movie S1 depicts a tubular liposome observed over a period of 10 min. Initially the liposome contained multiple dim Z rings and no membrane constrictions (fig. S1, 0 and 100 s). These dim Z rings were found to slide back and forth along the length of the tube. When Z rings collided, they coalesced and produced brighter Z rings, and visible constrictions of the liposome wall appeared over them (fig. S1, 200 and 300 s). The constrictions in movie S1 are slight (see Fig. 3 and Fig. 4 and movie S2 and movie S3 for more prominent constrictions), but fig. S1 (taken from movie S1) illustrates that constrictions (i) appear only after the formation of bright Z rings and (ii) coincide with the locations of Z rings. We also observed that when dim Z rings coalesce to make bright ones, they never reemerge. This may be because the Z ring material is generating a constriction force, making it unfavorable to move uphill toward the larger diameter and away from the constriction.
Fig. 4.
A Z ring and its constriction abruptly relaxes. For this preparation, the GTP concentration was reduced from 400 to 100 µM and should have been exhausted in ~20 min. The first image was obtained ~10 min after mixing liposomes with FtsZ-mts and GTP, and images were taken every 10 s for another 60 min. Elapsed times after the first image are indicated on the frames. See movie S2 for the complete time series.
Whereas Fig. 3, movie S1, and fig. S1 show constrictions developing at the sites of bright Z rings, Fig. 4 illustrates an example of the opposite. In this experiment, we reduced the GTP from 400 to 100 µM, and we estimated that the GTP should be exhausted in ~20 min. The liposome in Fig. 4 showed a deep constriction centered on a bright Z ring at early time points (Fig. 4, 50 and 250 s). Eight minutes later, the constriction relaxed slightly for ~20 s (Fig. 4, 450 s) and then abruptly relaxed completely (Fig. 4, 460 s; this abrupt expansion is much more dramatic in movie S2). The Z ring did not collapse or disassemble but expanded uniformly to the larger diameter. The expansion occurred in less than 10 s and remained stable for more than 20 min. A second Z ring in the liposome abruptly relaxed its constriction 2 min later (movie S2). The remaining dim Z rings mostly disappeared over the next 20 min, consistent with the exhaustion of GTP. This series suggests that the early constrictions were maintained by a Z ring–dependent force and relaxed when these Z rings lost their ability to maintain the force. Movie S3 depicts another series where initially weak constrictions deepened over 10 min and then relaxed as the GTP was depleted. We also tested assembly in guanosine 5′-[(α,β)-methyleno] triphosphate (GMPCPP), which is hydrolyzed 3 to 10 times more slowly than GTP (12). GMPCPP supported the assembly of Z rings in the liposomes, and these liposomes had small constrictions.
Some tubular liposomes had Z rings, and others did not. This provided an additional opportunity to test the association of Z rings with constrictions. We measured by eye the frequency of visible indentations in tubular liposomes with inside diameters of <2.5 µm. In liposomes that did not contain Z rings, we counted 32 visible indentations over a total length of 2222 µm. In liposomes that contained Z rings, we counted 44 indentations over a total length of 575 µm. These indentations almost always coincidedwith a bright Z ring. Indentations or constrictions were thus five times more frequent in tubular liposomes that contained Z rings than in those without Z rings.
If the Z rings are generating a constriction force in the liposomes, they are doing this without a motor molecule. What could be the mechanism of force generation? We have previously reported that FtsZ can switch from a straight protofilament in GTP to a highly curved conformation in guanosine diphosphate (8, 13, 14). This conformational change is a candidate for force generation by FtsZ alone. Li et al. (4) also suggested that individual protofilaments may be generating a constriction force on the membrane segments to which they are attached.
A notable feature of the reconstituted Z rings is their strong tendency to form perfect closed rings that are oriented perpendicular to the axis of the tube. These features are consistent with the generation of a constriction force. If a filament grew longitudinally while attached to the membrane, in the absence of any force it might assume a loose helical shape or even more irregular course. If it is generating a constriction force, it would tend toward a minimum diameter, which would be a ring perpendicular to the axis (15).
The images of Li et al. (4) show individual protofilaments with few contacts between them. This raises the possibility that the assembly of protofilaments to form the long, thin Z ring is not propagated by direct lateral contacts between FtsZ protofilaments. An alternative mechanism is that a protofilament, by inserting amphipathic helices and exerting a force on the membrane, may produce distortions of the membrane that favor the attachment of additional protofilaments near the ends. This assembly, which would favor the formation of closed rings, might also be a source of constriction force.
Because FtsA is an actin homolog and is known to form dimers or oligomers (16, 17), we initially thought that FtsA self-association might play an essential role in Z ring assembly. However, our reconstitution shows that neither FtsA nor downstream division proteins are required for Z ring assembly. FtsZ, a membrane tether, and the interior curved surface of a tubular liposome are sufficient for this assembly and the generation of a constriction force.
The earliest cellular life forms probably contained a replicating system of macromolecules surrounded by a lipid membrane. A mechanism would be needed to divide this living liposome. Our simple system may recapitulate the primordial division machine (18).
Supplementary Material
www.sciencemag.org/cgi/content/full/1154520/DC1
Materials and Methods
Fig. S1
References
Movies S1 to S3
References and Notes
- 1.Chen Y, Erickson HP. J. Biol. Chem. 2005;280:22549. doi: 10.1074/jbc.M500895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanedar S, Margolin W. Curr. Biol. 2004;14:1167. doi: 10.1016/j.cub.2004.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters PC, Migocki MD, Thoni C, Harry EJ. Mol. Microbiol. 2007;64:487. doi: 10.1111/j.1365-2958.2007.05673.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Trimble MJ, Brun YV, Jensen GJ. EMBO J. 2007;22:4694. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DE, Gueiros-Filho FJ, Erickson HP. J. Bacteriol. 2004;186:5775. doi: 10.1128/JB.186.17.5775-5781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichoff S, Lutkenhaus J. Mol. Microbiol. 2005;55:1722. doi: 10.1111/j.1365-2958.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- 7.Pichoff S, Lutkenhaus J. EMBO J. 2002;21:685. doi: 10.1093/emboj/21.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson HP. Trends Cell Biol. 1997;7:362. doi: 10.1016/S0962-8924(97)01108-2. [DOI] [PubMed] [Google Scholar]
- 9.Dai K, Lutkenhaus J. J. Bacteriol. 1991;173:3500. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redick SD, Stricker J, Briscoe G, Erickson HP. J. Bacteriol. 2005;187:2727. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Methods are available as supporting material on Science Online.
- 12.Romberg L, Simon M, Erickson HP. J. Biol. Chem. 2001;276:11743. doi: 10.1074/jbc.M009033200. [DOI] [PubMed] [Google Scholar]
- 13.Erickson HP, Taylor DW, Taylor KA, Bramhill D. Proc. Natl. Acad. Sci. U.S.A. 1996;93:519. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Reedy M, Erickson HP. J. Bacteriol. 2000;182:164. doi: 10.1128/jb.182.1.164-170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews SS, Arkin AP. Biophys. J. 2007;93:1872. doi: 10.1529/biophysj.106.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feucht A, Lucet I, Yudkin MD, Errington J. Mol. Microbiol. 2001;40:115. doi: 10.1046/j.1365-2958.2001.02356.x. [DOI] [PubMed] [Google Scholar]
- 17.Lara B, et al. Mol. Microbiol. 2005;55:699. doi: 10.1111/j.1365-2958.2004.04432.x. [DOI] [PubMed] [Google Scholar]
- 18.Erickson HP. Bioessays. 2007;29:668. doi: 10.1002/bies.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.This work was supported by NIH grant GM66014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencemag.org/cgi/content/full/1154520/DC1
Materials and Methods
Fig. S1
References
Movies S1 to S3