Introduction
Microscopic hematuria is a frequent reason for referral to urology. It is often found incidentally as a result of routine examination in patients without urinary tract symptoms. Although there is generally no debate about the need to fully investigate patients with gross hematuria, there is often controversy regarding the approach to the patient with asymptomatic microscopic hematuria. The main issues disputed are related to the detection and definition of significant microscopic hematuria, which patients should be investigated, and how should they be evaluated.
In 1998, the Canadian Urological Association published patient guidelines for asymptomatic hematuria.1 A decade has passed since the initial development of these principles. Thus, the CUA Patient Guidelines Committee has been given the mandate from the CUA to update the asymptomatic microscopic hematuria patient guidelines.
Methods
A review of the literature was performed for publication years 1998 to 2008 using MEDLINE, limited to the English language. A review of the American Urological Association Best Practice Policy Panel on Asymptomatic Microhematuria was also undertaken.2,3
The following principles and issues concerning the diagnosis and management of the patient with asymptomatic microscopic hematuria (AMH) are reviewed:
Definition of AMH
Determination of which patients with AMH warrant further evaluation by a urologist a. Indications for nephrological assessment b.Indications for urological assessment
Urological evaluation of patients with AMH
Follow-up of the patient with benign AMH
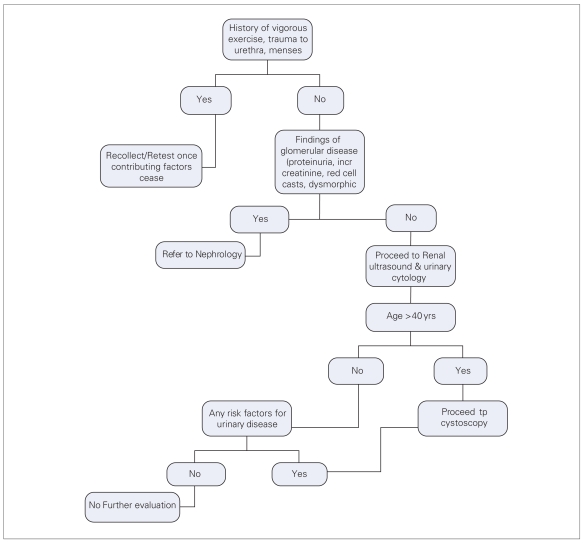
The revised guideline was reviewed and critiqued by the Guidelines Committee. Furthermore, due to the lack of evidence especially as it relates to which patients should be evaluated, a consensus opinion was developed based on an informal survey of Canadian urologists from across the country and a final algorithm was designed (Fig. 1).
Fig. 1.
Algorithm for the evaluation of the adult patient with asymptomatic microscopic hematuria.
Definition of microscopic hematuria
In the 1998 AMH guideline, significant microscopic hematuria was defined as greater than 2 RBCs/hpf on two microscopic urinalysis without recent exercise, menses, sexual activity or instrumentation. Review of the literature did not reveal any evidence to warrant changing this definition.
Which patients require evaluation for AMH?
Once microscopic hematuria has been confirmed, which patients require further evaluation? First, in patients with a history of recent exercise, menses, sexual activity or urethral trauma/instrumentation, a repeat microscopic exam should be done once the contributing factor has ceased.3 If the subsequent exam is negative, then further work-up is not required.
Next, it should be determined if the patient’s hematuria could be secondary to a glomerular cause. The presence of proteinuria, red cell casts, or dysmorphic red blood cells on microscopic exam and/or an elevated creatinine is suggestive of a glomerular cause of hematuria and these patients should be referred to a nephrologist for further investigation.3–5 Further urological work-up including cystoscopy may not be required in this situation. All other patients should be assessed for the need of further evaluation.
Evaluation of the upper and lower urinary tracts
There is inadequate evidence in the literature to definitively recommend which patients should undergo full or partial urological evaluation for their microscopic hematuria. The AUA Best Policy Panel recommends that all patients with microscopic hematuria undergo evaluation of the upper tracts while only high-risk patients have lower tract evaluation.2,3 Conversely, guidelines out of the UK endorse evaluation of the upper and lower urinary tracts for all patients with no risk stratification.6,7 The 1998 CUA guideline advocated evaluation of all patients over the age of 40 years only.1
The upper urinary tract is evaluated with diagnostic imaging. The aim of imaging is to detect neoplasms, urolithiasis, and obstructive or inflammatory lesions. Intravenous urography (IVU), ultrasonography (US), and enhanced computed tomography (CT) are the modalities used most commonly. Although there are numerous studies describing the application of these modalities in this setting,8–11 there are no comparative studies that can help establish an evidence-based policy.
Intravenous urography (IVU) is the study that has been traditionally used to investigate the upper urinary tract. It is widely available, easy to perform, and is able to detect transitional cell carcinoma of the upper urinary tract with an acceptable degree of sensitivity. However, fewer centres are offering IVU and the study has a limited sensitivity for diagnosing renal cell carcinoma.11
Ultrasound is also widely available. Moreover, it is noninvasive, does not require ionizing radiation or intravenous contrast and, compared to CT, is less expensive. It is superior to IVU for evaluating the renal parenchyma and renal cysts. However, like IVU, it has limited sensitivity for small renal masses.12 It also has a limited sensitivity for diagnosing transitional cell carcinoma.
CT is the optimal test for examining the renal parenchyma for masses. It is also the best test for diagnosing renal/ureteral calculi and renal and/or peri-renal infections. The disadvantages of CT include decreased availability in some centres, the need for ionizing radiation, exposure to intravenous contrast, and increase expense compared to IVU and US.
Due to the lack of evidence in this setting, any of these imaging modalities are acceptable for the evaluation of the patient with microscopic hematuria. However, taking patient safety (ionizing radiation and exposure to i.v. contrast), availability, and cost into consideration, it is recommended that US be used as the imaging test of first choice. CT and IVU are justified when additional tests are believed to be indicated.
The lower urinary tract is evaluated with urinary cytology and cystoscopy. Since cytology is an easy test to perform, is noninvasive and is relatively inexpensive, it is a recommended investigation for lower tract assessment that should be obtained early on in the evaluation of the patient with microscopic hematuria. The sensitivity for detecting transitional cell carcinoma is moderate and ranges from 40% to 75% depending on the number and type of urine specimens examined, the grade of the tumour, and the expertise of the cytopathologist.3
The 1998 CUA guidelines state
patients over the age of 40 have a significant incidence of pathology such as transitional cell carcinoma and renal cell carcinoma. These lesions are rare in patients under the age of 40. Therefore cystoscopy of young patients with asymptomatic microscopic hematuria is not warranted.13–16
The literature still supports this principle.3,4,17 However, in addition to an age over 40 years, there are other risk factors that should be considered significant for disease in patients with microscopic hematuria:3,18
Smoking history
Occupational exposure to chemicals or dyes (benzenes, aromatic amines)
History of irritative voiding symptoms
Analgesic abuse with phenacitin
History of pelvic irradiation
Cyclophosphamide exposure
Therefore, patients that are under the age of 40 years with none of the above risk factors do not require cystoscopy. Conversely, all patients over the age of 40 years need cystoscopy. Any patient with positive or atypical cytology, or those with one or more risk factors also need cystoscopic evaluation.
Recommended follow-up of the patient with AMH
Following a negative evaluation for AMH, between 1% and 3% of patients have been reported to be diagnosed with a urological malignancy within three years.16,19,20 A small proportion may also develop renal insufficiency due to glomerular disease.21–23 As a result, some form of patient follow-up is recommended after negative initial evaluation for AMH.
An evidence-based policy cannot be recommended at this time; however an approach similar to that recommended by the AUA Best Policy Panel is acceptable. Patients should be followed by their primary care physician with urinalysis, urinary cytology, and blood pressure checks at 6, 12, 24 and 36 months.3,18 If a patient develops gross hematuria, positive or atypical cytology, or irritative voiding symptoms without infection, then repeat urological evaluation is advised. The development of hypertension, proteinuria, or the finding of glomerular bleeding would necessitate referral to a nephrologist. If none of these occur after three years, then routine follow-up for persistent hematuria can be ceased.
Guideline summary
Definition of microscopic hematuria:
Greater than 2 RBCs/hpf on two microscopic urinalysis without recent exercise, menses, sexual activity or instrumentation (Grade C Recommendation; Level of Evidence 3b and 4).
Indications for nephrology referral:
The presence of proteinuria, red cell casts, or dysmorphic red blood cells on microscopic exam and/or an elevated creatinine is suggestive of a glomerular cause of hematuria (Grade C Recommendation; Level 3b and 4).
Indications and extent of urological evaluation:
All patients with microscopic hematuria should be investigated by urine cytology and upper tract imaging (Grade D Recommendation; Level 5 evidence).
Patients > 40 years of age, those with positive or atypical cytology, or any patient with the presence of any of the following risk factors should have their lower tract assessed by cystoscopy (smoking history, occupational exposure to chemicals or dyes, history of irritative voiding symptoms, analgesic abuse with phenacitin, history of pelvic irradiation, or cyclophosphamide exposure) (Grade C Recommendation; Level 3b and 4 evidence).
Upper tract evaluation:
There is limited evidence to strongly recommend one modality. Thus, although ultrasound, CT or IVP are acceptable, taking patient safety (ionizing radiation and exposure to i.v. contrast), availability, and cost into consideration, it is recommended that ultrasound be used as the imaging test of first choice (Grade C Recommendation; Level 3b and 4 evidence).
Follow-up after negative evaluation:
Patients should be followed by their primary care physician with urinalysis, urinary cytology, and blood pressure checks at 6, 12, 24 and 36 months (Grade C Recommendation; Level 3b and 4 evidence).
Repeat urological assessment is required if a patient develops gross hematuria, positive or atypical cytology, or irritative voiding symptoms without infection.
Nephrology referral is indicated with the development of hypertension, proteinuria, or the finding of glomerular bleeding.
If none of these occur after three years, then routine follow-up for persistent hematuria can be ceased.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
References
- 1.Canadian Urological Association. Asymptomatic microscopic hematuria. 1998 [Google Scholar]
- 2.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy — part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603. doi: 10.1016/s0090-4295(01)00919-0. [DOI] [PubMed] [Google Scholar]
- 3.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy — part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology. 2001;57:604–10. doi: 10.1016/s0090-4295(01)00920-7. [DOI] [PubMed] [Google Scholar]
- 4.Tomson C, Porter T. Asymptomatic microscopic or dipstick haematuria in adults: which investigations for which patients? A review of the evidence. BJU Int. 2002;90:185–98. doi: 10.1046/j.1464-410x.2002.02841.x. [DOI] [PubMed] [Google Scholar]
- 5.Savige J, Buzza M, Dagher H. Haematuria in asymptomatic individuals. BMJ. 2001;322:942–3. doi: 10.1136/bmj.322.7292.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scottish Intercollegiate Guidelin Network. SIGN Publication. 17. Edinburgh: 1997. Investigation of asymptomatic microscopic haematuria in adults. [Google Scholar]
- 7.Finlayson JA. Asymptomatic haematuria. Scottish guidelines are different from authors interpretation of best available evidence. [author reply 1599–600] BMJ. 2000;320:1598. [PubMed] [Google Scholar]
- 8.McNicholas MM, Raptopoulos VD, Schwartz RK, et al. Excretory phase CT urography for opacification of the urinary collecting system. AJR Am J Roentgenol. 1998;170:1261–7. doi: 10.2214/ajr.170.5.9574598. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi T, Muakami S, Shichijo Y, et al. Clinical and radiological aspects of infiltrating transitional cell carcinoma of the kidney. Urol Int. 1994;52:181–4. doi: 10.1159/000282604. [DOI] [PubMed] [Google Scholar]
- 10.Buckley JA, Urban BA, Soyer P, et al. Transitional cell carcinoma of the renal pelvis: a retrospective look at CT staging with pathologic correlation. Radiology. 1996;201:194–8. doi: 10.1148/radiology.201.1.8816543. [DOI] [PubMed] [Google Scholar]
- 11.Warshauer DM, McCarthy SM, Street L, et al. Detection of renal masses: sensitivities and specificities of excretory urography/linear tomography, US, and CT. Radiology. 1988;169:363–5. doi: 10.1148/radiology.169.2.3051112. [DOI] [PubMed] [Google Scholar]
- 12.Jamis-Dow CA, Choyke PL, Jennings SB, et al. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996;198:785–8. doi: 10.1148/radiology.198.3.8628872. [DOI] [PubMed] [Google Scholar]
- 13.Greene LF, O’Shaughnessy EJ, Jr, Hendricks ED. Study of five hundred patients with asymptomatic microhematuria. J Am Med Assoc. 1956;161:610–3. doi: 10.1001/jama.1956.02970070042011. [DOI] [PubMed] [Google Scholar]
- 14.Bard RH. The significance of asymptomatic microhematuria in women and its economic implications. A ten-year study. Arch Intern Med. 1988;148:2629–32. [PubMed] [Google Scholar]
- 15.Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol. 1987;138:1189–90. doi: 10.1016/s0022-5347(17)43545-2. [DOI] [PubMed] [Google Scholar]
- 16.Murakami S, Igarashi T, Hara S, et al. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol. 1990;144:99–101. doi: 10.1016/s0022-5347(17)39379-5. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Sasagawa I, Abe Y, et al. Indication of cystoscopy in patients with asymptomatic microscopic haematuria. Scand J Urol Nephrol. 2000;34:51–4. doi: 10.1080/003655900750016896. [DOI] [PubMed] [Google Scholar]
- 18.Grossfeld GD, Wolf JS, Jr, Litwan MS, et al. Asymptomatic microscopic hematuria in adults: summary of the AUA best practice policy recommendations. Am Fam Physician. 2001;63:1145–54. [PubMed] [Google Scholar]
- 19.Golin AL, Howard RS. Asymptomatic microscopic hematuria. J Urol. 1980;124:389–91. doi: 10.1016/s0022-5347(17)55461-0. [DOI] [PubMed] [Google Scholar]
- 20.Carson CC, III, Segura JW, III, Greene LF. Clinical importance of microhematuria. JAMA. 1979;241:149–50. [PubMed] [Google Scholar]
- 21.Dische FE, Weston MJ, Parsons V. Abnormally thin glomerular basement membranes associated with hematuria, proteinuria or renal failure in adults. Am J Nephrol. 1985;5:103–9. doi: 10.1159/000166914. [DOI] [PubMed] [Google Scholar]
- 22.Goel S, Davenport A, Goode NP, et al. Clinical features and outcome of patients with thin and ultrathin glomerular membranes. QJM. 1995;88:785–93. [PubMed] [Google Scholar]
- 23.Nieuwhof CM, de Heer F, de Leeuw P, et al. Thin GBM nephropathy: premature glomerular obsolescence is associated with hypertension and late onset renal failure. Kidney Int. 1997;51:1596–601. doi: 10.1038/ki.1997.219. [DOI] [PubMed] [Google Scholar]