Abstract
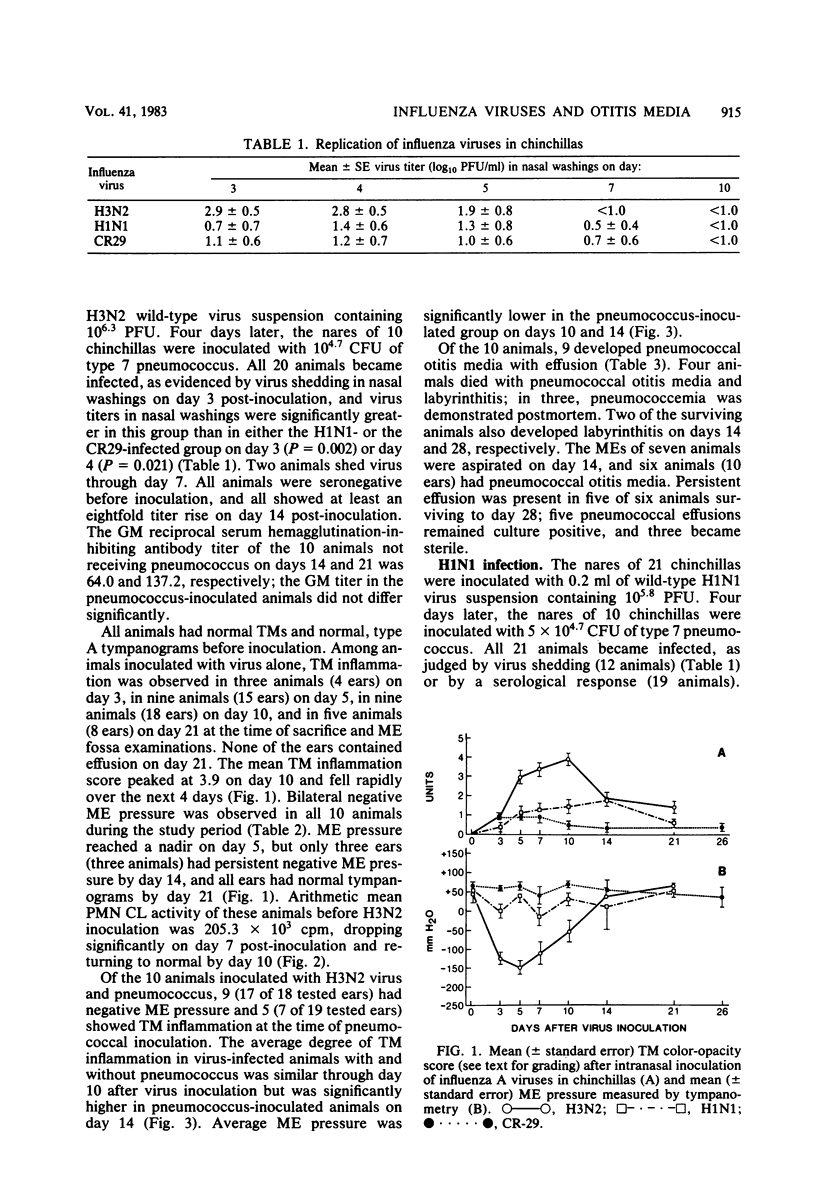
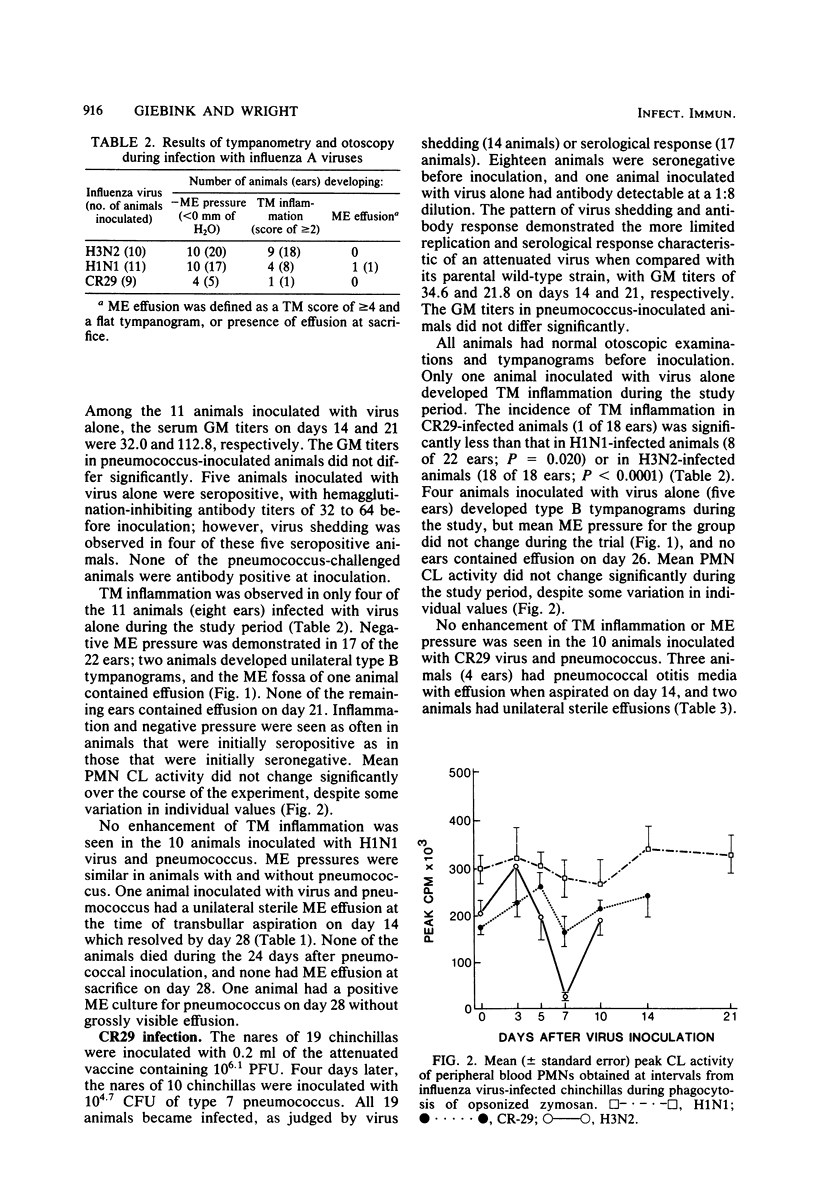
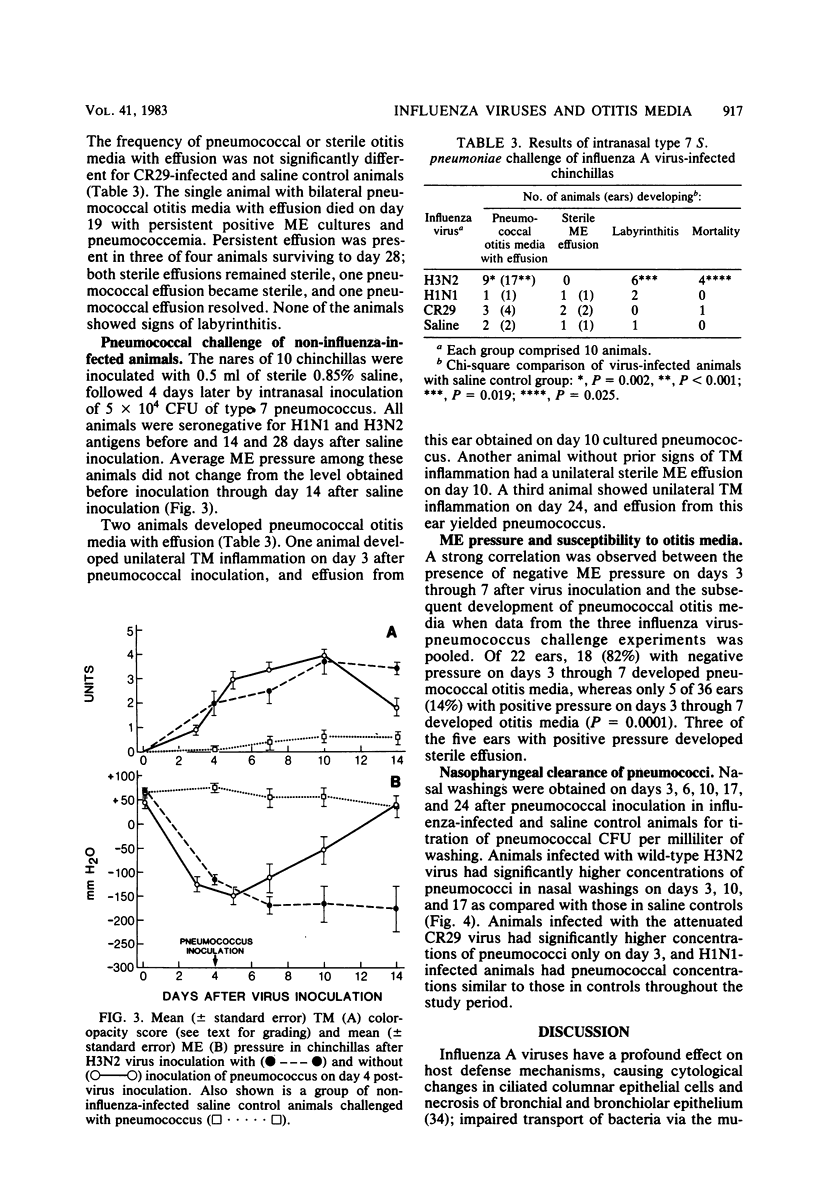
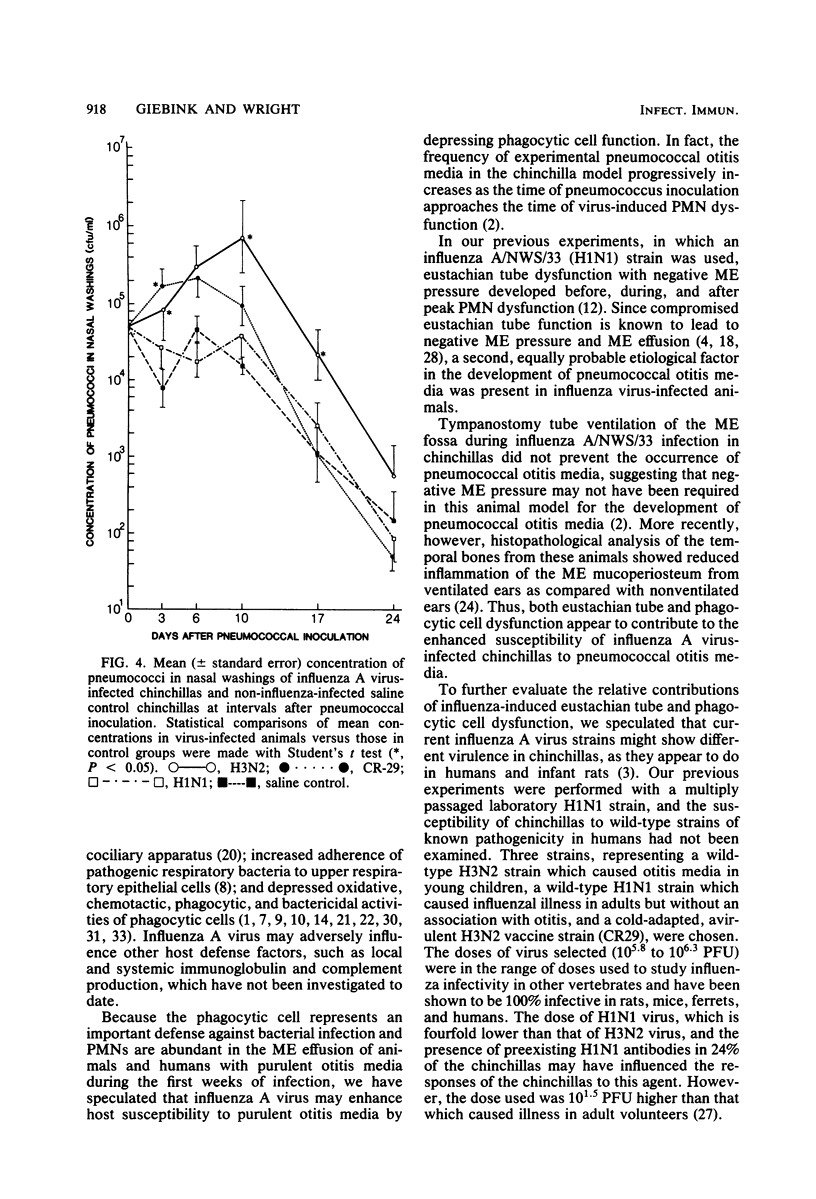
We have previously shown that chinchillas infected with a multiply passaged laboratory strain of influenza A/NWS/33 (H1N1) develop negative middle-ear pressure; polymorphonuclear leukocyte oxidative, bactericidal, and chemotactic dysfunction; and increased susceptibility to pneumococcal otitis media. Because influenza A virus strains show different virulence in humans, three such strains were compared in the chinchilla model. Negative middle-ear pressure and tympanic membrane inflammation developed significantly more often in chinchillas infected with wild-type H3N2 virus than with either wild-type H1N1 virus or an attenuated, cold-adapted H3N2 vaccine strain, CR29. Marked depression in polymorphonuclear leukocyte chemiluminescent activity also developed significantly more often in H3N2 infected animals than in H1N1- or CR29-infected animals. Intranasal challenge of influenza virus-infected animals with type 7 Streptococcus pneumoniae resulted in a significantly greater occurrence of pneumococcal otitis media in H3N2-infected animals than in H1N1-, CR29-, or non-influenza-infected control animals. Clearance of pneumococci from nasal washings of animals infected with wild-type H3N2 was significantly delayed in comparison with the other groups. Thus, the previously demonstrated increased susceptibility to otitis media among children infected with H3N2 influenza virus may relate to the capacity of this strain to induce negative middle-ear pressure, polymorphonuclear leukocyte dysfunction, and alteration in the mucosal clearance of pneumococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Giebink G. S., Mills E. L., Quie P. G. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981 Jun;143(6):836–845. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- Abramson J. S., Giebink G. S., Quie P. G. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect Immun. 1982 Apr;36(1):289–296. doi: 10.1128/iai.36.1.289-296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Maassab H. F., Jennings R., Potter C. W. Infant rat model of attenuation for recombinant influenza viruses prepared from cold-adapted attenuated A/Ann Arbor/6/60. Infect Immun. 1982 Nov;38(2):610–619. doi: 10.1128/iai.38.2.610-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone C. D. Eustachian tube function and allergy in otitis media. Pediatrics. 1978 May;61(5):753–760. [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Maassab H. F., Minuse E., Clark L. C., Abrams G. D., Mitchell J. R. Pilot studies on recombinant cold-adapted live type A and B influenza virus vaccines. J Infect Dis. 1977 Jul;136(1):17–25. doi: 10.1093/infdis/136.1.17. [DOI] [PubMed] [Google Scholar]
- Debets-Ossenkopp Y., van Dijk W. C., Mills E. L., Verbrugh H. A., Verhoef J. The effect of influenza virus on human polymorphonuclear leukocytes. Antonie Van Leeuwenhoek. 1980;46(1):103–103. doi: 10.1007/BF00422240. [DOI] [PubMed] [Google Scholar]
- Fainstein V., Musher D. M., Cate T. R. Bacterial adherence to pharyngeal cells during viral infection. J Infect Dis. 1980 Feb;141(2):172–176. doi: 10.1093/infdis/141.2.172. [DOI] [PubMed] [Google Scholar]
- GERONE P. J., WARD T. G., CHAPPELL W. A. Combined infections in mice with influenza virus and Diplococcus pneumoniae. Am J Hyg. 1957 Nov;66(3):331–341. doi: 10.1093/oxfordjournals.aje.a119906. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Berzins I. K., Marker S. C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infect Immun. 1980 Nov;30(2):445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Heller K. A., Harford E. R. Tympanometric configurations and middle ear findings in experimental otitis media. Ann Otol Rhinol Laryngol. 1982 Jan-Feb;91(1 Pt 1):20–24. doi: 10.1177/000348948209100106. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Heller K. A., Le C. T. Prediction of serous versus purulent otitis media by otoscopy and tympanometry in an animal model. Laryngoscope. 1983 Feb;93(2):208–211. doi: 10.1288/00005537-198302000-00017. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Payne E. E., Mills E. L., Juhn S. K., Quie P. G. Experimental otitis media due to Streptococcus pneumoniae: immunopathogenic response in the chinchilla. J Infect Dis. 1976 Dec;134(6):595–604. doi: 10.1093/infdis/134.6.595. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Schiffman G., Petty K., Quie P. G. Modification of otitis media following vaccination with the capsular polysaccharide of Streptococcus pneumoniae in chinchillas. J Infect Dis. 1978 Oct;138(4):480–487. doi: 10.1093/infdis/138.4.480. [DOI] [PubMed] [Google Scholar]
- Giebink G. S. The pathogenesis of pneumococcal otitis media in chinchillas and the efficacy of vaccination in prophylaxis. Rev Infect Dis. 1981 Mar-Apr;3(2):342–353. doi: 10.1093/clinids/3.2.342. [DOI] [PubMed] [Google Scholar]
- Goycoolea M. V., Paparella M. M., Goldberg B., Schlievert P. M., Carpenter A. M. Permeability of the middle ear to staphylococcal pyrogenic exotoxin in otitis media. Int J Pediatr Otorhinolaryngol. 1980 Feb;1(4):301–308. doi: 10.1016/0165-5876(80)90004-x. [DOI] [PubMed] [Google Scholar]
- Grönroos J. A., Vihma L., Salmivalli A., Berglund B. Coexisting viral (respiratory syncytial) and bacterial (pneumococcus) otitis media in children. Acta Otolaryngol. 1968 May;65(5):505–517. doi: 10.3109/00016486809120993. [DOI] [PubMed] [Google Scholar]
- Harboe M., Folling I. Sudden death caused by interaction between a macroglobulin and a divalent drug. Lancet. 1976 Aug 7;2(7980):285–289. doi: 10.1016/s0140-6736(76)90734-0. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Sanyal M. A., Watkins J. M., Fairclough D. L., Clyde W. A., Jr, Denny F. W. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982 Jun 10;306(23):1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- Kilburn K. H. Cilia and mucus transport as determinants of the response of lung to air pollutants. Arch Environ Health. 1967 Jan;14(1):77–91. doi: 10.1080/00039896.1967.10664699. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Parry R. P., Gilchrist C., Luquetti A., Tyrrell D. A. Influenza viruses and staphylococci in vitro: some interactions with polymorphonuclear leucocytes and epithelial cells. Br J Exp Pathol. 1977 Jun;58(3):281–288. [PMC free article] [PubMed] [Google Scholar]
- Meguro H., Bryant J. D., Torrence A. E., Wright P. F. Canine kidney cell line for isolation of respiratory viruses. J Clin Microbiol. 1979 Feb;9(2):175–179. doi: 10.1128/jcm.9.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff W. L., Giebink G. S., Shea D. A., Le C. T. Effect of tympanostomy tubes on the pathogenesis of acute otitis media. Am J Otolaryngol. 1982 May-Jun;3(3):189–195. doi: 10.1016/s0196-0709(82)80053-7. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Chanock R. M., Clements M. L., Anthony W. C., Sear A. J., Cisneros L. A., Rennels M. B., Miller E. H., Black R. E., Levine M. M. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect Immun. 1981 May;32(2):693–697. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise J. L. Otitis media in infants and children. Pediatrics. 1980 May;65(5):917–943. [PubMed] [Google Scholar]
- Reuman P. D., Ayoub E. M., Small P. A., Jr Influenza infection in the infant mouse. Pediatr Res. 1983 May;17(5):338–343. doi: 10.1203/00006450-198305000-00006. [DOI] [PubMed] [Google Scholar]
- Ruutu P., Vaheri A., Kosunen T. U. Depression of human neutrophil motility by influenza virus in vitro. Scand J Immunol. 1977;6(9):897–906. doi: 10.1111/j.1365-3083.1977.tb00410.x. [DOI] [PubMed] [Google Scholar]
- SELLERS T. F., Jr, SCHULMAN J., BOUVIER C., McCUNE R., KILBOURNE E. D. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961 Aug 1;114:237–256. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Schachern P. A., Paparella M. M., Goycoolea M., Goldberg B., Schlievert P. The round window membrane following application of staphylococcal exotoxin: an electron microscopic study. Laryngoscope. 1981 Dec;91(12):2007–2017. doi: 10.1288/00005537-198112000-00003. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Thompson J., Karzon D. T. Differing virulence of H1N1 and H3N2 influenza strains. Am J Epidemiol. 1980 Dec;112(6):814–819. doi: 10.1093/oxfordjournals.aje.a113053. [DOI] [PubMed] [Google Scholar]


