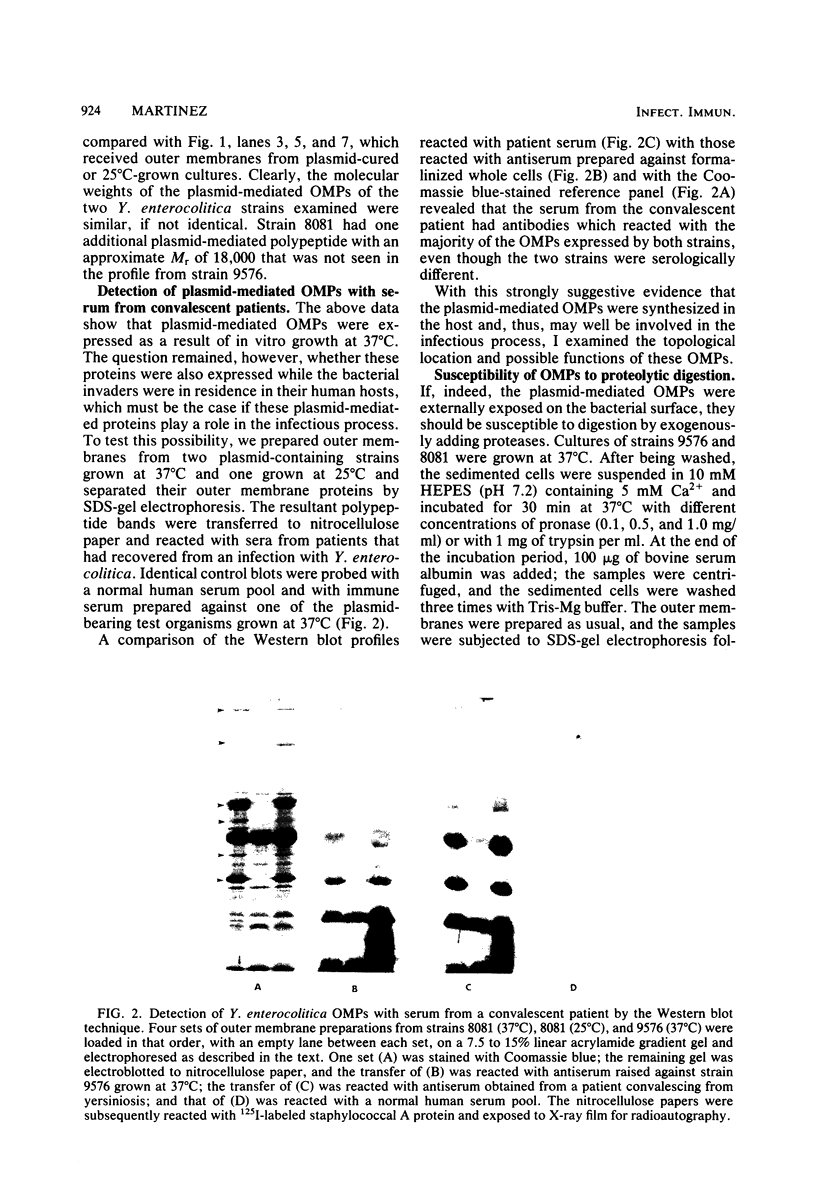
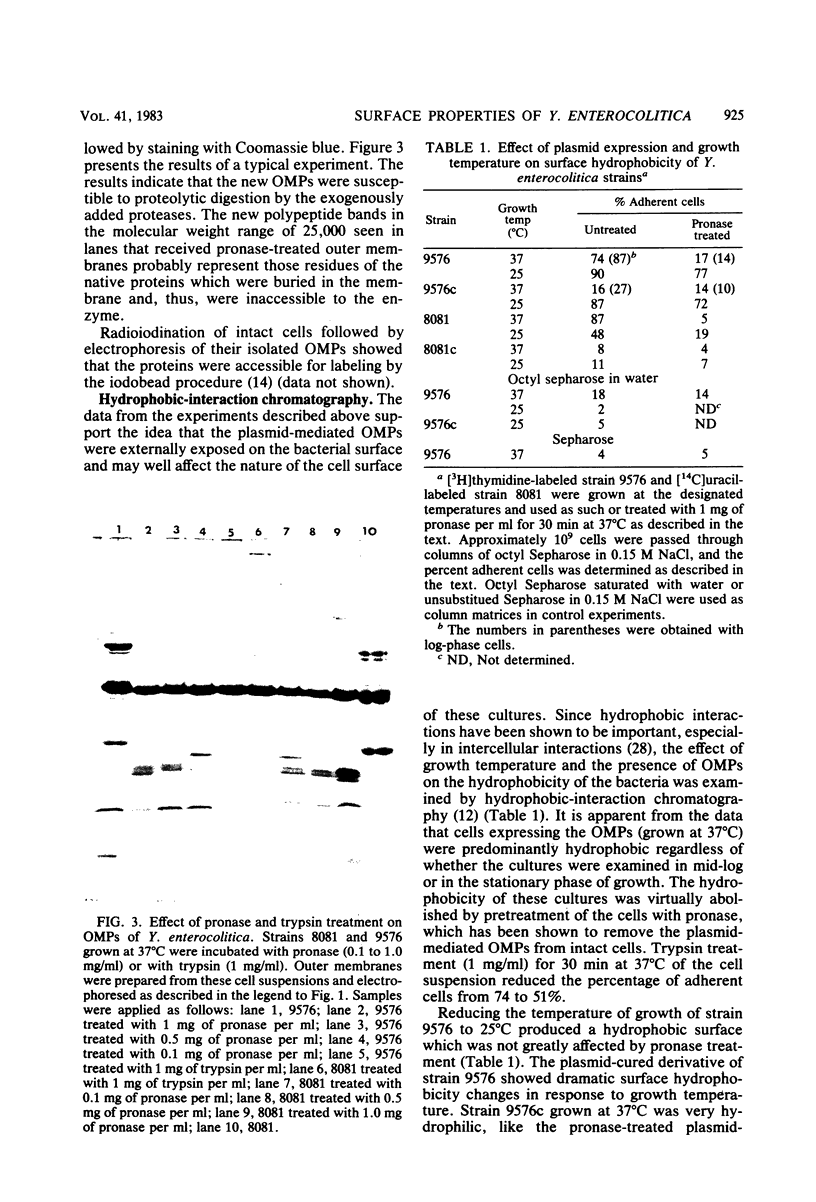
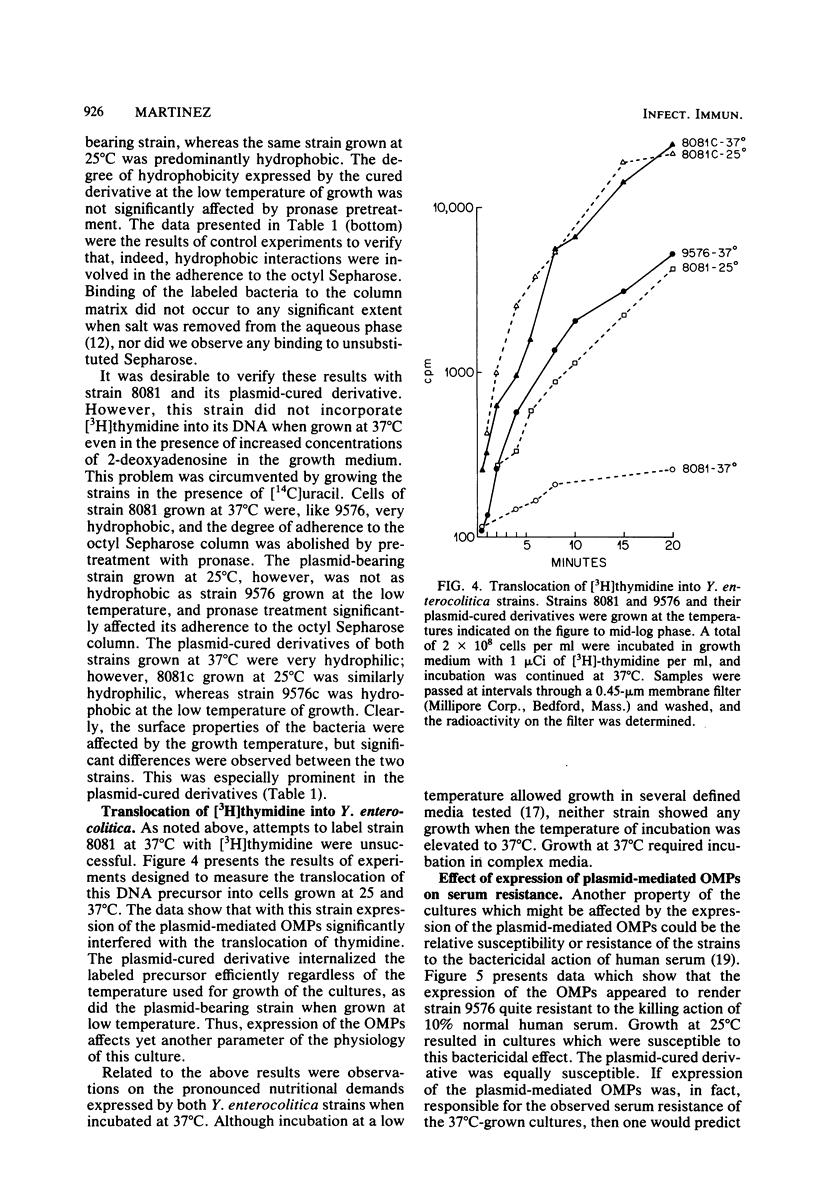
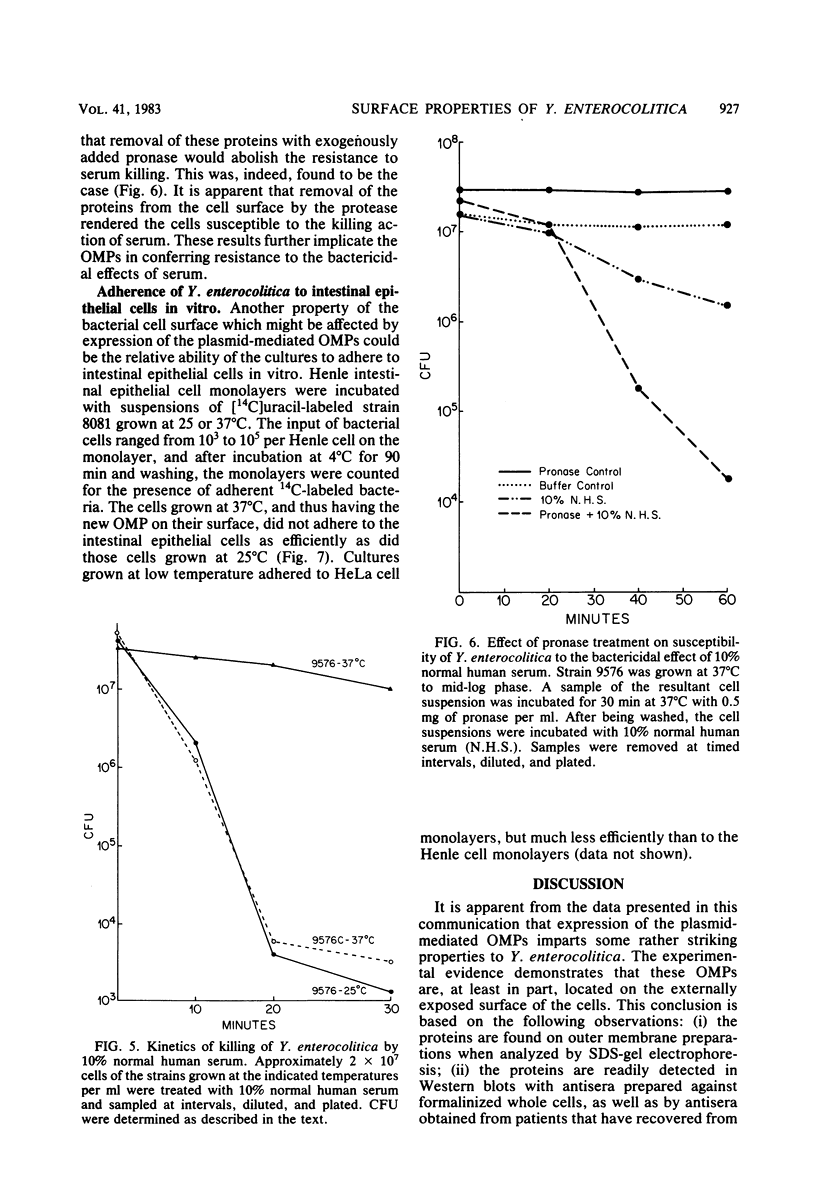
Abstract
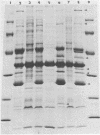
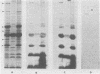
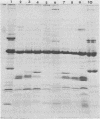
Enteropathogenic strains of Yersinia enterocolitica harbor a virulence plasmid which codes for a series of novel outer membrane proteins. The expression of these proteins on the outer membrane is temperature regulated: when cells are grown at 25 degrees C, these proteins are not exposed on the outer membrane, whereas they occur in high copy number when cells are grown at 37 degrees C. The majority of these proteins are externally exposed on the cell surface as evidenced by their susceptibility to proteolysis by exogenously added proteases. The expression of the plasmid-mediated proteins on the outer membrane does not favor adherence of the bacteria to intestinal epithelial cells in vitro. Cultures grown at 25 degrees C adhered to Henle cell monolayers, whereas those grown at 37 degrees C did so much less effectively. The presence of the proteins on the bacterial surface appears to be involved in rendering the cells resistant to the bactericidal effects of serum, i.e., 37 degrees C-grown cells were resistant to serum killing, and removal of the outer membrane proteins with pronase rendered them sensitive. Evidence is presented which strongly suggests that the plasmid-mediated proteins are synthesized and expressed on the cell surface either during or after transit of the ingested bacteria to the lamina propria. Some properties afforded to the cells by the outer membrane proteins are described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
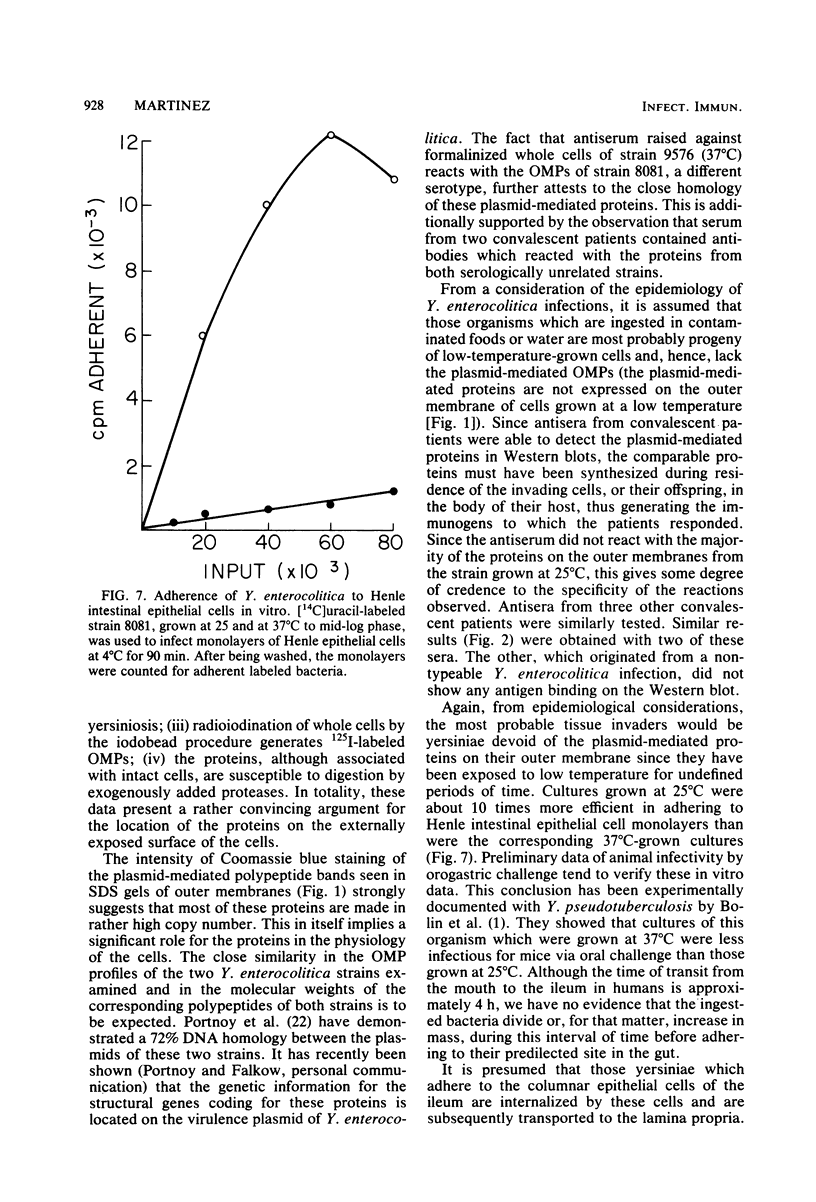
- Bottone E. J. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5(2):211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C., Bottone E. J. Serum resistance of Yersinia enterocolitica expressed in absence of other virulence markers. Infect Immun. 1983 Jan;39(1):469–472. doi: 10.1128/iai.39.1.469-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Bactericidal properties of human cell-free lymph. Infect Immun. 1976 Mar;13(3):768–775. doi: 10.1128/iai.13.3.768-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J., Carroll S. F. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980 Jun;28(3):735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V., Seemayer T. A. Experimental Yersinia enterocolitica enteritis in rabbits. Infect Immun. 1980 Apr;28(1):238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Toma S. Isolation of Yersinia enterocolitica from raw milk. Appl Environ Microbiol. 1978 Jan;35(1):54–58. doi: 10.1128/aem.35.1.54-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. I. Experimental infection in rabbits. Microbiol Immunol. 1977;21(7):341–363. [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]