Abstract
An ion pair receptor, 1, containing both cation- and anion-recognizing sites, has been synthesized and characterized. Single crystal X-ray diffraction structural studies and 1H NMR spectroscopic analyses confirm that 1 forms a stable 1:1 complex with CsF in solution and in the solid state in spite of the large separation enforced between the receptor-bound anion and cation. In 9:1 CDCl3/CD3OD, fluoride anion binding within the calix[4]pyrrole core of 1 is not observed in the absence of a co-bound cesium cation; however, it is seen in this solvent mixture under conditions where a Cs+ cation is bound to the crown ether strapped calix[4]arene subunit.
Introduction
Over the past several decades, a large number of macrocyclic compounds have been synthesized and studied as potential cation receptors.1 Moreover, as the importance of anions in biology, the environment, and medicine has become increasingly well recognized, attention has focused on the design and construction of anion receptors.2 However, in spite of their potential utility in such areas as salt solublization, ion extraction, and through-membrane transport, relatively little effort has been devoted to the synthesis and study of so-called ion pair receptors, species that are able to complex concurrently and with specificity both an anion and a cation.3-6 While a number of host systems are known that contain both an anion and cation binding site, enhanced binding of an ion pair, where binding of the cation enhances binding of the anion or vice versa, is generally seen only in systems wherein the two ion binding sites are held in close proximity.7 This has the consequence that in most cases it is so-called contact ion pairs, rather than solvent or spatially separated ion pairs, that are bound thereby avoiding the presumably unfavorable separation of two oppositely charged ions.5,6,8 In fact, we are aware of only two closely related examples of structurally characterized spatially separated ion pair complexes.6a-c However, in neither case was strong ion pair binding observed in solution.9 Therefore, we sought to explore whether it would be possible to produce a receptor that could bind a specific cationanion pair with high affinity and in the form of a solvent separated ion pair. We were particularly interested in a system that could be used to stabilize ion pairs involving the cesium cation, because of its importance in solvent separations targeted for use in radioactive waste purification.10 With such considerations in mind, we have now prepared the calix[4]arene crown-6 “capped” calix[4]pyrrole 1 and show here that it 1) forms a solvent separated ion pair complex with CsF in the solid state and 2) binds its constituent ions (Cs+ and F−) in a highly cooperative fashion in organic solvents (e.g., 9:1 CDCl3/CD3OD).
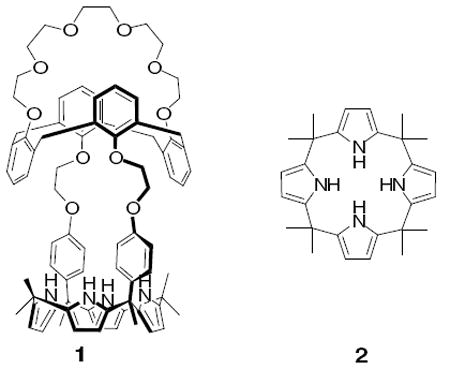
The ion pair receptor 1 was designed to bring together both an anion binding core and a cation-recognizing subunit in such a way that a large separation between the constituent ions of a bound ion pair would be enforced. Calix[4]pyrrole (2)11 and calix[4]arene crown-612 were chosen as the anion and cation binding species, respectively. Previous work had served to establish that these receptor systems, when studied individually, could be used to effect the binding of fluoride anion and cesium cation, at least in organic media. Accordingly, CsF was selected as the target salt for possible ion pair complexation.
Results and Discussion
The synthesis of receptor 1 is shown in Scheme 1. First, the calix[4]arene crown-6 ditosylate 313 was reacted with 4’-hydroxyacetophenone in acetonitrile in the presence of excess K2CO3 at reflux; this afforded diketone 4 in quantitative yield. Subsequent condensation of the latter species with pyrrole in the presence of excess trifluoroacetic acid at 65 °C then gave the dipyrromethane 5 in 46% yield. This key precursor was then condensed with acetone in the presence of a catalytic amount of BF3·OEt2 to give 1 in 18% yield.14
Scheme 1.
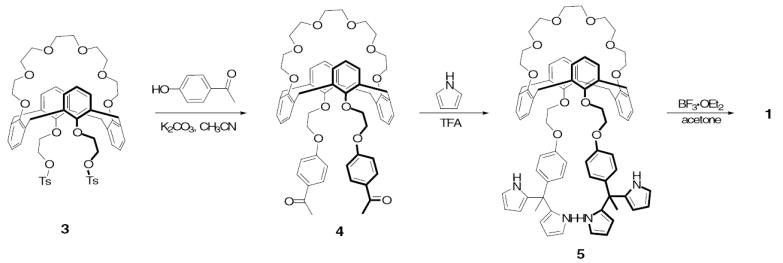
Synthesis of compound 1
Initial evidence that 1 can act as a receptor for CsF in the form of a solvent separated ion pair came from single crystal X-ray diffraction analysis. Suitable crystals were obtained by allowing a chloroform/methanol solution of receptor 1 to undergo slow evaporation in the presence of excess cesium fluoride. The resulting structure revealed that 1 forms a 1:1 complex with cesium fluoride, 1·CsF (cf. Figure 1). The Cs+ ion in 1·CsF is included in the calix[4]arene crown ether ring with the Cs+⋯O distances of 3.08 – 3.36 Å, while a distance of 3.43 – 3.63 Å characterizes the presumed π - cation interactions involving the Cs+ ion and the aromatic carbon atoms at the meta- and para-position to the phenoxy groups. On the other hand, the bound F− anion is hydrogen-bound to the NH’s of the calix[4]pyrrole subunit (the N⋯F− distances are in the range of 2.74 – 2.78 Å) and is also hydrogen-bound to a molecule of methanol. The presence of this bound methanol molecule serves to ensure that there is no direct interaction between the co-bound, spatially-separated Cs+ and F− ions in the solid state complex 1·CsF. This absence of interaction is likely reinforced by the large gap between the calix[4]pyrrole anion binding subunit and the crown-strapped calix[4]arene cation recognition site. In fact, the separation between the Cs+ and the F− ions seen in the solid state structure of 1·CsF, 10.92 Å, is much longer than the Cs+⋯F− distances seen in the solid state structure of the CsF complex of meso-octamethylcalix[4]pyrrole 2.15,16 This latter species, although capable of functioning as an ion pair receptor under certain biphasic extraction conditions,10a contains no independent cation recognition site. On the basis of these findings, we suggest that, at least in the solid state, the formation of a strong complex, 1·CsF, with individual, solvent-separated ions, is energetically favorable relative to other possible scenarios such as complexation of a contact ion pair.
Figure 1.
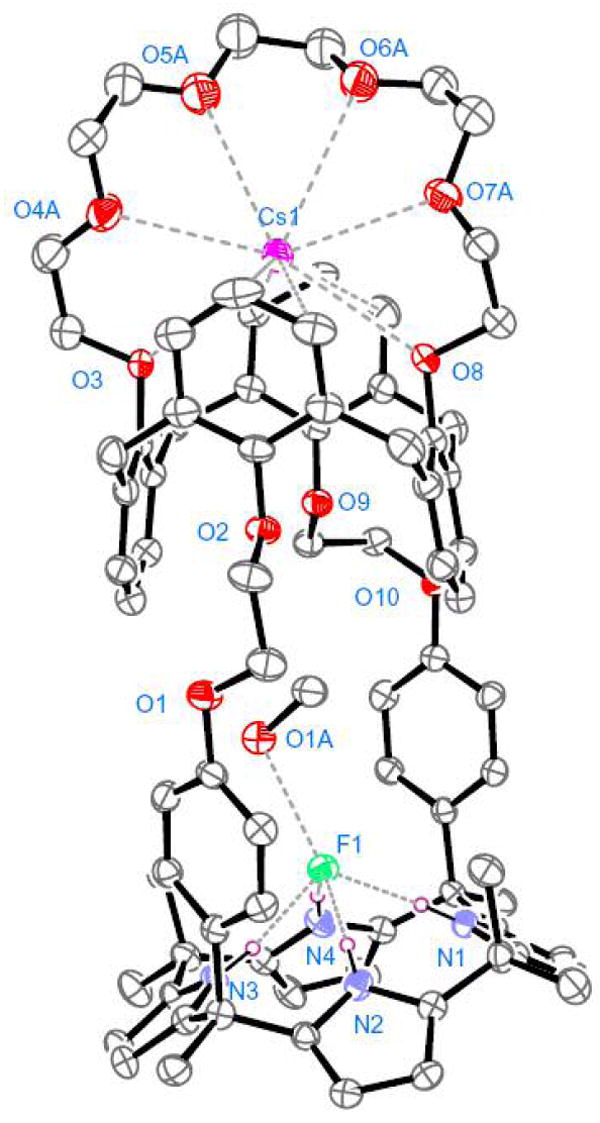
View of the 1·CsF complex showing a partial atom labeling scheme. Displacement ellipsoids are scaled to the 30% probability level. Most hydrogen atoms have been removed for clarity. Atoms in the ether linkage are disordered, with the higher occupancy atoms being shown.
The ability of 1 to bind halide anion salts in solution was probed via 1H NMR spectroscopy using initially CDCl3 as the solvent. In contrast to what was seen in the case of other calix[4]pyrrole derivatives, including the various other strapped calix[4]pyrroles prepared to date, in this solvent system only the addition of soluble fluoride anion salts (e.g., tetrabutylammonium fluoride, TBAF) served to engender spectroscopic changes consistent with anion binding (i.e., no other TBA halide anion salts had an effect on the 1H NMR spectrum). This apparent selectivity is thought to reflect a combination of a less accessible anion binding site and a more rigid calix[4]pyrrole core enforced by the rather inflexible phenoxy spacers (Figures S1 and S2).
The changes observed in the 1H NMR spectrum when 1 is subject to titration with TBAF in CDCl3 are shown in Figure S1. The anion-free form of 1 displays a broad singlet at δ = 6.74 ppm for the NH’s and two triplets at δ = 6.04 ppm and δ = 5.95 ppm, respectively, for the β-pyrrolic protons. Addition of 0.4 and 0.8 equivalents of TBAF gives rise to two sets of distinguishable resonances for all proton signals. These peaks are ascribed to the anion-free and fluoride bound forms of 1 and are consistent with the anion binding/decomplexation equilibrium being slow on the 1H NMR time scale. Such slow exchange kinetics are consistent with strong anion binding, a conclusion further supported by the observation of significant changes in the β-pyrrolic and, especially, the pyrrolic NH proton signals. The singlet associated with the NH proton resonance seen in free 1 is shifted to lower field by roughly 6 ppm (final δ ≈ 12.7 ppm) upon the addition of fluoride anion. The signal also becomes split into a doublet (J = 44.0 Hz), a finding that is ascribable to coupling between the bound fluoride anion and the NH protons.17
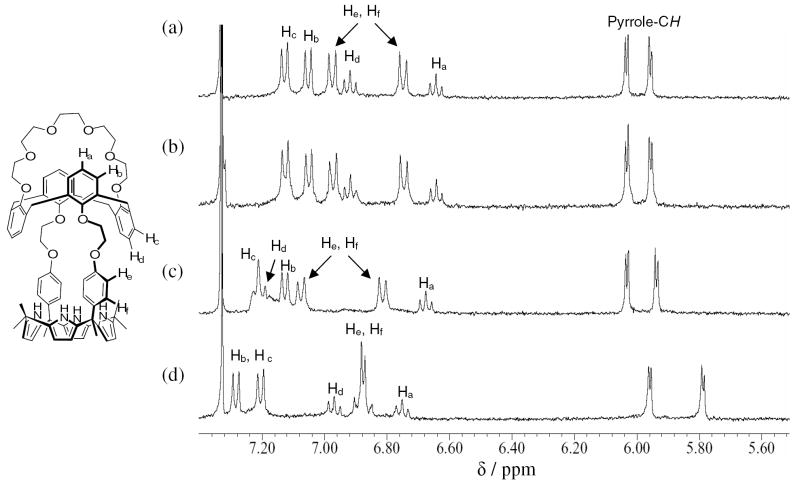
Very different behavior is seen when analogous 1H NMR spectroscopic analyses are carried out in 10% (v/v) CD3OD in CDCl3. Under these conditions, no evidence of fluoride anion binding is seen when 1 is treated with 5 equiv. of TBAF (cf. Figure 2).18 This lack of appreciable interaction is attributed to the stronger solvation of the fluoride ions by this more polar medium (Figures 2(b) and 3).
Figure 2.
Partial 1H NMR spectra of (a) 1 only, (b) 1 + 5 equiv. of TBAF, (c) 1 + 5 equiv. of CsClO4, and (d) 1 + 5 equiv. of CsF in CD3OD/CDCl3 (1/9, v/v).
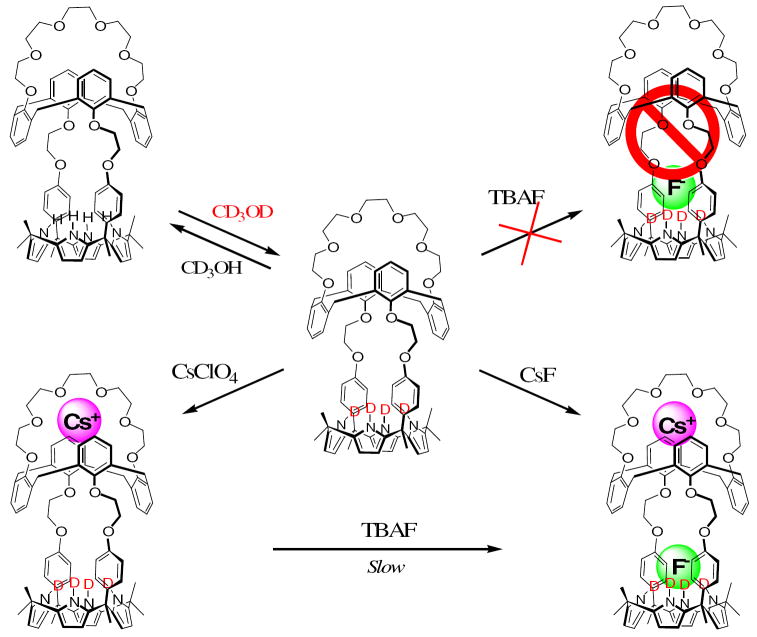
Figure 3.
Proposed binding interactions involving 1 and various Cs+ and F− salts in CD3OD/CDCl3 (1/9, v/v).
In contrast to what is seen with TBAF, the addition of 5 equiv. of cesium perchlorate induces remarkable changes in the signals for both the aromatic protons of the calix[4]arene core and in the aliphatic protons of the crown-6 ring. Particularly noteworthy is the considerable downfield shift of the Hd proton on the inverted phenoxy group, as would be expected if the oxygen atom of this moiety were involved in cesium cation complexation (Figure 2(c)). This stands in contrast to the β-pyrrolic and the meso-aromatic proton signals associated with the calix[4]pyrrole subunit; here, little appreciable change is seen. Taken together, these findings are consistent with the expectation that the addition of CsClO4 leads to the formation of a cation-bound complex, wherein the cesium cation is encapsulated in the calix[4]arene crown-6 ring and the perchlorate anion is either not bound strongly or bound at all by the calix[4]pyrrole core (cf. Figures 2(c), 3, and S4).
In analogy to what is seen with CsClO4, the addition of 5 equiv. of CsF to receptor 1 in 10% CD3OD in CDCl3 leads to downfield shifts in the proton signals of both the calix[4]arene and the crown-6 ring (see Figures 2(d) and S4(d)); this is as would be expected if the Cs+ cation were also being bound well in this case.12 However, in contrast to what was seen with TBAF, the use of CsF leads to significant upfield changes in the signals of both the β-pyrrolic protons and the meso-aromatic protons of the calix[4]pyrrole moiety (cf. Figures 2(d) and 3). Such observations are fully consistent with the fluoride anion being bound to the calix[4]pyrrole cavity of receptor 1 thus binding both Cs+ and F− ions as an ion pair complex, 1·CsF analogous to what is seen in the solid sate. It is thus concluded that the binding of the cesium cation to the crown ether ring plays a very critical role in inducing fluoride anion binding to the calix[4]pyrrole portion of receptor 1, anion binding that is otherwise not observed in the absence of Cs+ in this solvent system. No other cations tested, specifically Li+, Na+ and K+, were found to give rise to such an effect.
Further support for the above conclusion came from the finding that addition of TBAF to a preformed cesium complex (i.e., 1·Cs+, formed by Eq. 1) gives rise to the formation of a co-bound CsF complex analogous to that produced from CsF alone, albeit at a rate that is slow on the NMR time scale (cf. Figure S6); presumably, this reflects the slow kinetics associated with counter anion exchange (cf. Eq 2). In the event, it is noteworthy that a diffraction grade single crystal grown in the presence of both cesium perchlorate (CsClO4) and tetrabutylammonium fluoride (TBAF) gave rise to the exactly same structure as a datum crystal grown in the presence of CsF only (structure shown in Figure 1).
| (1) |
| (2) |
Isothermal titration calorimetry (ITC) was utilized to quantify the affinity of compound 1 for Cs+ and F− in a solvent mixture analogous to that used for the latter 1H NMR spectroscopic studies (i.e. 10% MeOH in CHCl3). The resulting titration of CsF [0.08 mM] with 1 [1.1 mM] was highly enthalpic ΔH = −16.2 kcal/mol. The data could be fit well to a 1:1 binding profile, yielding a binding energy of ΔG = −7.6 kcal/mol and a Ka = 3.8 × 105 M−1, while revealing a strong opposing entropy (TΔS = −8.6 kcal/mol) (Table 1). However, upon increasing the concentration of both 1 and CsF, a second event in the early stages of the titration becomes prevalent. While further study is in order, it is possible that this latter finding reflects changes in overall solvation that are not accounted for in simple receptor-free control experiments.
Table 1.
ITC titration data of 1, 1·F−, and 1·Cs+ measured at 298 Ka
| Host | Solvent | Guest | ΔH (kcal/mol) |
TΔS( kcal/mol) |
ΔG (kcal/mol) |
Ka (M-1) |
|---|---|---|---|---|---|---|
| 1 | CH3CN | CsTPBb | −6.7 | 1.3 | −8.1 | 8.0 × 105 |
| 1·F− | CH3CN | CsTPBb | −6.0 | 2.3 | −8.3 | 1.2 × 106 |
| 1 | CH3CN | TBAFc | −6.2 | 0.8 | −7.0 | 1.3 × 105 |
| 1·Cs+ | CH3CN | TBAFc | −7.2 | −0.4 | −6.8 | 1.1 × 105 |
| 1 | CH3OH/CHCl3 (1/9, v/v) |
CsF | −16.2 | −8.6 | −7.6 | 3.4 × 105 |
Errors estimated to be less than 15%.
CsTPB = cesium tetraphenylborate.
TBAF = tetrabutylammonium fluoride.
Efforts to analyze the individual ion binding events were also made using ITC. In this case, titrations using TBAF and cesium tetraphenylborate (CsTPB) were carried out, albeit in acetonitrile due to solubility considerations.19 First CsTPB was titrated into 1; this resulted in a Ka value of 8.0 × 105 M−1. Next, CsTPB was titrated into a 3:1 mixture of TBAF:1; this gave rise to first a set of exothermic signals, followed by a series of endothermic traces towards the end of the titration. Fitting to a 1:1 profile proved clean and gave rise to a Ka value of 1.2 × 106 M−1, a small increase in the affinity as compared to what was observed in the absence of fluoride.
In a separate experiment, the interaction of TBAF with 1 was studied; this yielded a Ka of 1.3 × 105 M−1. TBAF was then titrated into a 2:1 mixture of CsTPB:1, and the resulting isotherm again showed an initial exothermic interaction, followed by endothermic signals towards the end of the titration. However, as above, this data could be fit well to a 1:1 binding isotherm, yielding a Ka value of 1.1 × 106 M−1. Thus, in acetonitrile it appears that the binding of the individual ions is virtually independent of one another and that the affinity of 1 for cesium is about an order of magnitude greater than for fluoride. Such behavior stands in marked contrast to what is seen in 9:1 CDCl3/CD3OD (vide supra) and leads to the conclusion that the binding behavior of 1, like that of simple calix[4]pyrrole 2,11a,11e is subject to a strong solvent dependence. This is perhaps not surprising given the interplay of the relatively complicated and contradictory effects involved (e.g., receptor, salt, and individual ion solvation, ion-pairing, receptor-cation, receptor-anion, and receptor-ion pair interactions). However, the key point is that in all solvents tested to date, including acetonitrile, concurrent binding of both an anion (F−) and a cation (Cs+) can be effected using receptor 1.
Conclusions
An ion pair receptor 1 containing both cation- and anion-recognition sites has been synthesized. The X-ray crystal structure and the 1H NMR spectroscopic analysis provide support for the conclusion that 1 forms a stable 1:1 complex with CsF in spite of the large separation enforced between the anion and the cation. In more competitive media, such as 10% methanol in chloroform, little evidence of fluoride anion binding is observed in the absence of a co-bound cesium cation, on the basis of which it is suggested that binding of this cation to the crown ether strapped calix[4]arene makes possible complexation of a fluoride anion within the calix[4]pyrrole core of 1.
Supplementary Material
Synthetic details, NMR spectroscopic data, ITC analyses, and X-ray structural data for 1·CsF. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Institutes of Health (GM 58907 to J.L.S.) and by a grant from the Korea Science and Engineering Foundation (R01-2006-000-10001-0 to C.H.L) funded by the South Korean government (MOST).
References
- 1.Lehn J-M, Atwood JL, Davies JED, MacNicol DD, Vögtle F, editors. Comprehensive Supramolecular Chemistry. Vol. 1 Pergamon; Oxford: 1996. [Google Scholar]; (b) Lehn J-M. Supramolecular Chemistry Concepts and Perspectives. VCH; Weinheim: 1995. [Google Scholar]
- 2.(a) Beer PD, Gale PA. Angew Chem Int Ed. 2001;40:486–516. [PubMed] [Google Scholar]; (b) Sessler JL, Gale PA, Cho W-S. In: Anion Receptor Chemistry (Monographs in Supramolecular Chemistry) Stoddart JF, editor. The Royal Society of Chemistry; Cambridge: 2006. [Google Scholar]
- 3.Smith BD. In: Ion Pair Recognition by Ditopic Receptors Macrocyclic Chemistry Current Trends and Future Perspectives. Gloe K, Antonioli B, editors. Kluwer; London: 2005. pp. 137–152. [Google Scholar]
- 4.(a) Pfeifer JR, Reiβ P, Koert U. Angew Chem Int Ed. 2006;45:501–504. doi: 10.1002/anie.200502570. [DOI] [PubMed] [Google Scholar]; (b) Sisson AL, Shah MR, Bhosale S, Matile S. Chem Soc Rev. 2006;35:1269–1286. doi: 10.1039/b512423a. [DOI] [PubMed] [Google Scholar]; (c) Nakamura T, Akutagawa T, Honda K, Underhill AE, Coomber AT, Friend RH. Nature. 1998;394:159–162. [Google Scholar]; (d) Gokel GW, Leevy WM, Weber ME. Chem Rev. 2004;104:2723–2750. doi: 10.1021/cr020080k. [DOI] [PubMed] [Google Scholar]; (e) Davis AP, Sheppard DN, Smith BD. Chem Soc Rev. 2007;36:348–357. doi: 10.1039/b512651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Chrisstoffels LAJ, De Jong F, Reinhoudt DN, Sivelli S, Gazzola L, Casnati A, Ungaro R. J Am Chem Soc. 1999;121:10142–10151. [Google Scholar]; (b) Rudkevich DM, Mercer-Chalmers JD, Verboom W, Ungaro R, Reinhoudt DN. J Am Chem Soc. 1999;117:6124–6125. [Google Scholar]; (c) Schreeder J, van Duynhoven JPM, Engbersen JFJ, Reinhoudt DN. Angew Chem Int Ed Engl. 1996;35:1090–1093. [Google Scholar]
- 6.(a) Mahoney JM, Stucker KA, Jiang H, Carmichael I, Brinkmann NR, Beatty AM, Noll BC, Smith BD. J Am Chem Soc. 2005;127:2922–2928. doi: 10.1021/ja0440295. [DOI] [PubMed] [Google Scholar]; (b) Deetz MJ, Shang M, Smith BD. J Am Chem Soc. 2000;122:6201–6207. [Google Scholar]; (c) Mahoney JM, Beatty AM, Smith BD. Inorg Chem. 2004;43:7617–7621. doi: 10.1021/ic049066b. [DOI] [PubMed] [Google Scholar]; (d) Mahoney JM, Davis JP, Smith BD. J Org Chem. 2003;68:9819–6820. doi: 10.1021/jo035270p. [DOI] [PubMed] [Google Scholar]; (e) Mahoney JM, Beatty AM, Smith BD. J Am Chem Soc. 2001;123:5847–5858. doi: 10.1021/ja0156082. [DOI] [PubMed] [Google Scholar]; (f) Mahoney JM, Nawaratna GU, Beatty AM, Duggan PJ, Smith BD. Inorg Chem. 2004;43:5902–5907. doi: 10.1021/ic0494859. [DOI] [PubMed] [Google Scholar]
- 7.While a number of systems were prepared early on that contained both anion and cation binding subunits constrained at remote sites within the same molecular framework, few of these displayed cooperative anion plus cation binding in solution or coupled anion and cation complexation in the solid state. They are thus not considered as bona fide ion pair receptors. For reviews of these systems, see: Kirkovits GJ, Shriver JA, Gale PA, Sessler JL. J Incl Phenom Macrocycl Chem. 2001;41:69–75.Gale PA. Coord Chem Rev. 2003;240:191–221.
- 8.Marcus Y, Hefter G. Chem Rev. 2006;106:4585–4621. doi: 10.1021/cr040087x. [DOI] [PubMed] [Google Scholar]
- 9.Binding constants for the complexation of ion pairs were not actually recorded, perhaps due to a combination of poor solubility and slow binding kinetics. However, modest increases in the anion binding affinities were observed in the presence of cations.6a-c
- 10.(a) Wintergerst MP, Levitskaia TG, Moyer BA, Sessler JL, Delmau LH. J Am Chem Soc. 2008;130:4129–4139. doi: 10.1021/ja7102179. [DOI] [PubMed] [Google Scholar]; (b) Levitskaia TG, Bryan JC, Sachleben RA, Lamb JD, Moyer BA. J Am Chem Soc. 2000;122:554–562. [Google Scholar]; (c) Sachleben RA, Bryan JC, Engle NL, Haverlock TJ, Hay BP, Urvoas A, Moyer BA. Eur J Org Chem. 2003:4862–4869. [Google Scholar]
- 11.(a) Sessler JL, Gross DE, Cho W-S, Lynch VM, Schmidtchen FP, Bates GW, Light ME, Gale PA. J Am Chem Soc. 2006;128:12281–12288. doi: 10.1021/ja064012h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gale PA, Sessler JL, Král V, Lynch VM. J Am Chem Soc. 1996;118:5140–5141. [Google Scholar]; (c) Gale PA, Sessler JL, Král V. Chem Commun. 1998:1–8. [Google Scholar]; (d) Lee C-H, Miyaji H, Yoon D-W, Sessler JL. Chem Commun. 2008:24–34. doi: 10.1039/b713183f. [DOI] [PubMed] [Google Scholar]; (e) Gross DE, Schmidtchen FP, Antonius W, Gale PA, Lynch VM, Sessler JL. Chem–Eur J. 2008;14 doi: 10.1002/chem.200800899. Early View (Online) [DOI] [PubMed] [Google Scholar]
- 12.(a) Kim SK, Lee JK, Lee SH, Lim MS, Lee SW, Sim W, Kim JS. J Org Chem. 2004;69:2877–2880. doi: 10.1021/jo035567n. [DOI] [PubMed] [Google Scholar]; (b) Lee JK, Kim SK, Bartsch RA, Vicens J, Miyano S, Kim JS. J Org Chem. 2003;68:6720–6725. doi: 10.1021/jo034666y. [DOI] [PubMed] [Google Scholar]; (c) Kim SK, Sim W, Vicens J, Kim JS. Tetrahedron Lett. 2003;44:805–809. [Google Scholar]; (d) Kim SK, Vicens J, Park KM, Lee SS, Kim JS. Tetrahedron Lett. 2003;44:993–997. [Google Scholar]
- 13.(a) No K, Lee HJ, Park KM, Lee SS, Noh KH, Kim SK, Lee JY, Kim JS. Journal of Heterocyclic Chemistry. 2004;41:211–219. [Google Scholar]; (b) Kim JS, Shon OJ, Ko JW, Cho MH, Yu IY, Vicens J. J Org Chem. 2000;65:2386–2392. doi: 10.1021/jo9912754. [DOI] [PubMed] [Google Scholar]
- 14.Yoon D-W, Hwang H, Lee C-H. Angew Chem Int Ed. 2002;41:1757–1759. doi: 10.1002/1521-3773(20020517)41:10<1757::aid-anie1757>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.In the CsF complex of 2, the F- ion is symmetrically bound to the four NH’s of the calix[4]pyrrole via four hydrogen bonds at a N⋯F- distance of 2.79 Å, whereas the Cs+ is symmetrically encapsulated within the cone-like cavity of the calix[4]pyrrole via π - cation interactions with a distance of 3.39 Å between the Cs+ ion and the centroids of the pyrrole rings. The F- ion interacts both with the Cs+ ion within the same complex and with the one in an adjacent complex, and does so with separation distances of 3.69 Å and 2.77 Å, respectively.
- 16.Custelcean R, Delmau LH, Moyer BA, Sessler JL, Cho W-S, Gross D, Bates GW, Brooks SJ, Light ME, Gale PA. Angew Chem Int Ed. 2005;44:2537–2542. doi: 10.1002/anie.200462945. [DOI] [PubMed] [Google Scholar]
- 17.Sato W, Miyaji H, Sessler JL. Tetrahedron Lett. 2000;41:6731–6736. [Google Scholar]
- 18.In this solvent system, the pyrrolic NH proton signal, originally seen at δ = 6.74 in CDCl3, either shifts to lower field as the result of interaction with the CD3OD solvent or disappears as a consequence of D/H exchange (cf. Figures 2, 3, and S3).
- 19.CsTPB is not appreciably soluble in either chloroform or 10% methanol in chloroform.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic details, NMR spectroscopic data, ITC analyses, and X-ray structural data for 1·CsF. This material is available free of charge via the Internet at http://pubs.acs.org.