Abstract
Background
Longitudinal associations between patterns of crack cocaine use and progression of human immunodeficiency virus (HIV-1) disease are poorly understood, especially among women. This paper explores relationships between crack use and HIV-1 disease outcomes in a multi-center cohort of infected women.
Methods
Subjects were 1686 HIV-seropositive women enrolled at six U.S. research centers in the Women’s Interagency HIV Study. Approximately 80% were nonwhite and 29% used crack during the study period. Cox survival and random regression analysis examined bi-annual observations made April 1996 through September 2004. Outcome measures included: death due to AIDS-related causes; CD4 cell count; HIV-1 RNA level; and newly acquired AIDS-defining illnesses.
Results
Persistent crack users were over three times as likely as nonusers to die from AIDS-related causes, controlling for use of highly active antiretroviral viral therapies self-reported at >=95% adherence, problem drinking, age, race, income, education, illness duration, study site, and baseline virologic and immunological indicators. Persistent crack users and intermittent users in active phases showed greater CD4 cell loss and higher HIV-1 RNA levels controlling for the same covariates. Persistent and intermittent crack users were more likely than nonusers to develop new AIDS-defining illnesses controlling for identical confounds. These results persisted when controlling for heroin use, tobacco smoking, depressive symptoms, Hepatitis C virus co-infection, and intravenous drug use.
Conclusions
Use of crack cocaine independently predicts AIDS-related mortality, immunologic and virologic markers of HIV-1 disease progression, and development of AIDS-defining illnesses among women.
Recent research suggests that cocaine may directly affect the pathobiology of HIV by causing immune alterations in different lymphocytes such as helper T cells (CD4), suppressor/cytotoxic T cells (CD8), and natural killer (NK) cells.1 Studies show that cocaine interferes with the body’s ability to defend against infection by inhibiting the effector functions of neutrophils and macrophages, and by suppressing cytokine production, decreasing operation of important immune responses.2 Cocaine also enhances the replication of HIV in vitro.3 Cells from chronic cocaine users more readily support HIV replication and development of AIDS-defining opportunistic infections than cells from nonusers, suggesting a direct role for cocaine in the acquisition and progression of AIDS.2 Recently, cocaine has been shown to cause membrane permeability facilitating endothelial transmigration of infected dendritic cells across the blood brain barrier to the central nervous system.4 There is also evidence of cocaine-mediated alteration of immune responses and host resistance due to disturbances in the balance of Th1 pro-inflammatory versus Th2 anti-inflammatory cytokines and lipid bioeffectors.3
Epidemiologic research confirms that crack users are at high risk for HIV infection and progression.5-6 In a prospective study of HIV-seropositive drug users, crack use was significantly associated with progression to AIDS.7 A study of HIV-positive current and former drug users found that active cocaine use was the strongest predictor of failure to maintain viral suppression; 13% of active users maintained suppression vs. 46% of non-users.8 In a prospective cohort study, compared with nonusers and former users, active cocaine and heroin users experienced smaller median reductions in HIV-1 RNA and smaller median increases in CD4 from baseline, controlling for antiretroviral exposure, adherence, and socio-demographic factors.9 Compared to nonusers, the risk of AIDS-related opportunistic conditions was greater for persistent users and intermittent users during periods of active use, with no difference during periods of abstinence.10
Mixed results characterize studies of drug users in exclusively female U.S. cohorts. In a multi-center cohort of HIV-positive women, injection drug use was not associated with progression to AIDS.11 Another large multi-site cohort study found that hard drug use (i.e., cocaine, heroin, methadone, or injecting drugs) was significantly associated with AIDS-defining illnesses, but not with change in CD4, HIV-RNA, or mortality.12 In a third multi-site cohort of HIV+ women, non-injection drug use was associated with time to AIDS-defining event but not with AIDS-related mortality.13
While suggestive, these studies and others focusing on injection drug use do not uniformly demonstrate a link between illicit drug use and HIV-1 disease progression.14-17 Possible reasons include: diverse definitions of illness progression; failure to differentiate between active and nonactive users; and lack of distinction between mortality due to AIDS versus non-AIDS causes.17 Other reasons include lack of controls for highly active antiretroviral therapy (HAART) use and adherence, and inadequate follow-up periods. We addressed all of these issues by examining patterns of crack use and their association with four distinct measures of HIV/AIDS disease progression in a multi-center cohort over an eight-and-one-half-year period during the HAART era.
METHODS
Study Population
The Women’s Interagency HIV Study (WIHS) is a prospective cohort study of HIV disease progression among 2058 HIV-positive women at six consortium centers: Brooklyn, NY; the Bronx, NY; Chicago, IL; Los Angeles, CA; San Francisco/Bay Area, CA; and Washington, DC. Our analysis includes biannual observations from 4/1/96 (commercial availability of protease inhibitors) through 9/30/04.
We analyzed data from cohort members completing two or more study visits (not necessarily consecutive). All women provided institutional review board-approved written informed consent for research participation and use of their medical records. Analyses were adjusted for covariates identified in prior research as being associated with illicit drug use, HIV disease progression, and mortality both in the WIHS cohort13,18-19 and other cohorts.9-12, 14-16 These included age, race/ethnicity, education, income, baseline HIV-1 RNA and CD4, year of HIV+ diagnosis, and study site. We also controlled for problem drinking because of research reviewed by Cabral3 showing that cocaine in combination with alcohol places individuals at increased risk of infection with a number of pathogens, due to additive or synergistic effects resulting in impaired immune function.
Measurements
The first marker of disease progression was time-varying CD4 T lymphocyte levels of less than 200 cells/mm3. The second was time-varying HIV-1 RNA greater than 100,000 copies/ml. Lymphocyte subsets were determined using flow cytometry at laboratories participating in the AIDS Clinical Trials quality assurance program. Plasma HIV-RNA levels were measured using a nucleic acid sequence-based amplification technique (Organon Teknika, Durham, USA). Third, newly acquired AIDS-defining illnesses were identified through medical record review using a case file abstraction protocol for participants’ primary and specialty care records described elsewhere,20 along with respondent self-report. Conditions were defined according to the Centers for Disease Control and Prevention AIDS definition excluding the criterion of low CD4 cell count.21 Cause of death was obtained from death certificates and the National Death Index, local death registries, hospital records, physician reports, and information from friends/relatives. Deaths were classified as AIDS-related if the cause was an AIDS-defining illness, or if the stated cause was organ failure or nonspecific infection and the CD4 cell count was below 200 cells/mm,3 using procedures described elsewhere.22
At each study visit, women reported how often they took their regimens as prescribed during the past 6 months. Responses were classified as taking all drugs as prescribed >= 95% of the time vs. < 95%. This cutoff was based on past adherence research showing that HIV-1 RNA loads of <400 copies/ml occurred 80% of the time in patients with antiretroviral adherence of >= 95%.23 The construct validity of this measure is supported by WIHS research finding statistically significant relationships between adherence self-reports and subsequent virologic and immunologic parameters.24 For analysis, women were classified at each study visit as reporting HAART with >=95% adherence versus all others (i.e., non-adherent HAART use, other antiretroviral therapy use, and no therapy use).
Biannually, respondents reported the occurrence and frequency of alcohol and crack use in the past six months. Using National Institute on Alcohol Abuse and Alcoholism guidelines for women, at-risk drinking was defined as 8 or more drinks per week, and binge drinking as 4 or more drinks per day. Occurrence of either in the past six months was classified as problem drinking.
Following Lucas,10 four patterns of use were constructed and a value was assigned to the women’s reports for each visit, separately for crack and for alcohol use: 1) intermittent use with current abstinence (crack use or problem drinking reported previously with abstinence reported at the current visit), and 2) intermittent but currently active use (use reported at current visit but not all previous ones), 3) persistent use (use reported at every visit), and 4) nonuse (no reports of crack or problem drinking).
Statistical Analysis
Time to AIDS-related death and time to AIDS-defining illness were each examined using Kaplan–Meier survival analysis to test for differences in survival and hazard function according to patterns of crack use. Data from women with non-AIDS-related mortality were retained in the analysis until the date of death, when they were right-censored. Women lost to follow-up were censored at their last interview date. We used the Cox proportional hazards model to examine whether different patterns of crack use were associated with mortality and with AIDS-defining illnesses controlling for illness duration, baseline immunologic and virologic factors, use of HAART at greater than or equal to 95% adherence, socio-demographic characteristics, and study site. We used random effects logistic regression analysis (MIXOR)25 to examine the effects of different crack use patterns on CD4 cell count and HIV-1 RNA level controlling for the same covariates. Random effects analysis modeled intrasubject associations as a Gaussian process representing an individual’s propensity to develop an outcome indicating virologic, immunologic or clinical disease progression. Two random effects, for intercept and slope, fit the data better than one random effect.
RESULTS
Data from 1686 women were analyzed: 1203 (71.4%) were categorized as nonusers, 429 (25.4%) as intermittent users, and 54 (3.2%) as persistent users of crack. Their characteristics are presented in Table 1.
Table 1.
Characteristics of 1686 HIV+ women in a multi-site cohort according to longitudinal patterns of crack use, 1996-20041
| Variable | Nonusers n = 1,203 |
Intermittent users n = 429 |
Persistent users n = 54 |
Chi Square/ANOVA Significance2 | Linear Trend Significance |
|---|---|---|---|---|---|
| African American | 600 (50%) | 305 (71%) | 36 (67%) | *** | *** |
| Hispanic/Latina | 331 (28%) | 60 (14%) | 12 (22%) | *** | *** |
| Caucasian/other | 272 (23%) | 64 (15%) | 6 (11%) | ** | *** |
| Less than high school education at baseline | 414 (34%) | 179 (42%) | 33 (61%) | *** | *** |
| Baseline income <= $12,000/year | 651 (55%) | 334 (78%) | 46 (85%) | *** | *** |
| Age in years at baseline | 36.5 (8.3) | 37.4 (6.6) | 38.0 (7.9) | n.s. | -- |
| Baseline CD4 count (cells/mm3) | 364 (264) | 433 (305) | 257 (194) | *** | -- |
| Baseline CD4<200 cells/mm3 | 338 (29%) | 91 (22%) | 20 (40%) | ** | ** |
| Baseline CD4 Percentiles | |||||
| 25th | 175 | 227 | 70 | ||
| 50th | 328 | 374 | 274 | ||
| 75th | 508 | 564 | 366 | ||
| Baseline HIV-1 RNA level (copies/ml) | 89424 (344409) | 54815 (157806) | 205160 (373550) | ** | -- |
| Baseline HIV-1 RNA>100,000 (copies/ml) | 164 (15%) | 52 (13%) | 18 (37%) | *** | ns |
| Baseline HIV-1 RNA Percentiles | |||||
| 25th | 4,000 | 4,000 | 4,250 | -- | |
| 50th | 8,100 | 8,950 | 38,000 | ||
| 75th | 53,250 | 44,000 | 265,000 | ||
| Baseline log10HIV-1 RNA | 4.05 (0.93) | 4.01 (.090) | 4.53 (1.04) | ** | ** |
| Baseline log10HIV-1 RNA Percentiles | |||||
| 25th | 3.60 | 3.60 | 3.63 | -- | |
| 50th | 3.91 | 3.95 | 4.58 | ||
| 75th | 4.73 | 4.64 | 5.42 | ||
| Year of HIV+ diagnosis | 1991 (2.5) | 1991 (2.8) | 1991 (2.5) | ns | *** |
| Ever reported HAART | 903 (75%) | 307 (72%) | 17 (32%) | *** | *** |
| HAART adherence >=95% at all reports | 346 (29%) | 69 (16%) | 4 (7%) | *** | *** |
| Baseline problem alcohol use3 | 123 (10%) | 114 (27%) | 17 (32%) | *** | *** |
| Number study visits completed | 12 (6) | 13 (5) | 6 (6) | *** | -- |
| Date of first study visit (mo/year) | 8/96 | 9/96 | 9/96 | ** | -- |
| Follow-up time, (months) | 86 (20) | 85 (18) | 66 (33) | *** | -- |
| Deceased during study (all cause mortality) | 278 (23%) | 104 (24%) | 37 (68%) | *** | *** |
p <.01,
p <.001,
n.s.= not statistically significant
Values are expressed as frequency (%) for discrete variables and as mean (standard deviation) for continuous variables.
For discrete variables, significance refers to chi-square and linear by linear associations, for continuous variables, to analysis of variance.
Problem alcohol use is defined as >7 drinks/week and/or >=4 drinks per day
Source: Women’s Interagency HIV Study: 1994-2004
There were 419 deaths during the follow-up period: 197 (47.0%) were AIDS-related, 138 (33.0%) were non-AIDS related, and 84 (20.0%) were indeterminate. Time to death was assessed with a Kaplan-Meier function (Figure 1). The estimated survival rates at 8.2 years (3000 days) were 89% for nonusers, 90% for intermittent users, and 65% for persistent users (log-rank test=6.6, p<.05). In a Cox proportional hazards model (Table 2) adjusting for age, race, income, education, problem drinking, adherent HAART use, CD4 count < 200 cells/mm3 at baseline, HIV-1 RNA level > 100,000 copies/ml at baseline, illness duration, and study site, compared with that for nonusers, the risk of AIDS-related death was significantly higher for persistent users (hazard ratio=3.61, p<.001), but not for intermittent users.
Figure 1.
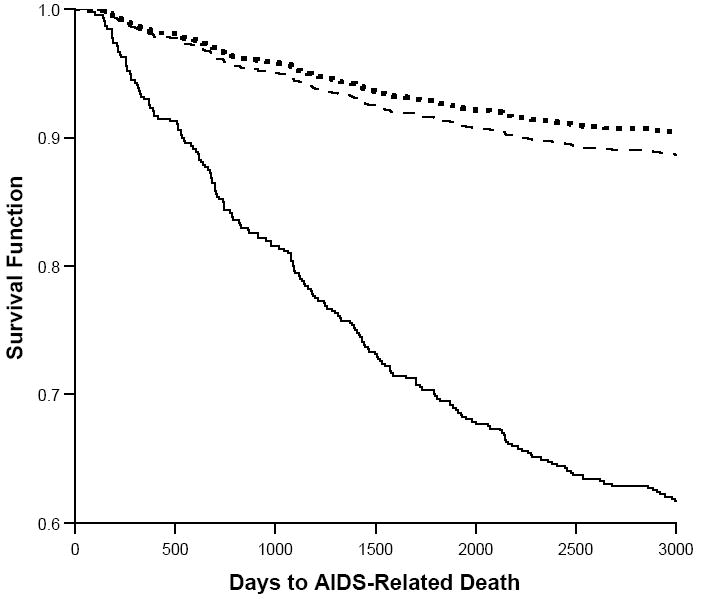
Survival by patterns of crack use in a cohort of HIV-1 infected women. Compared with nonusers (dashed line), and intermittent users (dotted line), days to death for persistent crack users (solid line) was significantly shorter and survival rates significantly lower (p<.05).
Table 2.
Cox proportional hazards models of effects of patterns of crack use on AIDS-related mortality and AIDS-defining illnesses, N=1686: Models control for study site.
| Variable | Dependent variable: AIDS-related mortality (200/1686 = 11.9%) Hazard Ratio | Dependent variable: Newly acquired AIDS-defining illness1 (543/1686 = 32.2%) Hazard Ratio |
|---|---|---|
| Crack Use | *** | *** |
| Intermittent | 0.93 | 1.57*** |
| Persistent | 3.61*** | 1.65* |
| Problem Drinking2 | ** | |
| Intermittent | 0.54** | 1.05 |
| Persistent | 0.38 | 0.69 |
| HAART & >=95% adherent | 0.52** | 1.13 |
| CD4 lymphocyte count <200 cells/mm3at baseline | 5.70*** | 1.05 |
| HIV-1 RNA >100,000 copies/ml at baseline | 2.46*** | 0.94 |
| Year of HIV+ diagnosis | 0.99 | 1.05** |
| African American | 1.44 | 0.90 |
| Latina | 1.16 | 1.01 |
| Low income (<$12K/yr) | 1.27 | 1.06 |
| Less than high school education | 1.16 | 1.10 |
| Age (10 year increments) | 1.04 | 1.01 |
p<.05,
p<.01,
p<.001
Defined in accordance with the Centers for Disease Control and Prevention 1993 clinical surveillance conditions, excluding the criterion of low CD4 cell count (CDC, 1993).
Problem drinking defined as = >7 drinks per week and/or binge drinking >=4 drinks per day.
Source: Women’s Interagency HIV Study: 1994-2004
Of the total group of 1686 women, 543 (32.2%) were found to have a newly-acquired AIDS-defining illness during the follow-up period. Significantly higher proportions of intermittent users (42.0%, n=180) and persistent users (38.9%, n=21) reported a new illness during this time period than did nonusers (28.4%, n=342) (chi square=27.6, p<.001). The most frequently reported AIDS-defining illnesses were bacterial pneumonia (n=98, 18% of all cases), pneumocystis carinii pneumonia (n=52, 10%), herpes simplex virus-non-pulmonary (n=49, 9%), esophogeal candidiasis (n=48, 9%), cryptosporidiasis (n=30, 6%), dementia/encephalopathy (n=27, 5%), wasting syndrome (n=27, 5%), and tuberculosis (n=20, 4%). Among these, persistent and/or intermittent users were significantly more likely than nonusers to report bacterial pneumonia (chi square=18.8, p<.001), tuberculosis (chi square=16.6, p<.01), and esophogeal candidiasis (chi square=6.4, p<.05). Time to new AIDS-defining illness was assessed in the three groups with a Kaplan-Meier function (Figure 2). The average days to illness or censoring was 2592 days for nonusers, 2305 days for intermittent users, and 2211 days for persistent users (log-rank test=27.5, p<.001). In a Cox proportional hazards model (Table 2) the risk of AIDS-defining illness was significantly higher for intermittent crack users (hazard ratio=1.57, p<.001) and consistent users (hazard ratio=1.65, p<.05) than for nonusers, adjusting for all covariates.
Figure 2.
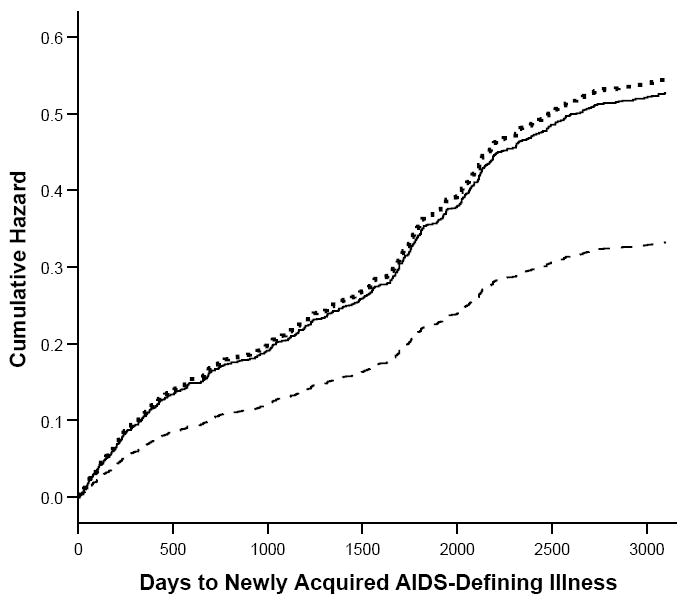
Time to newly acquired AIDS-defining illness by patterns of crack use in a cohort of HIV-1 infected women. Compared with nonusers (dashed line), days to illness for intermittent users (dotted line) and persistent crack users (solid line) were significantly shorter and hazard rates significantly higher (p<.001).
Figures 3 and 4 present the unadjusted proportions over time by pattern of crack cocaine use of women with CD4 count<200 cells/mm3, and HIV-1 RNA>100,000 copies/ml. Throughout most of the study period, those reporting persistent crack use had higher viral load concentrations and poorer immune function, while those reporting no use had the lowest HIV-1 RNA levels and best immune health, with intermittent crack users falling in between.
Figure 3.
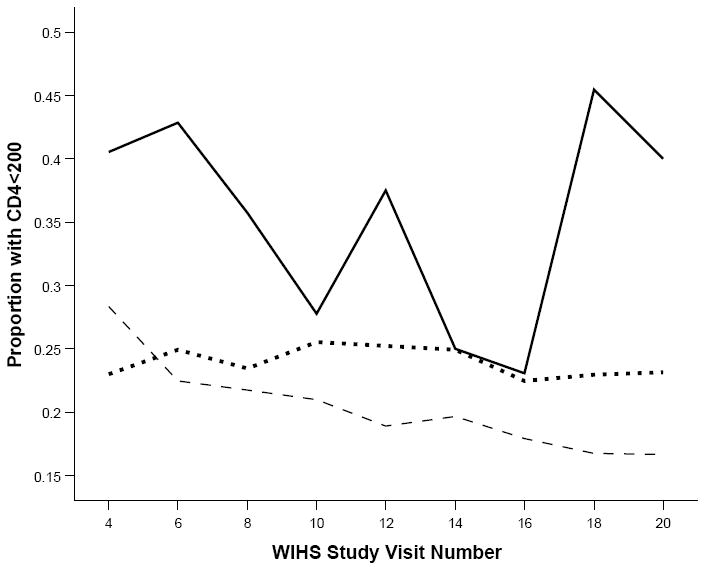
Unadjusted proportions of women with CD4 lymphocyte count <200 copies/mm3 over 18 semi-annual study visits. Nonusers (dashed line) had generally lower proportions, while persistent users (solid line) typically had the highest proportions, with intermittent users (dotted line) falling in between.
Figure 4.
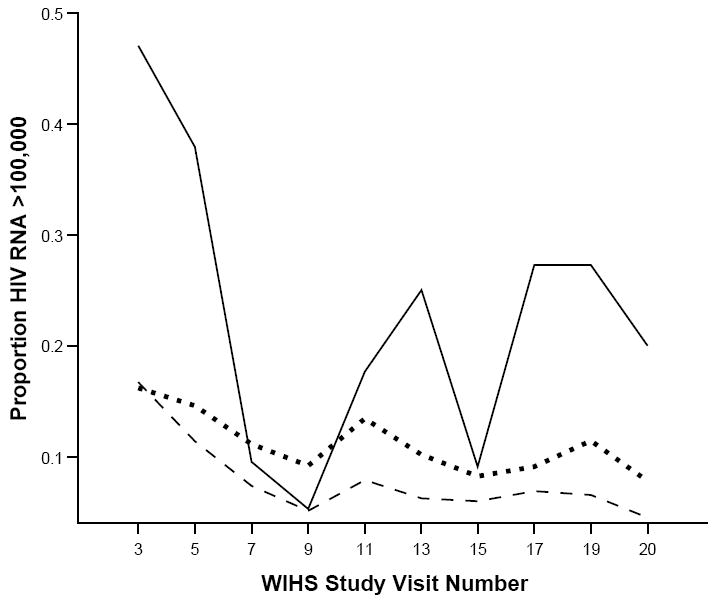
Unadjusted proportions of women with HIV-1 RNA viral load >100,000 copies over 18 semi-annual study visits. Nonusers (dashed line) had consistently lower proportions, while persistent users (solid line) generally had the highest proportions, with intermittent users (dotted line) falling in between.
Table 3 presents the results of a time-varying random regression analysis of the effects of persistent and intermittent crack cocaine use on CD4 < 200 and HIV-1 RNA > 100,000. Across both models, persistent crack use, intermittent-active, and intermittent-abstinent crack use were significantly associated with HIV disease progression, controlling for adherent HAART use, problem drinking, women’s socio-demographic characteristics, study site, illness duration, baseline viral load (in the CD4 model), and baseline CD4 (in the viral load model). Persistent problem drinking was positively associated with disease progression defined by high viral load but not low CD4. In both models, adherent HAART use was protective against disease progression.
Table 3.
Random regression analysis of effects of time-varying patterns of crack use on markers of HIV disease progression, N=1686: Models control for study site.
| Variable | Dependent Variable: Time Varying CD4<200 cells/mm3 Estimate1 | Dependent Variable: Time Varying HIV-1 RNA > 100,000 copies/ml Estimate1 |
|---|---|---|
| Intercept | 1.87 | -1.00 |
| Time (study visit number) | 0.05*** | 0.03*** |
| Crack use | ||
| Crack use intermittent – abstinent | 0.67*** | 0.45** |
| Crack use intermittent – active | 0.98*** | 0.58*** |
| Crack use - persistent | 0.82** | 2.24*** |
| Problem drinking2 | ||
| Problem drinking intermittent - abstinent | -0.18 | -0.22 |
| Problem drinking intermittent – active | 0.08 | -0.01 |
| Problem drinking – persistent | -1.08 | 1.91* |
| CD4 lymphocyte count <200 cells/mm3at baseline | -- | 2.21*** |
| HIV-1 RNA >100,000 copies/ml at baseline | 2.60*** | -- |
| Year of HIV+ diagnosis | -0.09*** | -0.04 |
| HAART >=95% adherence | -1.10*** | -2.13*** |
| Caucasian | -- | -- |
| African American | 0.24 | 0.16 |
| Latina | 0.69*** | -0.16 |
| Low income (<$12K/yr) | 0.20** | 0.26** |
| Less than high school education | -0.10 | 0.22 |
| Age (10 year increments) | 0.42*** | -0.05 |
p<.05,
p<.01,
p<.001
Effect shown as unstandardized parameter estimate where negative sign indicates that outcome was less likely and positive sign indicates that outcome was more likely.
Problem drinking defined as >= 7 drinks per week and/or binge drinking >= 4 drinks per day.
Source: Women’s Interagency HIV Study: 1994-2004
We tested five additional covariates that could account for the relationship between crack use and disease progression, with the same models used in the Cox proportional hazards and random regression analyses. Results (not shown) remained highly similar controlling, separately, for heroin use, intravenous drug use, tobacco smoking, Hepatitis C virus co-infection, and depressive symptoms (using the Center for Epidemiologic Studies-Depression Scale clinical cutoff of 16).26 The only exceptions were for intermittent-abstinent crack use, which became non-significant in the viral load models when controlling for smoking and for depression.
Finally, to explore the impact of crack use on immune reconstitution, we conducted a supplementary analysis of associations between patterns of use (nonuse, inactive use, and active use) and immunologic response. Following Lucas and colleagues,9 for all women remaining in the cohort at the end of the study period (n=1,053), we defined change in HIV-1 RNA (log10 copies/ml) as the difference between the most recent viral load and peak HIV-1 RNA level, and change in CD4 as the difference between the most recent and nadir CD4 lymphocyte counts. We found that the median reduction in HIV-1 RNA level was highest in nonusers, at 1.7 log10 copies/ml, compared to 1.4 log10 copies/ml in inactive crack users and 1.0 log10 copies/ml in active users (F=4.94, df=2/1035, p<.01). The median CD4 increase was highest in nonusers, at 161 cells/mm3, compared with 123 cells/mm3 in inactive users, and 100 cells/mm3 in active users (F=6.99, df=2/1035, p<.01). In multivariate linear regression models (not shown), active and inactive crack use remained significant after adjustment for race/ethnicity, use of HAART, HAART adherence 95% of time, prior HAART exposure, nadir CD4 count, and peak HIV-1 RNA level. Here, compared with nonusers, active and inactive crack users had smaller median reductions in HIV-1 RNA from baseline, and smaller median increases in CD4. These results confirm that inferior virologic and immunologic responses are associated with both active and inactive use of crack.
DISCUSSION
Ours is the first study to show that use of crack cocaine in a large, national cohort of HIV-positive women is longitudinally associated with subsequent deterioration in immune status, failure of virologic suppression, development of AIDS-defining conditions, and mortality due to AIDS-related causes, even among those who reported adhering to HAART regimens 95% of the time or more. Likely confounds such as heroin use, intravenous drug use, tobacco smoking, Hepatitis C virus co-infection, and depression do not appear to account for these significant associations, nor do socio-demographic factors, illness duration, or baseline immunologic or virologic indicators. Unlike prior research on a predominantly male sample,10 we did not consistently find that progression was less likely during periods of abstinence among women crack users, providing support for the notion that effects of cocaine on the immune system may vary by gender, as others have suggested.3
Even in the face of this evidence, our analysis does not conclusively demonstrate that crack use causes AIDS-related morbidity and mortality. We have not ruled out other processes that could account for these associations, such as greater sexual risk taking, poorer diet and nutrition, substandard living conditions, and other unknown confounds.
Our findings suggest that a multi-pronged research agenda is needed to understand the effects of crack cocaine on HIV disease progression. In vivo studies can illuminate the specific role of the drug in HIV pathogenesis.2 In vitro research, such as the human lymphocyte/SCID (huPBL/SCID) mouse model,1 can shed light on how cocaine upregulates HIV and also acts as a co-factor in HIV pathogenesis.4 In vitro studies of peripheral blood samples from crack users can examine alterations in T cell and dendritic cell subsets, immune function, cytokine and chemokine expression, indicating predisposition to HIV infection. Studies of alveolar macrophages from the lungs of chronic crack users can help to understand impaired cytokine production2 and how intrapulmonary accumulation of contaminants may promote chronic lung diseases.17 However, neither in vivo nor in vitro research can control for the complex interactions that occur in human beings with repeated exposure to crack over time, necessitating rigorous, large-scale epidemiologic studies of morbidity and mortality among HIV+ and at-risk users, and potential differences associated with frequency, quantity, and mode of administration.17
While prior research highlights difficulties crack users confront in using the medical care system,27 this was not true in our cohort. At their last interview, 100% of participants reported seeing a health care provider in the past 6 months: 93% said they saw the same health care provider consistently, including 94% of persistent crack users. Related to this are the findings of a recent study of HIV-positive African Americans in which women crack users reported more positive relationships with their physicians than did male crack users.28 Use of HAART in the WIHS cohort is significantly related to higher satisfaction with both medical care and with health care providers.29 This is a foundation on which to build care delivery models that are effective in engaging and retaining women crack users. One recommendation is that women using crack receive sustained follow-up with periodic reevaluations of therapy regimens to promote greater use of and adherence to HAART.30 Another suggestion is co-location of rapid HIV testing, risk reduction counseling, HIV therapies, psychiatric and substance abuse treatment, and other services to promote successful engagement and seamless access to multiple interventions.30-31 Finally, the importance of cultural competence is paramount, calling for diversity in clinical staff, attention to patient-provider communication, and sensitivity to the multiple vulnerabilities faced by these women.32-33
One caveat to our findings is the non-representativeness of our longitudinal cohort, limiting the generalizability of our results. Another study limitation is use of self-report rather than urine or toxicology screening to measure crack cocaine exposure. The same is true for self-reported measures of alcohol use, HAART use, and adherence, which may be subject to distortion due to recall errors or positive response bias. Study intervals were lengthy, number of time-points varied by participant, and respondents entered the study at different stages of illness. The use of death certificates and other administrative records to establish AIDS-related deaths, and reliance on case record review to identify AIDS-defining illnesses also introduced measurement error into our dependent variables. Finally, without direct measures of pathobiology, the effect of crack on disease progression can only be assessed using proxy variables.
The challenges involved in treating crack addiction are well documented, and include high rates of treatment drop-outs, treatment repeaters, and relapse.34 This suggests the need for models that acknowledge crack users’ diverse levels of readiness for change and recognize that work can be done, even with women who are not yet ready to alter their behavior or are just beginning to consider doing so. Helping individuals move from pre-contemplation to readiness for change requires approaches that are assertive and “strengths-based,”35 building on low-income, minority women’s intrinsic resources such as resilience and street smarts.27,35 Finally, culturally competent care is needed to promote the trust necessary for personal risk taking that accompanies willingness to change through addiction treatment and commitment to antiretroviral regimens.33,36
Acknowledgments
This research was supported by supplemental funding provided by the National Institute on Drug Abuse. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). Funding agencies did not participate in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Judith A. Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. There are no conflicts of interest for any of the paper’s coauthors. J.A. Cook originated the study, supervised the data analyses and interpretation, and led the writing, J.K. Burke-Miller and D.D. Grey conducted and interpreted the data analyses. All of the authors helped to conceptualize ideas, interpreted findings, and reviewed drafts of the article.
Contributor Information
Judith A. Cook, University of Illinois at Chicago, Department of Psychiatry
Jane K. Burke-Miller, University of Illinois at Chicago, Department of Psychiatry
Mardge H. Cohen, Core Center and Stroger (formerly Cook County) Hospital
Robert L. Cook, University of Florida, College of Public Health and Health Professions
David Vlahov, New York Academy of Medicine
Tracey E. Wilson, State University of New York Downstate Medical Center, Department of Preventive Medicine and Community Health
Elizabeth T. Golub, Johns Hopkins School of Public Health, Department of Epidemiology
Rebecca M. Schwartz, State University of New York Downstate Medical Center, Department of Preventive Medicine and Community Health
Andrea A. Howard, Montefiore Medical Center, Albert Einstein College of Medicine
Claudia Ponath, University of California at San Francisco, Department of Medicine
Michael W. Plankey, Georgetown University Medical Center, Department of Medicine
Dennis D. Grey, University of Illinois at Chicago, Department of Psychiatry
References
- 1.Xu W, Flick T, Mitchel J, Knowles C, Ault K. Cocaine effects on immunocompetent cells: an observation of in vitro cocaine exposure. Int J Immunopharmacol. 1999;21:463–472. doi: 10.1016/s0192-0561(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–138. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 3.Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1:280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 4.Nair MP, Schwartz SA, Mahajan SD, et al. Drug abuse and neuropathogenesis of HIV infection: role of DC-SIGN and IDO. J Neuroimmunol. 2004;157:56–60. doi: 10.1016/j.jneuroim.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. Am J Public Health. 1998;88:108–113. doi: 10.2105/ajph.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vittinghoff E, Hessol NA, Bacchetti P, Fusaro RE, Holmberg SD, Buchbinder SP. Cofactors for HIV disease progression in a cohort of homosexual and bisexual men. J Acquir Immune Defic Syndr. 2001;27:308–314. doi: 10.1097/00126334-200107010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten JH, Dernas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 11.Rompalo AM, Shah N, Margolick JB, Farzadegan H, Arnsten J, Schuman P, Rich JD, Gardner LI, Smith DK, Vlahov D. Evaluation of possible effects of continued drug use on HIV progression among women. Int J STD AIDS. 2004;15:322–327. doi: 10.1177/095646240401500510. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe LE, Frederick M, Pitt J, et al. Effect of hard-drug use of CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37:1423–1430. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- 13.Kapadia F, Cook JA, Cohen MH. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use,”. Addiction. 2005;100:990–1002. doi: 10.1111/j.1360-0443.2005.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galai N, Vlahov D, Bareta JC, Wang C, Cohn S, Sterling TR. Prognostic factors for survival differ according to CD4+ cell count among HIV-infected injection drug users. J Acquir Immune Defic Syndr. 2005;38:74–81. doi: 10.1097/00126334-200501010-00014. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Madge S, Johnson AM, et al. A comparison of exposure groups in the EuroSIDA study: starting highly active antiretroviral therapy (HAART), response to HAART, and survival. J Acquir Immune Defic Syndr. 1999;22:369–378. doi: 10.1097/00126334-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Pezzotti P, Galai N, Vlahov D, Reza G, Lyles CM, Astemborski J. Direct comparison of time to AIDS and infectious disease between HIV seroconverter injection drug users in Italy and the Unites States: results from the ALIVE and ISS studies. J Acquir Immune Defic Syndr. 1999;20(1):275–282. doi: 10.1097/00042560-199903010-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41:1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- 18.Anastos K, Kalish LA, Hessol N, et al. The relative value of CD4 cell count and quantitative HIV-1 RNA in predicting survival in HIV-1-infected women: results of the Women’s Interagency HIV Study. AIDS. 1999;13:1717–1726. doi: 10.1097/00002030-199909100-00016. [DOI] [PubMed] [Google Scholar]
- 19.Cook JA, Grey DD, Burke JK, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study: WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 21.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 22.Cohen MH, French AL, Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson D, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 24.Wilson T, Barron Y, Cohen MH, et al. Adherence to antiretroviral therapy and associations with sexual behavior. Clin Infect Dis. 2002;34:529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]
- 25.Hedeker D, Gibbons RD. MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed. 1996;49:157–176. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1979;1:385–401. [Google Scholar]
- 27.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 28.Crisp BR, Williams M, Timpson S, Ross MW. Medication compliance and satisfaction with treatment for HIV disease in a sample of African-American crack cocaine smokers. AIDS Behav. 2004;8:199–206. doi: 10.1023/B:AIBE.0000030250.33931.af. [DOI] [PubMed] [Google Scholar]
- 29.Burke-Miller JK, Cook JA, Cohen MH, et al. Longitudinal relationships between use of highly active antiretroviral therapy and satisfaction with care among women living with HIV/AIDS. Am J Public Health. 2006;96:1044–1051. doi: 10.2105/AJPH.2005.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004;29:117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 31.Cook JA, Grey DD, Burke-Miller JK, Cohen MH, Vlahov D, Kapadia F, Wilson TE, Cook R, Schwartz RM, Golub ET, Anastos K, Ponath C, Goparaju L, Levine AM. Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug Alcohol Depend. 2007;89:74–81. doi: 10.1016/j.drugalcdep.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone VE. Optimizing the care of minority patients with HIV/AIDS. Clin Infect Dis. 2004;38:400–404. doi: 10.1086/380969. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SD, Cunningham-Williams RM, Cottler LB. A tripartite of HIV-risk for African American women: the intersection of drug use, violence, and depression. Drug Alcohol Depend. 2003;70:169–175. doi: 10.1016/s0376-8716(02)00345-9. [DOI] [PubMed] [Google Scholar]
- 34.Reiber C, Ramirez A, Parent D, Rawson RA. Predicting treatment success at multiple timepoints in diverse patient populations of cocaine-dependent individuals. Drug Alcohol Depend. 2002;68:35–48. doi: 10.1016/s0376-8716(02)00103-5. [DOI] [PubMed] [Google Scholar]
- 35.Fisk D, Rakfeldt J, McCormack E. Assertive outreach: an effective strategy for engaging homeless persons with substance use disorders into treatment. Am J Drug Alcohol Abuse. 2006;32:479–486. doi: 10.1080/00952990600754006. [DOI] [PubMed] [Google Scholar]
- 36.Gielen AC, McDonnell KA, Wu AW, O’Campo P, Faden R. Quality of life among women living with HIV: the importance of violence, social support, and self care behaviors. Soc Sci Med. 2001;52:315–322. doi: 10.1016/s0277-9536(00)00135-0. [DOI] [PubMed] [Google Scholar]


