Abstract

We report the synthesis of silver nanoparticles with grain sizes down to electron Fermi wavelength. These nanoparticles exhibit bright luminescence and large Raman enhancement effect. The number of photons emitted from these nanoparticles exceeded that from quantum dots or dye molecules by approximately 2 or 5 orders of magnitude, respectively.
Noble metal nanostructures on different length scales exhibit a variety of optical properties ranging from plasmon resonance1 and Raman enhancement2 to fluoescence3, providing novel opportunities for bioimaging and sensing4. In a regime where the particle size is on the same order of the electron Fermi wavelength (~0.5nm for silver and gold), metal nanoparticles (NPs) often display strong single-electron excitations and emit fluorescence3. However, as particle size approaches electron mean free path length (~50nm for silver and gold), fluorescence usually disappears and instead, collective excitations of electrons become dominant, leading to plasmon resonance. Large Raman enhancements have also been observed from nanoclusters5 and NPs6 of noble metals. It has been shown recently that electron–phonon interactions within metal nanoparticle can be modified by the crystallinity7. Thus, control of the particle size and structure is a powerful strategy to modulate electronic structures and optical properties of metal nanostructures. Here, we reported the observation that polycrystalline silver NPs with grain sizes down to electron Fermi wavelength exhibit both bright luminescence and a large enhancement effect on the Raman scattering signals of proximal molecules (an effect being referred to as “Raman activity” hereafter). The number of photons emitted from these NPs exceeded that from quantum dots or dye molecules by approximately 2 or 5 orders of magnitude, respectively.
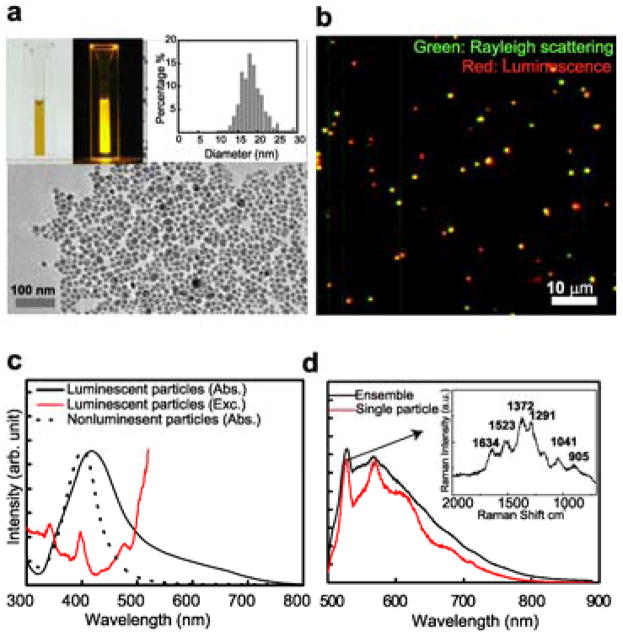
We synthesized luminescent and Raman active metal NPs by thermal reduction of silver ions in glycine matrix, taking advantage of the solid-state matrix to control the nucleation and migration of reduced silver atoms (see supporting information (SI) for synthesis protocol). The glycine-coated NPs remained stable in aqueous solution for more than two years at ambient conditions (Fig. 1a). Transmission electron microscopy (TEM) revealed particle diameters ranging from 2 to 30 nm. Different fractions of mono-dispersed NPs can be separated using centrifugation (see SI). TEM images of two such fractions, with particle diameters of 18 ± 3 nm and 3 ± 1 nm, are shown in Fig. 1a and Fig. S1a – c.
Figure 1.
Solid-phase synthesis creates silver NPs with bright and robust luminescence. a, Upper left panel: An aqueous solution of the 18 nm glycine-coated silver NPs. The photos were taken without (left) and with (right) 532 nm laser excitation; in the latter case, a 545nm long pass filter was used to block the scattered laser light. Lower panel: TEM image of the NPs. Upper right panel: Size distribution of the NPs determined from TEM. b, An overlay Rayleigh scattering (Green) and luminescent (Red) image of the 18 nm NPs. NPs with yellow or orange color exhibit both luminescent and Rayleigh scattering. c, Absorption (solid black) and excitation (red) spectra of the 18 nm luminescent silver NPs. As a comparison, the black dashed line shows the absorption spectrum of the 20 nm nonluminescent silver NPs produced by solution phase synthesis. d, Ensemble (black) and single-particle (red) emission spectra of luminescent silver NPs. Inset: using a high-density grating, the Raman peak of the single-particle emission spectrum was further resolved into multiple lines.
Luminescence from these NPs can be readily detected at the single-particle level. Comparison of the Rayleigh scattering (dark field) and luminescence images showed that more than 95% percent of the NPs were luminescent (Fig. 1b). This was in contrast to colloidal silver NPs prepared by typical solution phase methods, of which only ~2% of the particles emit luminescence (Fig. S2). TEM characterization indicated that the great majority of the NPs appear as individual particles rather than aggregates (Fig. S3). These NPs created by solid-phase synthesis are remarkably bright and photostable: under the same excitation conditions; the half lifetime of the 18 nm silver NPs was 660 s, while the half lifetimes of the Rhodamine 6G (R6G) molecules and the commercially available quantum dots (CdSe/ZnS core-shell, emission maximum at 605 nm) were only 0.4 s and 37 s, respectively (Fig. S4). The average luminescence intensity from individual silver NPs was 40 and 4 times larger than those from single R6G molecules and quantum dots, respectively. Thus the total number of photons emitted from individual silver NPs is nearly 70,000 times larger than that from R6G molecules and 70 times larger than that from quantum dots. Using the previously determined number of photons emitted from R6G (~106)8, we estimated that a single silver NP emitted on average 7 × 1010 photons before photobleaching. The average number of photons emitted from the 3 nm silver NPs was estimated to be 4 × 109 using a similar approach (Fig. S1d).
To further explore the optical properties of these NPs, we measured the absorption, excitation and emission spectra of the 18 nm NPs, which can be readily produced in high concentration to allow ensemble spectral characterization. The absorption spectrum displayed a peak near the plasmon resonance of nonluminescent silver NPs, but with a significantly broader width (Fig. 1c, black line), suggesting the possible presence of additional optical transitions besides plasmons in the luminescent particles 1. The excitation spectrum adopted an almost opposite trend, exhibiting a minimum at the absorption peak (Fig. 1c, red line), Multiple narrow peaks were also found to superimpose on the broad trend of the excitation spectrum. These observations suggest that plasmons do not make major contributions to the luminescence, rather the luminescence likely arises from single-electron excitations between discrete energy states1. The luminescence spectra of individual NPs exhibited a narrow peak superimposed on a broad band (Fig. 1d). The frequency difference between the sharp peak and the excitation wavelength is independent on the excitation wavelength, indicative of its Raman scattering nature (Fig. S5). The Raman peak can be further resolved into multiple lines (Fig. 1d inset), some of which are close to the previous reported surface enhanced Raman spectra (SERS) of glycine9 (Fig. S6). Therefore, the enhancement effect due to these silver NPs allows the Raman scattering signal of glycine coat molecules on individual NPs to be readily detected. To obtain a rough estimate of the Raman enhancement factor, we assumed that every silver atom on the 18 nm particle was bound to a glycine molecule and obtained a lower-limit estimate of 1010 for the enhancement factor (See SI for details). The Raman peak positions and intensities of individual particle fluctuated over time and heterogeneity among particles was also observed (Fig. S6). While the narrow-lines of the Raman signal was encoded with chemical information of glycine, the broad luminescence band from the silver NPs did not appear to require a specific glycine coating. With the same synthetic strategy, we also created acetate coated silver NPs, which also exhibited a broad luminescence band but different Raman signature (Fig. S7).
To investigate potential material structures responsible for the observed optical properties, we used high-resolution TEM to probe structural differences between the luminescent silver NPs created by solid-phase synthesis and the nonluminescent silver particles of similar sizes (~20 nm) produced by solution phase synthesis. The nonluminescent NPs typically showed single-crystalline or twined structures with domain sizes averaging around 8 nm (Figs. 2a, c). In contrast, the luminescent NPs displayed a polycrystalline structure with numerous small domains. The domain sizes were primarily in the 1 – 2 nm range (Figs. 2b, c). As an alternative approach to probe the domain size, we measured the binding energy (BE) of silver 3d5/2 electrons for both nonluminescent and luminescent NPs using X-ray photoelectron spectroscopy (Fig. 2d). The BE spectrum of the nonluminescent NPs showed predominantly a single peak at 368.3 eV, agreeing quantitatively with the bulk value for silver (368.1 eV)10a. In contrast, the spectrum of the luminescent NPs contained an additional peak at 369.9 eV, which is 1.8 eV blue-shifted from the bulk silver value. According to the previously determined correlation between the electron BE of silver and the particle size10b, the blue shift corresponds to an average domain size of 0.9 nm. We note that the BE of the 3d5/2 electrons of silver oxides are in the range of 367.3 to 368.4 eV10c, and cannot explain the blue-shifted 369.9 eV peak. The small domains present in the NPs likely result in discrete energy states that give rise to optical transitions and different sized sub-nm domains may result in different emission wavelengths (Fig. 1d), consistent with previous observations of size-dependent luminescence from small silver clusters3. Previous calculations indicate that sub-nm silver clusters and their junctions can give rise to a very large Raman enhancement due to a combination of electromagnetic field enhancement and chemical enhancement effect11. Thus strong Raman enhancement may also arise from the observed granular structure which contains many small nanocrystalline domains as well as domain junctions.
Figure 2.
Structural characterizations of silver NPs by high-resolution TEM. a and b, TEM images of a nonluminescent NP created by solution-phase synthesis (a) and a luminescent NP created by solid-phase synthesis (b). c, Domain size distributions obtained from luminescent (red) and nonluminescent (green) NPs. In each case, 15 NPs were characterized. Domain sizes were measured as the average of the long and short axes of the individual domains. d, X-ray photoelectron spectra (XPS) of luminescent and nonluminescent silver NPs. The spectrum of the nonluminescent NPs (green) was fit to a Gaussian centered at 368.3 eV (grey dashed line). The spectrum of the luminescent NPs (red) was fit with two Gaussians centered at 369.9 eV and 368.3 eV, respectively (black dashed lines).
In summary, we have created a new class of multi-domain silver nanostructures with individual domain sizes down to 1 nm or less using a simple solid-phase thermal reduction method. These NPs exhibit exceedingly bright luminescence and strongly enhanced Raman scattering. These optical properties potentially allow the NPs to function as new probes for bioimaging.
Supplementary Material
Detailed information for synthesis and characterizations of luminescent and Raman active silver NPs and supplementary figures. This material is available for free of charge via internet at http://pubs.acs.org/
Acknowledgments
This work was supported in part by Harvard NSEC center from NSF (to X.Z.) and Emory-Georgia Tech Center for Cancer Nanotechnology of Excellence from NIH to (Z.L.W.). X. Z. is a Howard Hughes Medical Institute investigator. Authors sincerely thank Professor Charles M. Lieber at Harvard University for insightful discussions and suggestions.
REFERNCES
- 1.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Vol. 25 Springer; 1995. [Google Scholar]
- 2.(a) Haynes CL, McFarland AD, Van Duyne RP. Anal Chem. 2005;77:338A–346A. [Google Scholar]; (b) Moskovits MJ. Raman Spectr. 2005;36:485–496. [Google Scholar]
- 3.(a) Fedrigo S, Harbich W, Buttet J. J Chem Phys. 1993;99:5712–5717. [Google Scholar]; (b) Peyser LA, Vinson AE, Bartko AP, Dickson R. M Science. 2001;291:103–106. doi: 10.1126/science.291.5501.103. [DOI] [PubMed] [Google Scholar]; (c) Zheng J, Dickson RM. J Am Chem Soc. 2002;124:13982–13983. doi: 10.1021/ja028282l. [DOI] [PubMed] [Google Scholar]
- 4.Mirkin CA, Letsinger RL, Mucic RC, Storhoff J. J Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]; (b) Sonnichsen C, Reinhard BM, Liphardt J, Alivisatos AP. Nature Biotech. 2005;23:741–745. doi: 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]; (c) Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. Nature Biotech. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 5.Capadona LP, Zheng J, Gonzalez JI, Lee TH, Patel SA, Dickson RM. Phys Rev Lett. 2005:94. doi: 10.1103/PhysRevLett.94.058301. [DOI] [PubMed] [Google Scholar]
- 6.(a) Nie SM, Emery SR. Science. 1997;275:1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]; (b) Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I, Dasari R, Feld MS. Phys Rev Lett. 1997;78:1667–1670. [Google Scholar]; (c) Jiang J, Bosnick KA, Maillard M, Brus LE. J Phys Chem B. 2003;107:9964–9972. [Google Scholar]; (d) Fromm DP, Sundaramurthy A, Kinkhabwala A, Schuck PJ, Kino GS, Moerner WE. J Chem Phys. 2006:124. doi: 10.1063/1.2167649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y, Min OY. Nature Mater. 2007;6:754–759. doi: 10.1038/nmat1982. [DOI] [PubMed] [Google Scholar]
- 8.Moerner WE, Fromm DP. Rev Sci Instrum. 2003;74:3597–3619. [Google Scholar]
- 9.(a) Suh JS, Moskovits M. J Am Chem Soc. 1986;108:4711–4718. [Google Scholar]; (b) Dou XM, Jung YM, Cao ZQ, Ozaki Y. Appl Spec. 1999;53:1440–1447. [Google Scholar]
- 10.(a) Luo K, St Clair TP, Lai X, Goodman DW. J Phys Chem B. 2000;104:3050–3057. [Google Scholar]; (b) Wertheim GK, Dicenzo SB. Phys Rev B. 1988;37:844–847. doi: 10.1103/physrevb.37.844. [DOI] [PubMed] [Google Scholar]; (c) Database, NIST. XPS. http://srdata.nist.gov/xps/
- 11.(a) Zhao LL, Jensen L, Schatz GC. J Am Chem Soc. 2006;128:2911–2919. doi: 10.1021/ja0556326. [DOI] [PubMed] [Google Scholar]; (b) Zhao LL, Jensen L, Schatz GC. Nano Lett. 2006;6:1229–1234. doi: 10.1021/nl0607378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information for synthesis and characterizations of luminescent and Raman active silver NPs and supplementary figures. This material is available for free of charge via internet at http://pubs.acs.org/