Abstract
Islet amyloid polypeptide (IAPP or amylin) is a 37-residue peptide secreted with insulin by beta-cells in the islets of Langerhans. The aggregation of the peptide into either amyloid fibers or small soluble oligomers has been implicated in the death of beta-cells during type 2 diabetes through disruption of the cellular membrane. The actual form of the peptide responsible for beta-cell death has been a subject of controversy. Previous research has indicated that the N-terminal region of the peptide (residues 1-19) is primarily responsible for the membrane disrupting effect of the hIAPP peptide and induces membrane disruption to a similar extent as the full-length peptide without forming amyloid fibers when bound to the membrane. The rat version of the peptide, which is both non-cytotoxic and non-amyloidogenic, differs from the human peptide by only one amino acid residue: Arg18 in the rat version while His18 in the human version. To elucidate the effect of this difference, we have measured in this study the effects of the rat and human versions of IAPP1-19 on islet cells and model membranes. Fluorescence microscopy shows a rapid increase in intracellular calcium levels of islet cells after the addition of hIAPP1-19 indicating disruption of the cellular membrane, while the rat version of the IAPP1-19 peptide is significantly less effective. Circular dichroism experiments and dye leakage assays on model liposomes show rIAPP1-19 is deficient in binding to and disrupting lipid membranes at low but not at high peptide to lipid ratios, indicating that the ability of rIAPP1-19 to form small aggregates necessary for membrane binding and disruption is significantly less than hIAPP1-19. At pH 6.0, where H18 is likely to be protonated, hIAPP1-19 resembles rIAPP1-19 in its ability to cause membrane disruption. Differential scanning calorimetry suggests a different mode of binding to the membrane for rIAPP1-19 compared to hIAPP1-19. Human IAPP1-19 has a minimal effect on the phase transition of lipid vesicles, suggesting a membrane orientation of the peptide in which the mobility of the acyl chains of the membrane is relatively unaffected. Rat IAPP1-19, however, has a strong effect on the phase transition of lipid vesicles at low concentrations suggesting the peptide does not easily insert into the membrane after binding to the surface. Our results indicate the modulation of the peptide orientation in the membrane by His18 plays a key role in the toxicity of non-amyloidogenic forms of hIAPP.
It has been known for many years that insoluble, highly aggregated amyloid deposits of human Islet Amyloid Polypeptide Protein (hIAPP, also known as amylin) are found post-mortem in the islets of Langerhans of pancreatic beta-cells in the majority (>90 %) of type 2 diabetic patients but not in non-diabetic patients of the same age cohort.(1) The high tissue visibility of the amyloid deposits and their prevalence in diabetic patients has led to the hypothesis that the formation of amyloid fibers plays a causative role in the development of the disease.(2, 3)
The hypothesis that extracellular amyloid fibers directly cause beta-cell apoptosis has been challenged by both in vitro and in vivo studies. Transgenic mice models have been particularly useful in this respect as rats do not spontaneously develop type 2 diabetes and have an IAPP variant that is both non-cytotoxic and non-amyloidogenic Fig. 1).(4) Transgenic mice expressing the human version of IAPP develop complications with metabolic characteristics similar to human type 2 diabetes.(5-8) However, there is poor spatial and temporal overlap between amyloid formation and beta-cell apoptosis in transgenic mice. Extracellular amyloid in homozygous transgenic mice is only detected after the phase of rapid beta-cell death has passed and the beta-cells undergoing apoptosis are not adjacent to amyloid fibers.(9-11) Furthermore, some transgenic mice models expressing hIAPP form extensive amyloid fiber deposits but do not develop type 2 diabetes.(12) The lack of correlation between islet amyloid formation and the pathology of type 2 diabetes in transgenic mice mirrors findings in humans, in that islet amyloid is found in people without diabetes as well as not found in all people with diabetes.(13, 14) Studies directly measuring the effect of IAPP upon beta-cells have confirmed the relatively low toxicity of mature amyloid fibers of IAPP.(15-19)
Figure 1.
The amino acid sequences of rat and human IAPP. The 1-19 fragment that is used in this study is shown in blue, and the differences between the two sequences are shown in red. A disulfide bond exists between residues 2-8. The N-termini are amidated like the naturally occurring peptide.
Unlike mature fibers, small hIAPP oligomers have been demonstrated to be cytotoxic to beta-cells.(5, 15-18, 20, 21) Soluble oligomers of IAPP, in common with other amyloidogenic proteins, have been implicated in the disruption of cellular homeostasis by either the formation of relatively non-selective ion channels or by direct fragmentation of the cellular membrane.(22, 23) The kinetics of oligomer formation is complex and is likely to involve multiple pathways.(24-26) Inhibition studies have suggested that the toxic oligomers are a distinct species of amyloid proteins and not simply direct intermediates of mature fibers. Inhibitors have been found that suppress the formation of toxic oligomers but not the formation of amyloid fibers.(27) Conversely, other inhibitors have been shown to have the opposite effect, suppressing the formation of amyloid fibers but not the formation of toxic oligomers.(18, 27) The ability of some inhibitors to suppress the formation of small oligomers independently of their effect on the formation of amyloid fibers suggests the toxic oligomers are at least partly off the kinetic pathway for the formation of amyloid fibers.
Although amyloidogenic proteins can disrupt membranes through the formation of toxic oligomers, new evidence suggests the formation of toxic oligomers may not be unique to amyloidogenic proteins. An antibody specific for the soluble oligomeric form of amyloidogenic proteins also recognizes several non-amyloidogenic pore-forming proteins.(28) More directly, the N-terminal fragment of human IAPP (residues 1-19) has been found to disrupt synthetic vesicles to nearly the same extent as full-length IAPP but does not form amyloid fibers while bound to the membrane.(25) Significantly, the sequences of human IAPP and rat IAPP are identical in this region of the protein except for residue 18, which is His in the human version and Arg in the rat version of the peptide (Fig. 1). In a companion paper of this issue, high-resolution structures of hIAPP1-19 and rIAPP1-19 in DPC micelles were solved by solution NMR. Although the structures are largely similar, rIAPP1-19 is more disordered at the N-terminus than the hIAPP1-19 peptide. More significantly, hIAPP1-19 is shielded from the paramagnetic quencher Mn+2 while rIAPP1-19 is exposed. The difference in the accessibility of Mn+2 to the two peptides when bound to DPC micelles suggests rIAPP1-19 adopts a different orientation in the membrane than hIAPP1-19. This effect is likely due to the difference in charge at residue 18, as hIAPP1-19 becomes significantly more solvent exposed at pH 6.0 where the histidine residue is likely to be ionized. To see the impact of this difference on the toxicity of the two peptides, we have performed in vivo and in vitro assays of the ability of the two peptides to permeabilize the membranes of both beta-cell islets and model membranes. We report here that the rIAPP1-19 peptide is significantly impaired in its ability to disrupt phospholipid membranes compared to the corresponding hIAPP1-19 peptide, highlighting the role of this key residue in controlling the toxicity of the peptide.
Materials and Methods
Materials
POPG (1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phospho-rac-(1-glycerol)), DMPG (1,2-Dimyristoyl-sn-Glycero-3-[Phospho-rac-(1-glycerol)]), and DMPC (1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine) phospholipids were obtained from Avanti (Alabaster, AL); DMSO (Dimethyl Sulfoxide), carboxyfluorescein and HFIP (1,1,1,3,3,3-Hexafluoro-2-Propanol) were obtained from Sigma-Aldrich; hIAPP1-19 and rIAPP1-19 with amidated C-termini (>95 % purity) were purchased from Genscript. Before use, lyophilized rIAPP1-19 and hIAPP1-19 were dissolved in HFIP at a concentration of 10 mg/ml for one hour to break up any pre-formed aggregates present in the solution. Aliquots of the peptide stock solution were flash-frozen in liquid nitrogen and then lyophilized again for more than 16 hours at less than 1 mTorr vacuum to completely remove HFIP. All experiments were conducted at room temperature (approximately 23°C). POPG vesicles for the circular dichroism and dye leakage experiments were prepared as previously described.(25)
Islet Isolation Protocol
Pancreatic islets were obtained from 20- to 30-g male CD-1 mice as previously described.(29) Briefly, mice were sacrificed by cervical dislocation and collagenase type XI was injected into the pancreas through the main pancreatic duct. The pancreas was removed and incubated in 5 mL of a collagenase solution at 37 °C. Islets that were used for experiments were 100-200 μm in diameter, had an intact islet membrane, and were oblong to spherical in shape. Islets were placed in tissue culture dishes and incubated in RPMI 1640 containing 10 % fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37 °C, 5% CO2, pH 7.4. Islets were used 1-6 days following isolation.
Intracellular Ca2+ Measurements
Calcium flux measurements were performed on islets after a 40-min incubation with 2 μM of the fluorescent calcium-sensitive dye, fura-2. Afterward, individual islets were loaded into an open cell chamber on a microfluidic chip. Islet media and stimulants were perfused over the islet at a rate of 0.6 μL/min. The chip was placed atop the stage of a Nikon Diaphot 300 microscope. The fura-2 was alternately excited using a filter wheel with 340 and 380 nm-light from a Xenon-arc lamp. The fluorescence emission from both excitation wavelengths was collected through a 510 ± 10 nm-bandpass filter. Images of the islet were collected at 1 Hz, and the intensity over islet area was integrated using Metamorph software. Intracellular calcium concentration was calculated by determining the ratio of the emission at 340 and 380 nm excitation after calibration of the system.
Circular Dichroism Spectroscopy
Lyophilized peptide was dissolved in sodium phosphate buffer (10 mM, pH 7.3), briefly vortexed and sonicated (approximately 15 seconds), and transferred to a 0.1 cm cuvette. After the initial spectrum of the peptide in solution was taken, POPG vesicles from a 40 mg/ml stock solution were titrated into the cuvette. Spectra were measured at 1 nm intervals from 185-260 nm at a scanning speed of 50 nm/min and a bandwidth of 5 nm. Each spectrum reported is the average of four scans after subtraction of the baseline spectrum of buffer and vesicles without peptide.
Dye leakage Assay
For the dye leakage experiments, carboxyfluorescein containing POPG vesicles were prepared by rehydrating the dried lipid film in 50 mM sodium phosphate buffer (pH 7.5) containing 40 mM carboxyfluorescein, adjusted to pH 7.5 by the addition of sodium hydroxide. Nonencapsulated carboxyfluorescein was removed from the vesicles through size exclusion chromatography using a PD-10 column (Amersham Pharmacia Biotech, Uppsala, Sweden). Vesicle solutions were used immediately; and a fresh vesicle solution was used for each experiment. Fluorescence readings were taken at an excitation wavelength of 493 nm and an emission wavelength of 518 nm. A baseline reading was taken on the solutions prior to the addition of the peptide. After injection, the fluorescence intensity was recorded after 100 seconds of interaction. The fluorescence signal given by the addition of peptide was then normalized by the addition of Triton X detergent, causing all vesicles present to release any remaining dye to obtain the total possible fluorescent signal.
Differential Scanning Calorimetry
Multilamellar vesicles of DMPC and DMPG (70% DMPC; 30% DMPG) were prepared as described for the dye leakage experiments. Sodium phosphate buffer (10 mM) with 150 mM NaCl at pH 7.3 was used to hydrate the samples. The total molar concentration of lipid was kept constant (5.9 mM) for each sample while the molar peptide concentration was varied as indicated. Scans were run on a Calorimetry Sciences N-DSC II over a temperature range of 5 to 45°C with a total of four heating and four cooling scans. The heating scans were run at 0.25°C/min while the cooling scans were run at 1.0°C/min, in between each of which there was a 10 minutes equilibration period. The buffer solution without the sample was used as the reference cell. The data was converted to molar heat capacity using the average molecular weight of the lipids, the lipid concentration, and a partial specific volume of 0.988 ml/g. Excess heat capacity was calculated by subtracting a baseline with buffer in both the reference and sample cells at the same scanning rate.
Results and Discussion
Membrane disruption induced by hIAPP1-19 and rIAPP1-19 in POPG vesicles
To test for differences in the extent of membrane disruption induced by hIAPP1-19 and rIAPP1-19 peptides, leakage of the dye carboxyfluorescein from large unilamellar POPG vesicles (Fig. 2) was measured as a function of the concentration of POPG at three different concentrations of the peptide. Carboxyfluorescein in intact vesicles is at a high concentration (40 mM) and self-quenched. Disruption of the membrane of the vesicle by the peptide allows carboxyfluorescein to escape, eliminating the self-quenching effect and therefore increasing fluorescence of carboxyfluorescein. For each graph in Fig. 2, the peptide-to-lipid-ratio was varied by the addition of empty POPG vesicles (that is vesicles not containing carboxyfluorescein) while the concentrations of peptide and carboxyfluorescein-containing vesicles were kept constant. Decreasing the peptide-lipid ratio in this manner has two effects. First, peptide binding and membrane disruption for empty vesicles not containing carboxyfluorescein is not detected. Binding of peptide to empty vesicles reduces the amount of peptide available to bind to carboxyfluorescein-containing vesicles. The membrane disruption induced by the peptide should accordingly decrease in a near-linear fashion as the amount of empty vesicles is increased (values to the right on the x-axis in Fig. 2). Second, the amount of peptide bound to each vesicle is decreased as the total lipid concentration is increased. If the process of membrane disruption is non-linearly dependent on the concentration of the peptide bound to the membrane, the amount of membrane disruption can also be expected to be non-linearly dependent on the peptide to lipid ratio. This can occur if either the peptide oligomerizes to form peptide channels in the membrane or if membrane disruption occurs by a weakening of the membrane via a carpet type mechanism as reported for antimicrobial peptides.(30-32)
Figure 2.
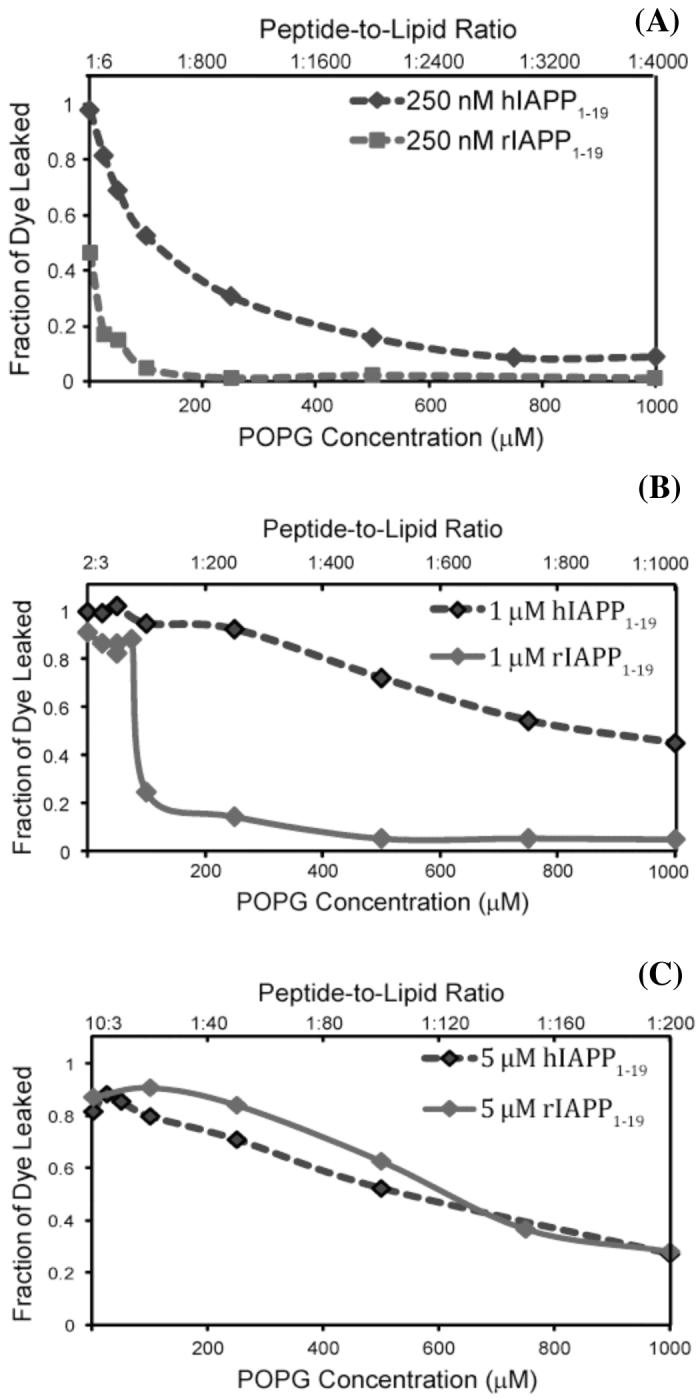
Liposome leakage induced by hIAPP1-19 (blue diamonds) and the rIAPP1-19 fragment (green diamonds) at pH 7.5. The peptide-to-lipid ratio was varied by adding 250 nM (A), 1 μM (B), and 5 μM (C) solutions of either rIAPP1-19 or hIAPP1-19 to POPG liposomes containing carboxyfluorescein (1.5 μM) and enough empty POPG liposomes (not containing carboxyfluorescein) to create the indicated molar ratio of peptide to lipid. The fluorescence signal was recorded at 100 seconds after peptide injection and normalized to the total possible signal upon addition of detergent.
Human IAPP1-19 strongly induced membrane disruption at all concentrations tested (Fig. 2). Membrane disruption decreased as the peptide-to-lipid ratio is decreased, but the plots are relatively linear for peptide concentrations of 1 μM and 5 μM (Fig. 2B and 2C). Only at the lowest peptide concentration of 250 nM is a non-linear dependence of membrane disruption on the concentration of POPG apparent. At a 5 μM peptide concentration, rIAPP1-19 is slightly more effective than hIAPP1-19 at disrupting the membrane (Fig. 2C). This effect is most likely due to the greater charge on the rIAPP1-19 peptide at pH 7.3 (+4 vs. +3 for the hIAPP1-19 assuming H18 is uncharged at pH 7.5) which facilitates partitioning into membranes containing anionic lipids. At a lower peptide concentration (1 μM, Fig. 2B), rIAPP1-19 shows a striking non-linear dependence on the peptide-to-lipid ratio. At high peptide to lipid molar ratios, the rIAPP1-19 peptide is nearly as effective as the hIAPP1-19 peptide. However, as the peptide to lipid ratio is decreased, the membrane disrupting activity of rIAPP1-19 is abruptly lost, dropping off suddenly as a critical peptide-to-lipid ratio is surpassed (approximately 1:100). At the lowest peptide concentration tested in this study, rIAPP1-19 induces significant membrane disruption only at the highest peptide-to-lipid ratio (1:6) measured (Fig. 2C).
The physiologically relevant peptide-to-lipid ratio is difficult to establish exactly, as IAPP is stored at high concentrations (0.8 -4 mM) in secretory granules before being released into the bloodstream.(33) After release from the secretory granule, the peptide is diluted in the bloodstream to quite low concentrations.(34) While the equilibrium concentration of IAPP in the bloodstream is quite low, islet cells are briefly exposed to high concentrations of IAPP immediately upon the release of IAPP from the secretory granule. The amount of IAPP bound to the islet membrane and the consequent peptide-to-lipid ratio therefore depends on a variety of factors that are poorly understood at present, including the dynamics of the release of IAPP from the secretory granule, the reversibility of the membrane binding process, and the affinity for IAPP for the cellular membrane. It is also important to note that cell membranes, unlike model membranes, are highly heterogeneous and it is probable that IAPP is concentrated in certain regions of the cell membrane, an effect seen previously for the related Aβ1-40 peptide.(35, 36) In light of the uncertainty about the kinetics of the process of IAPP release from the secretory granule and also the binding of IAPP to the beta-cell membrane, it is difficult to establish a narrow range of peptide-to-lipid ratios as being physiologically relevant. Nevertheless, low peptide-to-lipid ratios are more likely to be physiologically relevant than high peptide-to-lipid ratios as the equilibrium concentration of IAPP is low.
Human IAPP differs from rIAPP1-19 only by the H18R substitution. At the slightly alkaline pH of the extracellular matrix (∼7.3), H18 will be deprotonated while R18 will remain charged. This ionization state of H18 has previously been shown to have an effect on the fibrillization of full-length hIAPP, with hIAPP fibrillizing faster at acidic pH where H18 is charged.(37, 38) To test the effect of the ionization state of H18 on the membrane disruption, the vesicle disruption assay was repeated at pH 6.0 using 1 μM of either hIAPP1-19 or rIAPP1-19 (Fig. 3). The ionization state at residue 18 appears to be critical for membrane disruption, as hIAPP1-19 causes significantly less disruption at lower peptide-to-lipid ratios at pH 6.0 than at pH 7.3 (compare Fig. 2B and Fig. 3). The amount of disruption induced by hIAPP1-19 at pH 6.0 is very similar to rIAPP1-19, suggesting hIAPP1-19 disrupts liposomes by a similar mechanism as rIAPP1-19 at pH 6.0 but not at pH 7.3 where histidine is deprotonated.
Figure 3.
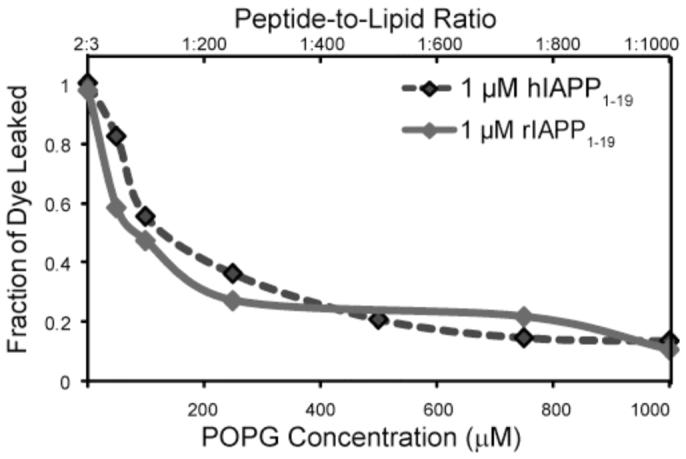
Liposome leakage induced by hIAPP1-19 (blue diamonds) and the rIAPP1-19 fragment (green diamonds) at pH 6.0. Except for the pH, the liposome leakage assay was performed as indicated for the pH 7.3 sample using 1 μM solutions of either rIAPP1-19 or hIAPP1-19.
The pH dependence of membrane disruption for hIAPP1-19 is supported by the paramagnetic quenching experiments detailed in the companion paper.(39) Human IAPP1-19 in DPC micelles is strongly quenched by Mn +2 at pH 6.0 but not at pH 7.3. Rat IAPP1-19, by contrast, is strongly quenched at pH 7.3. As the Mn+2 ion quenches the signals of residues near the membrane-solution interface, it is expected that a surface-associated peptide will be more strongly quenched than a peptide more deeply inserted into the membrane. The enhancement of paramagnetic quenching of hIAPP1-19 at pH 6.0 indicates hIAPP1-19 binds closer to the surface of the micelle when H18 is protonated, adopting a surface-associated binding mode similar to rIAPP1-19.
Membrane disruption induced by hIAPP1-19 and rIAPP1-19 in pancreatic islets
The results of the dye leakage assay indicate hIAPP1-19 strongly disrupts phospholipid membranes; on the other hand, rIAPP1-19 also disrupts phospholipid membranes if the peptide-lipid-ratio is high enough. These results are in agreement with our previous study which showed that hIAPP1-19 fragment, which is non-amyloidogenic if bound to a membrane, disrupted POPG vesicles to a similar extent as the strongly amyloidogenic full-length IAPP peptide.(25) However, all of these assays were performed under conditions that maximized peptide binding (low ionic strength, high content of anionic lipids) and differ from the conditions found in the in vivo setting. A more accurate assessment of the damage induced by IAPP could be done by the use of cells loaded with a calcium sensitive dye to monitor changes in intracellular calcium levels due to the membrane permeabilization induced by IAPP.(15, 40-43) Full-length hIAPP is known to disrupt the calcium homeostasis of neuroblastoma (SH-SY5Y) cells in a conformation-dependent manner. (15) Demuro et al have shown that the addition of monomeric hIAPP and mature fibers of hIAPP had little effect on intercellular calcium levels but the addition of a prefibrillar oligomeric form of hIAPP caused the influx of calcium into the cell. The rise in intercellular calcium concentrations induced by prefibrillar oligomeric hIAPP was dependent on the concentration of hIAPP used, with a maximal response at 12 μg/ml and an EC50 of approximately 3.5 μg/mL.(15) The membrane disruption induced by hIAPP was large enough to permit leakage of calcein dye from the cell, indicating that prefibrillar oligomeric hIAPP does not simply disrupt calcium homeostasis by the activation of endogenous calcium channels (15) as has been reported for the fibrillar form of hIAPP.(43)
To test the toxic effect of hIAPP1-19 and rIAPP1-19 peptides on pancreatic islets, we performed an analogous experiment by measuring the influx of calcium into single islets upon the addition of hIAPP1-19 and rIAPP1-19. Figure 4 shows the effect of hIAPP1-19, rIAPP1-19, and the full-length rIAPP1-37 peptide on the intracellular calcium levels of pancreatic islets. Both hIAPP1-19 and rIAPP1-19 are toxic at relatively low concentrations, as measured by their ability to disrupt the cellular membrane and allow the influx of calcium into the cell. However, hIAPP1-19 is significantly more effective than rIAPP1-19 at disrupting the islet membrane as shown in Fig. 4. The rise in intracellular calcium levels occurred immediately, without the presence of a lag phase that has been detected for the full-length peptide.(15) Full-length rat IAPP prepared in the same manner does not induce the influx of intracellular calcium into the cell, in agreement with the low cytotoxicity of full-length rIAPP reported in previous studies.(9, 15, 16)
Figure 4.
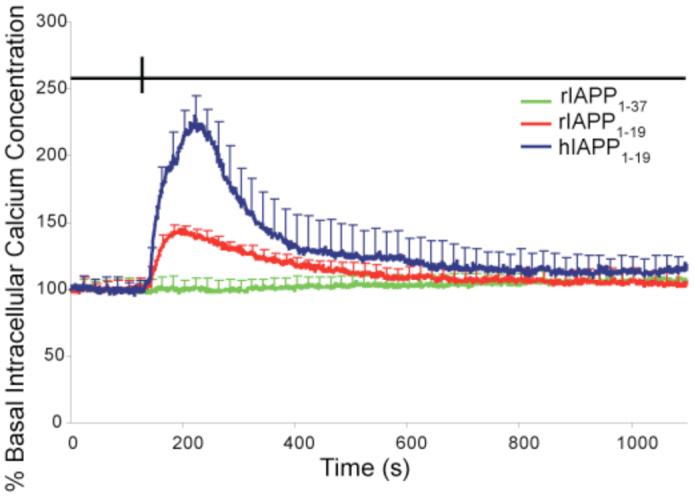
Membrane permeabilization in pancreatic islets induced by IAPP. Whole mouse pancreatic islets were loaded 45 minutes prior to the experiment with 2 μM of the calcium-sensitive dye fura-2 AM. At 140 seconds (indicated by a vertical line at the top) 12 μg/ml hIAPP1-19, rIAPP1-19 or rIAPP1-37 was perfused over the cells. The values given are the average of 5 islet samples; error bars indicate standard error of the mean.
Comparison of the binding affinity of hIAPP1-19 and rIAPP1-19 for POPG vesicles
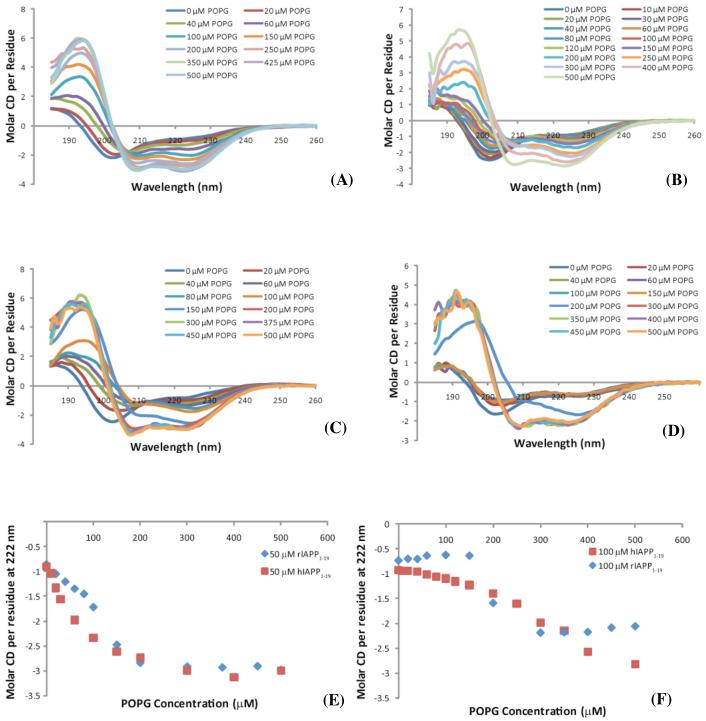
The differences in the membrane disruption induced by hIAPP1-19 and rIAPP1-19 suggest a difference may exist in the degree of cooperativity in binding to the membrane. Figure 5 shows the binding of rIAPP1-19 and hIAPP1-19 to POPG vesicles as approximated by the measurement of the conformational changes occurring upon binding to lipid membranes. This method is well established for the qualitative measurement of peptide membrane binding,(44) although conformational changes occurring after membrane binding can complicate quantitative analysis. Like the full-length peptide, both hIAPP1-19 and rIAPP1-19 exist in a random coil conformation in solution and adopt an alpha-helical conformation upon binding to the membrane.(33, 45) However, noticeable differences can be seen in the binding curves of hIAPP1-19 and rIAPP1-19 titrated with increasing concentrations of POPG vesicles (Fig. 5).
Figure 5.
CD Spectra of 50 μM hIAPP1-19 (A), 100 μM hIAPP1-19(B), 50 μM rIAPP1-19 (C), and 100 μM rIAPP1-19 (D) at the indicated concentrations of POPG. Plots of the molar CD per residue at 222 nm for 50 μM hIAPP1-19 and rIAPP1-19 (E) and 100 μM hIAPP1-19 and rIAPP1-19 (F) with the indicated concentrations of POPG. All spectra were obtained in 10 mM sodium phosphate buffer, pH 7.3.
As shown in Figure 5, rIAPP1-19 is nearly as effective in binding as hIAPP1-19 at saturating concentrations of POPG but is somewhat less effective at lower concentrations. The binding of full-length human and rat IAPP is known to be a cooperative process with membrane binding proceeding much more efficiently after the formation of small aggregates of IAPP on the membrane.(33) Full-length rat IAPP differs from full-length human IAPP in that the nucleation of small aggregates by rat IAPP is noticeably impaired as compared to that of human IAPP.(33) The lower affinity of rIAPP1-19 for the membrane at lower concentrations of POPG suggests the rat 1-19 fragment, like full-length rIAPP, is impaired in forming the small aggregates that enhance membrane binding.
Interaction of hIAPP1-19 and rIAPP1-19 with membrane determined by differential scanning calorimetry
In the accompanying paper detailing the structures of hIAPP1-19 and rIAPP1-19 in dodecylphosphocholine (DPC) micelles, we show a significant difference in the accessibility of hIAPP1-19 and rIAPP1-19 to manganese ions.(39) This indicates hIAPP1-19 is located significantly deeper within the hydrophobic core of the micelle than rIAPP1-19 and suggests a different mode for the binding to phospholipid bilayers. To test this model, the interaction of hIAPP1-19 and rIAPP1-19 peptides with membrane was characterized by differential scanning calorimetry (DSC). In general, the phase behavior of lipid membrane systems is highly affected by the presence of guest molecules such as peptides.(46, 47) In particular, the effect on the thermally-induced gel to liquid crystalline phase transition and the related thermodynamic variables (melting temperature (Tm), enthalpy change (ΔH), and entropy change (ΔS)) depends on the nature of the interactions between the peptide and the membrane and on the topology of the peptide relative to the lipid bilayer.(48, 49) A peptide randomly distributed on the surface of the bilayer disorders the surrounding lipids when the membrane is in the gel phase, lowering the ΔH and Tm associated with the gel to liquid crystalline phase transition. The disruption of the lipid-lipid interactions within the membrane also decreases the stability and rigidity of the membrane and therefore decreases the cooperativity of the phase transition. This results in a decreased sharpness of the peak relative to the pure lipid system. The pretransition, reflective of a change in the orientation of the headgroup of phospholipids, is affected as well. A peptide inserted in a transmembrane orientation has less effect on the physical properties of the membrane because the lipid-lipid interactions are disrupted to a much lower degree than with a surface associated peptide.(49) The aggregation of the peptide decreases the perturbation on the membrane by decreasing the surface area of the membrane in contact with the peptide.(50, 51)
Figure 6 shows the effect of hIAPP1-19 on mixed DMPC/DMPG vesicles (7/3 ratio). The DSC of pure DMPC/DMPG vesicles shows a single main transition at 24.5°C indicative of the gel to liquid crystalline phase transition and a smaller pretransition at 5.4°C indicative of the rippled gel-to-gel phase transition. The addition of hIAPP1-19 up to 2 mole percent had a very little effect on the thermodynamics of the gel to liquid crystalline phase transition. This indicates the lipid-lipid interactions stabilizing the membrane are largely intact and could suggest that the peptide binds in a transmembrane orientation in an aggregated state, as previously inferred for the full-length human peptide.(33, 52, 53) Self-association of the peptide reduces the number of lipid molecules in contact with the peptide and is therefore expected to decrease the influence of the peptide on the physical properties of the membrane.
Figure 6.
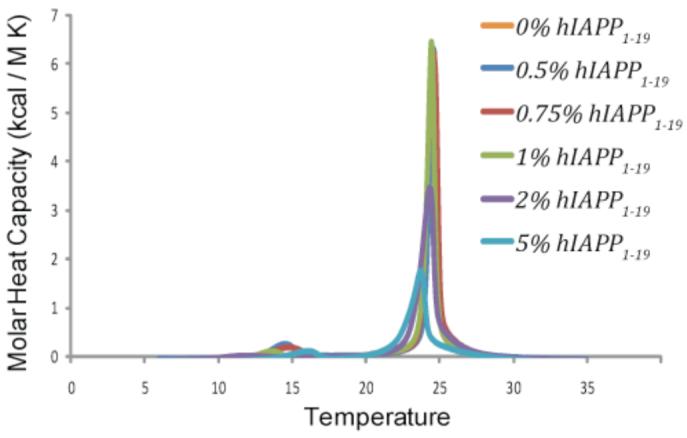
Differential scanning calorimetry of the pretransition and the main gel to liquid-crystalline phase transition of DMPC : DMPG (7:3) vesicles at the indicated molar ratio of hIAPP1-19 to lipid. Peptide and lipids were co-dissolved in a chloroform/ethanol solution, dried and resuspended in sodium phosphate buffer, pH 7.3 with 150 mM NaCl.
Rat IAPP1-19 has a much different effect on the membrane (Figure 7). At low concentrations of peptide (0.5-0.75 mole %) the peptide only slightly reduces the ΔH of the main phase transition, but the peptide has a large effect on the pretransition peak reflective of the rippled gel-to-gel phase transition, shifting it to higher temperatures and dramatically increasing the height of the peak for membranes containing 0.5 mole % rIAPP1-19. An increase in the pretransition peak height is unusual as most substances tend to reduce the enthalpy change associated with the rippled gel-to-gel phase transition. The rippled gel phase is similar to the gel phase except for the existence of point defects in the bilayer caused by a small percentage of molecules in the fluid phase.(54) The existence of these defects in turn leads to periodic ripples in the membrane surface. Most peptides disorder the gel phase therefore reducing the enthalpy change associated with the rippled gel-to-gel phase transition as defects are present in both phases. An increase in ΔH for the pretransition can be associated with a decrease in the ripple periodicity, which in turn is related to curvature changes in the membrane induced by the mismatch in size between the headgroup region and hydrophobic core of the bilayer induced by the peptide binding to the surface of the membrane.(54, 55) The formation of ripples in a lipid bilayer has been detected with a surfactant that induces positive curvature in the membrane.(56) Unlike hIAPP1-19, the gel to liquid crystalline phase transition of membranes containing rIAPP1-19 is significantly different at higher concentrations of peptide. At higher concentrations the gel to liquid crystalline transition is greatly reduced in height and splits into two peaks, one peak with a phase transition at a lower temperature than the pure lipid peak and one peak at a higher temperature than the pure lipid peak. The splitting of a phase transition peak is usually indicative of lipid domain formation, with one peak corresponding to peptide-enriched domains of lipids and the other to peptide-poor domains of lipid.(57, 58) The greater perturbation of the lipid bilayer by rIAPP1-19 indicated by the DSC results suggests that, unlike hIAPP1-19, rIAPP1-19 may be bound to the surface of the bilayers and not in the active conformation necessary to form pores. This is consistent with the membrane orientation of IAPP peptides determined from our NMR studies.(39)
Figure 7.
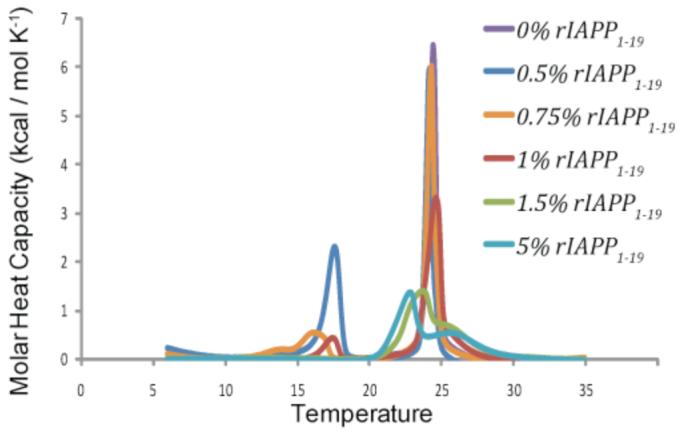
Differential scanning calorimetry of the pretransition and the main gel to liquid-crystalline phase transition of DMPC : DMPG (7:3) vesicles at the indicated molar ratio of rIAPP1-19 to lipid. Peptide and lipids were co-dissolved in a chloroform/ethanol solution, dried and resuspended in 10 mM sodium phosphate buffer, pH 7.3 with 150 mM NaCl.
Conclusions
The 1-19 peptide fragment of human IAPP (or amylin) is of particular interest in amyloidogenic protein research as it disrupts model anionic membranes to a near-identical extent as the full-length IAPP peptide but is conformationally stable; once bound to the membrane in an active alpha-helical form, it does not proceed to form amyloid fibers.(25) This feature is important for the study of the IAPP peptide, whose rapid and complex aggregation process leads to considerable difficulties in biophysical studies. One of the key arguments for the importance of IAPP amyloid fibers, rather than other oligomeric states of the peptide, in the death of beta-cells during type II diabetes is the lack of toxicity of the non-amyloidogenic rat version of the peptide. In light of the nearly identical sequences of the rat and human versions of IAPP sequence in the 1-19 region of the peptide, the membrane disrupting effect of hIAPP1-19 is surprising. In this study, we confirmed the membrane disrupting activity previously reported for highly charged model membranes also exists for islet cells under physiological conditions. Our results suggest an important role for His18 in the early stages of aggregation and membrane binding. Histidine-18 has previously been implicated in the rate of assembly of amyloid fibers, with amyloid fiber formation proceeding much more slowly when His18 is protonated at a low pH.(38) In fact, the R18H substitution in the rIAPP1-37 is sufficient to turn the peptide from being non-amyloidogenic to slightly amyloidogenic, which is most likely due to the disruption of the stacking of the beta sheets forming the amyloid fiber by the protonated arginine residue.(59) However, mature amyloid fibers show relatively little toxicity to beta-cells and a different mechanism is likely to operate during the earlier stages of aggregation where the toxicity of the peptide is highest and large aggregates are absent. Our results show, for the relatively non-amyloidogenic but toxic 1-19 fragments of the peptide, that the His18 residue also plays a key role in controlling the toxicity of the peptide by modulating the interactions of IAPP with the phospholipid membrane.
Acknowledgments
This study was supported by research funds from Michigan Diabetes Research Training Center at the University of Michigan and NIH (AI054515 to A.R., DK046960 to R.T.K). The authors thank Professor Ari Gafni and his research group for their interest in this work and for generous access to their CD spectrometer.
Abbreviations
- CD
Circular Dichrosim
- DMPC
1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine
- DMPG
1,2-Dimyristoyl-sn-Glycero-3-Phospho-rac-(1-glycerol)
- DMSO
Dimethyl Sulfoxide
- DSC
Differential Scanning Calorimetry
- HFIP
1,1,1,3,3,3-Hexafluoro-2-Propanol
- hIAPP
human Islet Amyloid Polypeptide
- IAPP
Islet Amyloid Polypeptide
- NMR
Nuclear Magnetic Resonance
- POPG
1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phospho-rac-(1-glycerol)
- rIAPP
rat Islet Amyloid Polypeptide
Footnotes
References
- 1.Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- 2.Hoppener JWM, Lips CJM. Role of islet amyloid in type 2 diabetes mellitus. Int. J. Biochem. Cell Biol. 2006;38:726–736. doi: 10.1016/j.biocel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo A, Yankner BA. Neurobiology of Alzheimer’s Disease. 1996. Amyloid fibril toxicity in Alzheimer’s disease and diabetes; pp. 89–95. [DOI] [PubMed] [Google Scholar]
- 4.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 5.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet Amyloid in Type 2 Diabetes, and the Toxic Oligomer Hypothesis. Endocr. Rev. 2008;29:302–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppener JWM, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JHH, Vroom TM, Ahren B, Lips CJM. Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42:427–434. doi: 10.1007/s001250051175. [DOI] [PubMed] [Google Scholar]
- 7.Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW, Kreutter DK, Butler PC. Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes. 1998;47:743–750. doi: 10.2337/diabetes.47.5.743. [DOI] [PubMed] [Google Scholar]
- 8.Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- 9.Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1656–1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- 10.Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes - Evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 12.Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet antyloid deposition in mice expressing the gene for human islet antyloid polypeptide. Diabetes. 2005;54:2235–2244. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]
- 13.Tasaka Y, Nakaya F, Matsumoto H, Iwamoto Y, Omori Y. Pancreatic Amylin Content in Human Diabetic Subjects and Its Relation to Diabetes. Pancreas. 1995;11:303–308. doi: 10.1097/00006676-199510000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Bell ET. Hyalinization of the Islets of Langerhans in Nondiabetic Individuals. Am. J. Pathol. 1959;35:801–805. [PMC free article] [PubMed] [Google Scholar]
- 15.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 16.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 17.Konarkowska B, Aitken JF, Kistler J, Zhang SP, Cooper GJS. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, Kayed R, Lin CY, Gurlo T, Haataja L, Jayasinghe S, Langen R, Glabe CG, Butler PC. Inhibition of human IAPP fibril formation does not prevent beta-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am. J. Physiol. 2006;291:E1317–E1324. doi: 10.1152/ajpendo.00082.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ritzel RA, Butler PC. Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes. 2003;52:1701–1708. doi: 10.2337/diabetes.52.7.1701. [DOI] [PubMed] [Google Scholar]
- 20.Anguiano M, Nowak RJ, Lansbury PT. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 21.Lin CY, Gurlo T, Kayed R, Butler AE, Haataja L, Glabe CG, Butler PC. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes. 2007;56:1324–1332. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- 22.Quist A, Doudevski L, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]; Brender JR, DÜrr UHN, Heyl D, Budarapu MB, Ramamoorthy A. Membrane fragmentation by human islet amyloid polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. BBA Biomembranes. 2007;1768:2026–2029. doi: 10.1016/j.bbamem.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers ET, Powers DL. Mechanisms of protein fibril formation: Nucleated polymerization with competing off-pathway aggregation. Biophys. J. 2008;94:379–391. doi: 10.1529/biophysj.107.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. Amyloid Fiber Formation and Membrane Disruption are Separate Processes Localized in Two Distinct Regions of IAPP, the Type-2-Diabetes-Related Peptide. J. Am. Chem. Soc. 2008;130:6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khemtemourian L, Killian JA, Hoppener JW, Engel MF. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp. Diabetes Res. 2008. 2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshiike Y, Kayed R, Milton SC, Takashima A, Glabe CG. Pore-forming proteins share structural and functional homology with amyloid oligomers. Neuromol. Med. 2007;9:270–275. doi: 10.1007/s12017-007-0003-6. [DOI] [PubMed] [Google Scholar]
- 29.Pralong WF, Bartley C, Wollheim CB. Single Islet Beta-Cell Stimulation by Nutrients - Relationship between Pyridine-Nucleotides, Cytosolic Ca2+ and Secretion. EMBO J. 1990;9:53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochem. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 32.Hoskin DW, Ramamoorthy A. Studies on Anticancer Activities of Antimicrobial Peptides. BBA Biomembranes. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 34.Vanhulst KL, Hackeng WHL, Hoppener JWM, Vanjaarsveld BC, Nieuwenhuis MG, Blankenstein MA, Lips CJM. An Improved Method for the Determination of Islet Amyloid Polypeptide Levels in Plasma. Ann. Clin. Biochem. 1994;31:165–170. doi: 10.1177/000456329403100209. [DOI] [PubMed] [Google Scholar]
- 35.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease - a review. J. Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Liyanage U, Bickel PE, Xia WM, Lansbury PT, Kosik KS. A detergent-insoluble membrane compartment contains A beta in vivo. Nat. Med. 1998;4:730–734. doi: 10.1038/nm0698-730. [DOI] [PubMed] [Google Scholar]
- 37.Jaikaran E, Higham CE, Serpell LC, Zurdo J, Gross M, Clark A, Fraser PE. Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 2001;308:515–525. doi: 10.1006/jmbi.2001.4593. [DOI] [PubMed] [Google Scholar]
- 38.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 39.Nanga RPR, Brender JR, Xu J, Veglia G, Ramamoorthy A. Structures of rat and human islet amyloid polypeptide proteins (IAPP)1-19 in dodecylphosphocholine micelles by solution NMR spectroscopy. Biochemistry. 2008 doi: 10.1021/bi8014357. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J. Biol. Chem. 2000;275:14077–14083. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- 41.Mattson MP, Goodman Y. Different Amyloidogenic Peptides Share a Similar Mechanism of Neurotoxicity Involving Reactive Oxygen Species and Calcium. Brain Res. 1995;676:219–224. doi: 10.1016/0006-8993(95)00148-j. [DOI] [PubMed] [Google Scholar]
- 42.Casas S, Gomis R, Gribble FM, Altirriba J, Knuutila S, Novials A. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes. 2007;56:2284–2294. doi: 10.2337/db07-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas S, Novials A, Reimann F, Gomis R, Gribble FM. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia. 2008 doi: 10.1007/s00125-008-1111-z. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladokhin AS, White SH. Folding of amphipathic alpha-helices on membranes: Energetics of helix formation by melittin. J. Mol. Biol. 1999;285:1363–1369. doi: 10.1006/jmbi.1998.2346. [DOI] [PubMed] [Google Scholar]
- 45.Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44:12113–12119. doi: 10.1021/bi050840w. [DOI] [PubMed] [Google Scholar]
- 46.Pappalardo G, Milardi D, Magri A, Attanasio F, Impellizzeri G, La Rosa C, Grasso D, Rizzarelli E. Environmental factors differently affect human and rat IAPP: Conformational preferences and membrane interactions of IAPP17-29 peptide derivatives. Chem.-Eur. J. 2007;13:10204–10215. doi: 10.1002/chem.200700576. [DOI] [PubMed] [Google Scholar]
- 47.Grasso D, Milardi D, La Rosa C, Rizzarelli E. DSC study of the interaction of the prion peptide PrP106-126 with artificial membranes. New J. Chem. 2001;25:1543–1548. [Google Scholar]
- 48.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 49.McElhaney RN. Differential Scanning Calorimetric Studies of Lipid Protein Interactions in Model Membrane Systems. Biochim. Biophys. Acta. 1986;864:361–421. doi: 10.1016/0304-4157(86)90004-3. [DOI] [PubMed] [Google Scholar]
- 50.Ivanova VP, Makarov IM, Schaffer TE, Heimburg T. Analyzing heat capacity profiles of peptide-containing membranes: cluster formation of gramicidin A. Biophysical J. 2003;84:2427–2439. doi: 10.1016/S0006-3495(03)75047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramamoorthy A, Lee DK, Santos JS, Henzler-Wildman KA. Nitrogen-14 Solid-State NMR Spectroscopy of Aligned Phospholipid Bilayers to Probe Peptide-Lipid Interaction and Oligomerization of Membrane Associated Peptides. J. Am. Chem. Soc. 2008 doi: 10.1021/ja802210u. In Press. [DOI] [PubMed] [Google Scholar]
- 52.Knight JD, Miranker AD. Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 2004;341:1175–1187. doi: 10.1016/j.jmb.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 53.Mishra R, Bulic B, Sellin D, Jha S, Waldmann H, Winter R. Small-Molecule Inhibitors of Islet Amyloid Polypeptide Fibril Formation. Angew. Chem. Int. Ed. Engl. 2008;47:4679–4682. doi: 10.1002/anie.200705372. [DOI] [PubMed] [Google Scholar]
- 54.Heimburg T. A model for the lipid pretransition: Coupling of ripple formation with the chain-melting transition. Biophys. J. 2000;78:1154–1165. doi: 10.1016/S0006-3495(00)76673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun XQ, Gezelter JD. Dipolar ordering in the ripple phases of molecular-scale models of lipid membranes. J. Phys. Chem. B. 2008;112:1968–1975. doi: 10.1021/jp0762020. [DOI] [PubMed] [Google Scholar]
- 56.Brasseur R, Braun N, El Kirat K, Deleu M, Mingeot-Leclercq MP, Dufrene YF. The biologically important surfactin lipopeptide induces nanoripples in supported lipid Bilayers. Langmuir. 2007;23:9769–9772. doi: 10.1021/la7014868. [DOI] [PubMed] [Google Scholar]
- 57.Epand RM. Detecting the presence of membrane domains using DSC. Biophys. Chem. 2007;126:197–200. doi: 10.1016/j.bpc.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Epand RF, Schmitt MA, Gellman SH, Epand RM. Role. of membrane lipids in the mechanism of bacterial species selective toxicity by two alpha/beta-antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1343–1350. doi: 10.1016/j.bbamem.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Green J, Goldsbury C, Min T, Sunderji S, Frey P, Kistler J, Cooper G, Aebi U. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J. Mol. Biol. 2003;326:1147–1156. doi: 10.1016/s0022-2836(02)01377-3. [DOI] [PubMed] [Google Scholar]