Abstract
Infection with Plasmodium falciparum during pregnancy results in the adherence of infected red blood cells (IRBCs) in placenta, causing pregnancy-associated malaria with severe health complications in mothers and fetuses. The chondroitin 4-sulfate (C4S) chains of very low-sulfated chondroitin sulfate proteoglycans (CSPGs) in placenta mediate the IRBC adherence. While it is known that partially sulfated but not fully sulfated C4S effectively binds IRBCs, structural interactions involved remain unclear and are incompletely understood. In this study, structurally defined C4S oligosaccharides of varying sulfate contents and sizes were evaluated for their ability to inhibit the binding of IRBCs from different P. falciparum strains to CSPG purified from placenta. The results clearly show that, with all parasite strains studied, dodecasaccharide is the minimal chain length required for the efficient adherence of IRBCs to CSPG and two 4-sulfated disaccharides within this minimal structural motif are sufficient for maximal binding. Together these data demonstrate for the first time that the C4S structural requirement for IRBC adherence is parasite strain-independent. We also show that the carboxyl group on nonreducing end glucuronic acid in dodecasaccharide motif is important for IRBC binding. Thus, in oligosaccharides containing terminal 4,5-unsaturated glucuronic acid, the nonreducing end disaccharide moiety does not interact with IRBCs due to the altered spatial orientation of carboxyl group. In such C4S oligosaccharides, 14 mer but not 12 mer constitutes the minimal motif for inhibition of IRBC binding to placental CSPG. These data have important implications for the development and evaluation of therapeutics and vaccine for placental malaria.
In Plasmodium falciparum infected individuals, parasite-infected red blood cells (IRBCs)1 sequester in the microvascular capillaries of vital organs, including brain, liver, kidney, causing cerebral and other organ-related fatal malaria (1–3). Many studies have demonstrated that the sequestration of IRBCs is mediated by cell adhesion molecules, such as CD36, ICAM-1, VCAM-1, E-selectin and P-selectin, expressed on the endothelial surface (4–7). In pregnant women, however, IRBCs selectively adhere in the placenta, causing a number of severe adverse clinical conditions, including low birth weight, abortion, still birth, and maternal anemia and death (8–11). IRBC adherence in the placenta is mediated by a low-sulfated CSPG present abundantly in the intervillous space and at low levels on the syncytiotrophoblast surface (12–14). The occurrence and distribution pattern of low-sulfated CSPGs in human placenta mirror the pattern of IRBC adherence in P. falciparum infected placentas (14).
Previously, we and others have studied C4S-IRBC interactions involved in the adherence of IRBCs in placenta (15–19). Although the presence of both 4-sulfated and non-sulfated disaccharide moieties within the C4S binding motif is known to be critical for IRBC binding, the minimum chain length and number of 4-sulfate groups required for IRBC binding still remain unclear. Earlier studies have suggested that either a tetradecasaccharide/higher oligosaccharide motif or a dodecasaccharide is required for IRBC binding (15–18). Recently, it was reported that, var2-CSA PfEMP1, parasite protein suggested to mediate IRBC adherence to C4S, binds to lipid-conjugated octasaccharides, and that decasaccharides could maximally inhibit IRBC binding to placental tissues (20). Thus, it was suggested that an octa- and a decasaccharide, respectively, are the minimal motifs involved in PfEMP1 binding and in inhibition of IRBC adherence. Further, the level of sulfate group required for IRBC binding was reported to vary with polymorphic PfEMP1 expressed by different parasite strains (20). However, since the C4S chains of placental CSPG are very low sulfated with sulfate groups located exclusively at the C-4 position of N-acetylgalactosamine, thus presenting similar receptor structural features, it is likely that the C4S-IRBC structural interactions are conserved. In this study, to gain further insight into C4S structural requirements for IRBC binding to CSPG, we performed a detailed study using structurally defined oligosaccharides prepared from three different methods, including those obtained by an enzymatic procedure, CSPG purified from placenta, and parasites selected for high binding strength to placental CSPG. Our data clearly demonstrate that, in all parasite strains studied, dodecasaccharide (12 mer) is the minimum C4S chain length for IRBC binding to placental CSPG. The data also show that, regardless of parasite strains, two 4-sulfated disaccharides within the 12 mer minimal motif is sufficient for maximal inhibition of IRBC binding and that if C4S oligosaccharides contain 4,5-unsaturated GlcA at the nonreducing termini, 14 mer is the minimal chain length for efficient interaction with IRBCs.
EXPERIMENTAL PROCEDURES
Materials
Proteus vulgaris chondroitinase ABC (120 units/mg) and Streptococcus dysgalactiae hyaluronidase (0.5 units/vial), sturgeon notochord C4S (98% 4-sulfated, 1.5% 6-sulfated, and 0.5% non-sulfated disaccharides) were purchased from Seikagaku America (Falmouth, MA). Bovine testicular hyaluronidase (400–1000 units/mg), bovine tracheal CSA (bCSA, 53% 4-sulfated, 39% 6-sulfated and 8% non-sulfated disaccharides) and N,O-bis(trimethylsilyl)acetamide were obtained from Sigma-Aldrich. DEAE-Sepharose and PD-10 columns were from Amersham Pharmacia; Bio-Gel P-4 and Bio-Gel P-6 were from Bio-Rad; Polystyrene Petri dishes (Falcon 1058) were from Becton-Dickinson Labware. Human term placentas were obtained from the Maternity Section of the Hershey Medical Center Hospital, Hershey. Human blood and serum for parasite culturing were obtained from the Blood Bank of the Hershey Medical Center.
Purification of Low-sulfated CSPG from Human Placenta
The low-sulfated CSPG was purified from human term placentas as described earlier (12, 13).
Preparation of C4S with Different 4-Sulfate Content
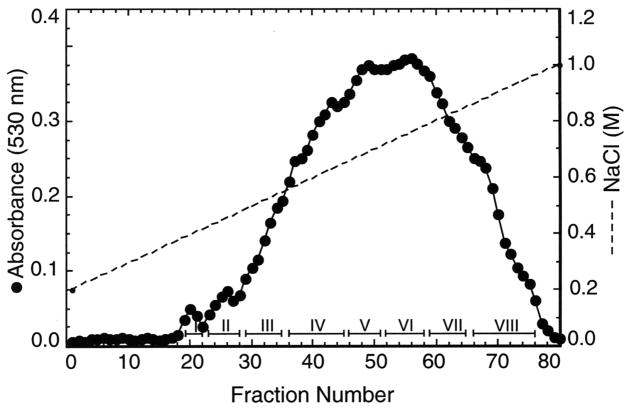
bCSA (1 g), a heterogeneous mixture of CS copolymers differing in 4- and 6-sulfate contents, was regioselectively 6-O-desulfated as reported previously (17). Briefly, the pyridinium salt of bCSA, prepared by chromatography on Dowex 50 W-X 8 (H+) followed by neutralization with pyridine and lyophilization, was 6-O-desulfated using 40 ml of N,O-bis(trimethylsilyl)acetamide in 200 ml of anhydrous pyridine (17). The partially sulfated C4S (500 mg) was chromatographed on a DEAE-Sepharose column (2.5 X 17 cm) equilibrated with 50 mM sodium acetate, 200 mM NaCl, pH 5.5. The bound C4S was eluted with a linear gradient of 0.2–1.0 M NaCl in the same buffer. Fractions (4.5 ml) were collected and aliquots analyzed for uronic acid content by the carbazole method (21). The C4S-containing fractions were pooled as indicated in Figure 2, dialyzed, and lyophilized.
FIGURE 2.
Fractionation of the partially sulfated C4Ss obtained by the regioselective 6-O-desulfation of bCSA by DEAE-Sepharose chromatography. The disaccharide composition of the C4S fractions is given in Table 1.
Preparation of Oligosaccharides By Digestion of C4S with S. dysgalactiae Hyaluronidase
Partially sulfated C4S (~10 mg each), obtained from regioselective 6-O-desulfation of bCSA, were treated with S. dysgalactiae hyaluronidase (200 milliunits) in 600 μl of 100 mM sodium phosphate buffer, pH 6.2, containing 0.02% BSA at 37 ºC for 36 h (13, 22). The enzyme digests were heated in a boiling water bath for 5 min, centrifuged, and chromatographed on Bio-Gel P-6 columns (1.5 X 70 cm) using 0.1 M acetic acid, 0.1 M pyridine, pH 5.5. Two-ml fractions were collected and aliquots analyzed by polyacrylamide gel electrophoresis. Selected oligosaccharide fractions were fractionated further on a 4.6 X 250 mm amine-bonded YMC-Pack PA HPLC column (YMC, Kyoto, Japan) using Hitachi L-6200 HPLC (Hitachi, Tokyo, Japan). The column was eluted with linear gradients of 0 to 1 M NaCl in water at a flow rate of 0.5 ml/min for 90 min. The elution of oligosaccharides was monitored by recording absorbance at 215 nm, and fractions of 0.5 ml were collected. The separated oligosaccharide fractions were desalted using PD-10 columns and dried using Speed-Vac concentrator. The oligosaccharides were analyzed by mass spectrometry to determine the chain length and level of sulfation. The oligosaccharides were also analyzed for disaccharide composition, and quantified by hexosamine analysis.
Preparation of CSA Oligosaccharides of Varying Sizes
Bovine CSA (10 mg), dissolved in 1 ml of 100 mM sodium acetate, 150 mM sodium chloride, pH 5.0, was treated with testicular hyaluronidase (800 units) at 37 ºC for 3 h. The oligosaccharides of varying sizes formed were fractionated on Bio-Gel P-6 column and analyzed by gel electrophoresis as described (17).
Preparation of C4S Dodecasaccharides of Varying Sulfate Contents
C4S (250 mg) consisting of 75% 4-sulfated, 1% 6-sulfated and 24% non-sulfated disaccharides (Fraction VII in Figure 2 and Table 1) was treated with testicular hyaluronidase (24 mg) in 5 ml of 100 mM sodium acetate, 150 mM sodium chloride, pH 5.0, at 37 ºC for 24 h. The digest was chromatographed in four aliquots on calibrated Bio-Gel P-4 columns (1.8 X 110 cm) using 0.1 M acetic acid, 0.1 M pyridine, pH 5.5. Fractions (3 ml) were collected and aliquots analyzed for uronic acid content (21). The fractions corresponding to 12 mers were pooled, dried, and chromatographed on Amberlite IR-120 columns. The 12 mers were neutralized with pyridine, lyophilized, and partially desulfated by solvolysis in 10% Me2SO as reported previously (17). The oligosaccharide mixture was fractionated by HPLC on a 4.6 X 250 mm amine-bonded YMC-Pack PA-II (YMC, Kyoto, Japan) coupled to inline UV detector. The elution was at a flow rate of 1 ml/min with a linear gradient of 0.1–1 M NaH2PO4 for 90 min and at 1 M NaH2PO4 for 5 min. The oligosaccharide fractions were desalted on PD-10 columns and analyzed by mass spectrometry. The composition was determined by HPLC analysis of disaccharides formed by chondroitinase ABC digestion. The oligosaccharides were quantified by hexosamine analysis.
Table 1.
Disaccharide composition of partially sulfated C4S fractionsa
| Disaccharide composition
|
|||
|---|---|---|---|
| C4S fraction | Δdi-4S | Δdi-0S | Δdi-6S |
| % mol proportionb | |||
| I | 3 | 97 | 0 |
| II | 9 | 91 | 0 |
| III | 14 | 86 | 0 |
| IV | 26 | 74 | 0 |
| V | 35 | 65 | 0 |
| VI | 52 | 48 | 0 |
| VII | 75 | 24 | 1 |
| VIII | 86 | 9 | 5 |
The partially 4-sulfated C4S fractions obtained by DEAE-Sepharose chromatography (see Figure 2).
calculated from peak areas in the chromatogram assuming that molar extinction coefficients of non-sulfated, 4-sulfated and 6-sulfated disaccharides are similar.
Enzymatic Synthesis of C4S Dodecasaccharides with Different Sulfate Contents
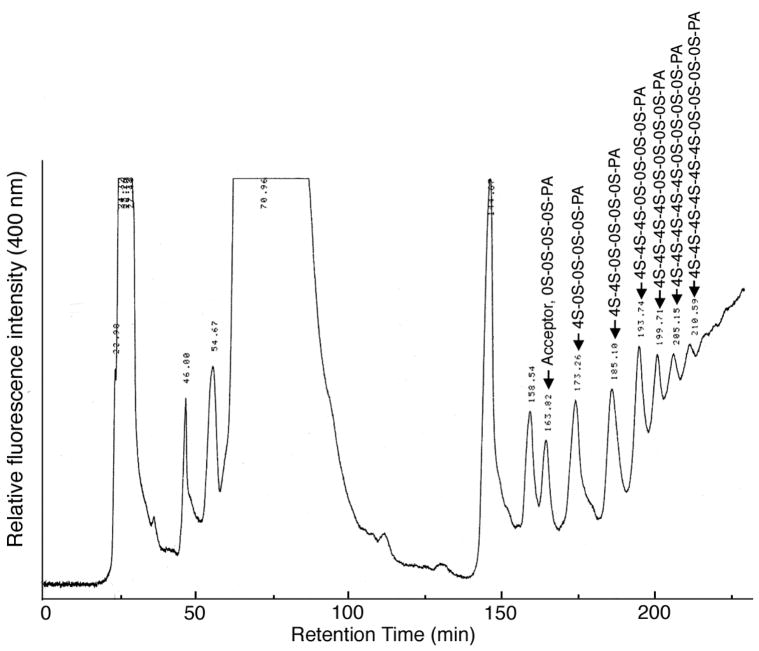
The oligosaccharides were prepared by testicular hyaluronidase-catalyzed transglycosylation involving transfer of C4S disaccharide to acceptor oligosaccharides as reported previously (23–26). Briefly, pyridylaminated (PA) chondroitin oligosaccharides (See Table 2, 10–20 nmol) were incubated with 50–100 μg of C4S from Sturgeon notochord and 5–10 U of bovine testicular hyaluronidase in 250 μl 100 mM TrisHCl, pH 7.0, at 37°C for 1 h. The reaction mixture was heated on a boiling water bath for 3 min and chromatographed using Hitachi L-6200 HPLC system with a fluorescence detector on a TSKgel Amide-80 (21.5 X 300 mm) TOSOH column equilibrated with 0.1 M sodium acetate-acetic acid buffer, pH 4.0, acetonitrile (20:80, v/v) at 40°C at a flow rate of 3 ml/min. After sample injection, the above solvent was mixed linearly with 0.1 M sodium acetate-acetic acid buffer, pH 4.0, acetonitrile (50/50, v/v) over a period of 330 min and the eluted oligosaccharides were detected by 320 nm excitation and 400 nm emission. The oligosaccharides fractions were pooled, lyophilized, and quantified by determining GalN content by Dionex HPLC after acid hydrolysis (see below).
Table 2.
The C4S oligosaccharides synthesized by testicular hyaluronidase transglycosylation reaction
| Donor | Acceptor | Productsa |
|---|---|---|
| C4S | 0S-0S-0S-0S-0S-PA | 4S-0S-0S-0S-0S-0S-PA |
| 4S-4S-0S-0S-0S-0S-0S-PA | ||
| 4S-4S-4S-0S-0S-0S-0S-0S-PA | ||
| 4S-4S-4S-4S-0S-0S-0S-0S-0S-PA | ||
| C4S | 0S-0S-0S-0S-PA | 4S-0S-0S-0S-0S-PA |
| 4S-4S-0S-0S-0S-0S-PA | ||
| 4S-4S-4S-0S-0S-0S-0S-PA | ||
| 4S-4S-4S-4S-0S-0S-0S-0S-PA | ||
| 4S-4S-4S-4S-4S-0S-0S-0S-0S-PA | ||
| C4S | 0S-0S-0S-PA | 4S-0S-0S-0S-PA |
| 4S-4S-0S-0S-0S-PA | ||
| 4S-4S-4S-0S-0S-0S-PA | ||
| 4S-4S-4S-4S-0S-0S-0S-PA | ||
| 4S-4S-4S-4S-4S-0S-0S-0S-PA | ||
| C4S | 4S-0S-0S-PA | 4S-4S-0S-0S-PA |
| 4S-4S-4S-0S-0S-PA | ||
| 4S-4S-4S-4S-0S-0S-PA | ||
| 4S-4S-4S-4S-4S-0S-0S-PA | ||
| C4S | 0S-4S-4S-PA | 4S-0S-4S-4S-PA |
| 4S-4S-0S-4S-4S-PA | ||
| 4S-4S-4S-0S-4S-4S-PA | ||
| 4S-4S-4S-4S-0S-4S-4S-PA | ||
| 4S-4S-4S-4S-4S-0S-4S-4S-PA | ||
| 4S-4S-4S-4S-4S-4S-0S-4S-4S-PA |
The oligosaccharides indicated in the bold are used for the inhibition analysis.
Gel Electrophoresis
The oligosaccharide fractions (4–6 μg each/well) obtained from the Bio-Gel P-6 column chromatography of S. dysgalactiae hyaluronidase digest of C4S or testicular hyaluronidase-treated CSA were analyzed by electrophoresis on 10% polyacrylamide gels (15 X 16 cm) using 100 mM borate buffer, 2 mM EDTA, pH 8.3. The gels were stained with 0.03% Alcian blue followed by ammoniacal silver (17).
Disaccharide Compositional Analysis of C4S Oligosaccharides
The oligosaccharides (5–10 μg) were digested with chondroitinase ABC (5 milliunits) and the released unsaturated disaccharides were analyzed on a 4.6 X 250 mm amine-bonded silica PA03 column (YMC Inc., Milford, MA) using Waters 600E HPLC system (Milford, MA) as described previously (12, 17).
Determination of GalNAc Contents in C4S Oligosaccharides
Aliquots of the purified oligosaccharide fractions (2–4 μg each) were hydrolyzed with 400 μl of 3 M HCl at 100 ºC for 4 h. The hydrolysates were dried in a Speed-Vac and analyzed by high-pH anion-exchange chromatography on a CarboPac PA1 column (4 X 250 mm) using Dionex BioLC system equipped with pulsed amperometric detector (27). The column was eluted with 20 mM sodium hydroxide and the GalN was quantified by determining the response factor obtained by analyzing a standard solution of GalN.
Mass Spectrometry
The mass spectral analysis of C4S oligosaccharides was performed on API-100 LC/MS (single quadruple; PE-Sciex, Thornhill, Ontario, Canada) using nitrogen as ionization gas source as described earlier (28). The mass spectrometer was operated in the negative ion mode. The ion-spray voltage was set at −3500 V, and the orifice voltage was −55 V. The samples were introduced in 50% acetonitrile/0.05% formic acid. A JASCO amilic 100N micro HPLC syringe pump was used to deliver the samples at a flow rate of 3 μl/min. The scanning was performed from m/z 300 to 1200 during the 1-min scan. The reconstruction of mass spectrogram was done using AP1 100/300 SWV1.1.1 data processing system.
P. falciparum Parasites
The parasite strains used in this study were: FCR3, CS2, NF54 (all obtained from MR4, ATCC), a 3D7 clone used in previous studies (13, 17), and a placental isolate obtained as described previously (29). The C4S-adherent parasites from laboratory strains were selected for high binding strengths by several rounds of panning on plastic Petri dishes coated with CSPG purified from human placenta. The adherent parasites were cultured using type O-positive human red blood cells and human plasma at 3% hematocrit in RPMI 1640 medium. The parasites were synchronized with 5% sorbitol as described previously (17). IRBCs at the early trophozoite stage were used for the assays.
IRBC-CSPG Adhesion Inhibition Assays
The purified placental CSPG (0.2 μg/ml in PBS, pH 7.2) was coated onto plastic Petri dishes as circular spots (0.2–0.4 cm diameter), incubated at 4°C overnight, and blocked with 1% BSA at room temperature for 2 h. The spots were overlaid with a 2% suspension of cell pellet (parasite culture with 20–30% parasitemia) in PBS, pH 7.2, pre-incubated for 30 min at room temperature with the C4S oligosaccharides or C4S polymer at the indicated concentrations. After 30 min at room temperature, the unbound cells were washed, and the bound cells fixed with 2% glutaraldehyde, stained with Giemsa, and counted under light microscope (17).
Statistical Analysis
Oligosaccharide inhibitory data are plotted as mean values + standard error of means (SEM). Analysis of results was performed by one-way ANOVA using Prism GraphPad 3.0. p values of <0.05 were considered statistically significant.
RESULTS
C4S Chain Length Requirement for Binding of IRBCs from Different P. falciparum Strains to Placental CSPG
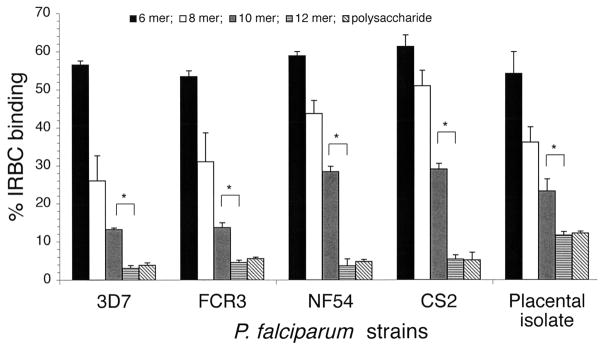
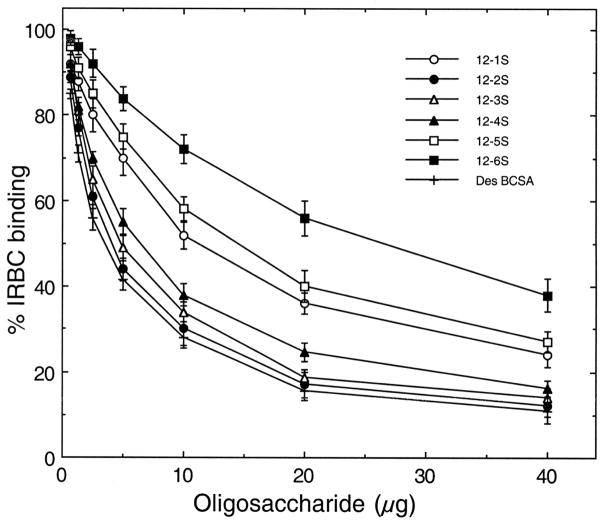
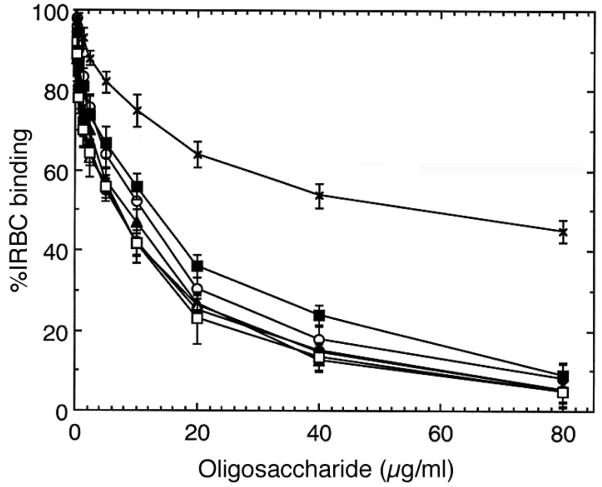
Oligosaccharides obtained by partial depolymerization of bovine tracheal CSA have been shown to inhibit IRBC binding to placental CSPG or to bovine tracheal CSA in a size-dependent manner (17, 18, 20). To determine whether C4S chain length requirement for IRBC binding to placental CSPG varies with different parasite strains, we prepared CSA oligosaccharides of varying sizes and characterized by gel electrophoresis (17). The oligosaccharides were evaluated for their ability to inhibit binding of IRBCs from four laboratory P. falciparum strains and one placental parasite isolate to placental CSPG. With all parasites strains analyzed, only 12 mers and higher oligosaccharides could maximally inhibit IRBC binding and their inhibitory capacity was similar to that of C4S polysaccharide containing 35% 4-sulfated disaccharide moiety (Figure 1). In all cases, the extent of inhibition by deca- (10 mers), octa- (8 mers) and hexasaccharides (6 mers) were significantly lower. These results suggest that the structural elements of parasite adhesive protein(s) involved in IRBC binding to placental CSPG are conserved and are independent of parasite strains.
FIGURE 1.
Inhibition of P. falciparum IRBC binding to placental CSPG by C4S oligosaccharides. Inhibition was performed using 80, 40, 20, or 10 μg/ml solutions of oligosaccharides or polysaccharide. The assays were performed two times each in duplicates. The extent of inhibition by oligosaccharides is expressed as IRBC binding relative to that of control and values are plotted as means + SEM. Shown is representative graph for the inhibitory activity of oligosaccharides at 40 μg/ml concentration for all parasites. For placental isolate, inhibition was also performed at 100 and 120 μg/ml oligosaccharide concentration; 12 mers and polysaccharide completely inhibited IRBC binding only at 120 μg/ml. In the case of laboratory strains, 80 μg/ml of 12 mers and of polysaccharide could fully inhibit binding (not shown). *, p <0.01.
Sulfate Group Requirement for IRBCs from different P. falciparum Stains Binding to Placental CSPG
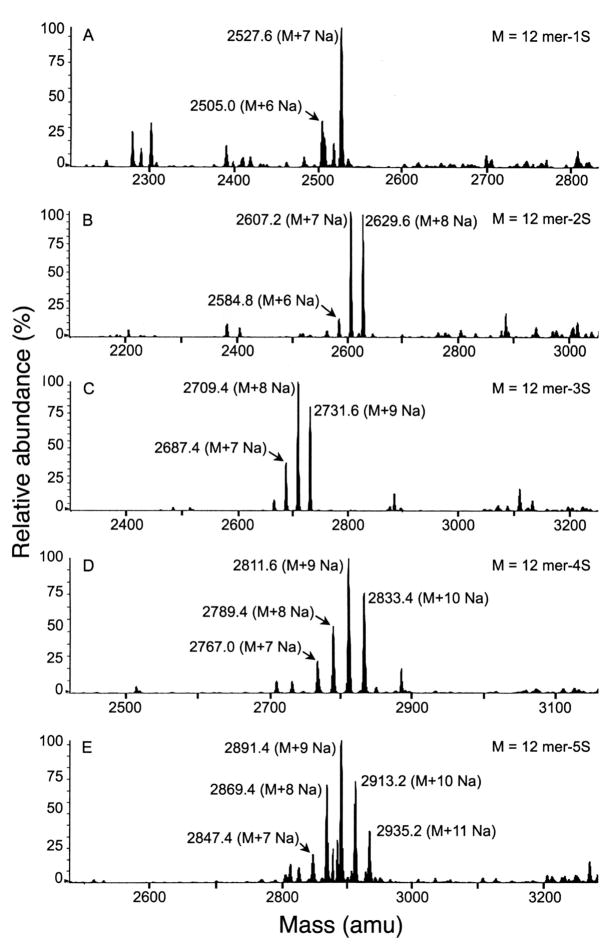
To conclusively determine the level of sulfation required for maximal interaction of IRBC binding with placental CSPG, we prepared oligosaccharides containing different levels of sulfate group by two different methods, structurally characterized the oligosaccharides, and analyzed their inhibitory capacities. bCSA was 6-O-desulfated regioselectively and the product was fractioned on DEAE-Sepharose chromatography (Figure 2). The C4S fraction (Fraction VII in Table 1) consisting of 75% 4-sulfated and 24% non-sulfated disaccharides was partially depolymerized with testicular hyaluronidase and the oligosaccharide mixture thus obtained was fractionated by size-exclusion chromatography using Bio-Gel P-4 (not shown). The oligosaccharide fraction containing predominantly 12 mers was partially desulfated by solvolysis to yield a mixture of 12 mers with varying contents of 4-sulfate groups (17). HPLC fractionation of the oligosaccharide mixture furnished 12 mers containing one, two, three, four, or five sulfated disaccharides. The size and sulfate content of the purified oligosaccharides were determined by mass spectrometry (Figure 3; 28, 30, 31). Disaccharide compositional analysis showed that the sulfate groups in the oligosaccharides are exclusively at C4 of GalNAc residues. A fully 4-sulfated 12 mer was prepared by testicular hyaluronidase treatment of sturgeon notochord C4S followed by gel filtration chromatography (17). The oligosaccharide was further characterized by disaccharide compositional analysis and by gel electrophoresis (17).
FIGURE 3.
Mass Spectrometry of C4S dodecasaccharides obtained by the testicular hyaluronidase treatment of C4S with 75% 4-sulfate followed by Bio-Gel P-4 chromatography Panels A–E, respectively, represent mass spectrum of the dodecasaccharides containing 1, 2, 3, 4, or 5 sulfate groups. The identity of the peaks is also indicated in the spectra.
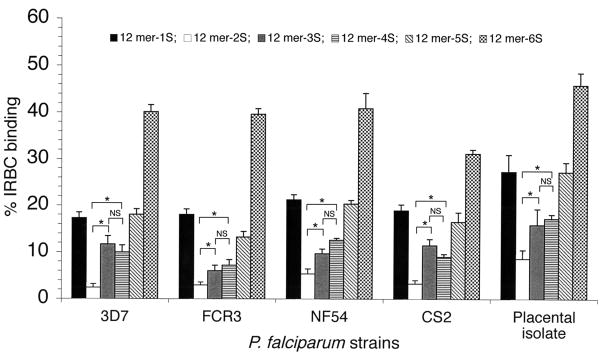
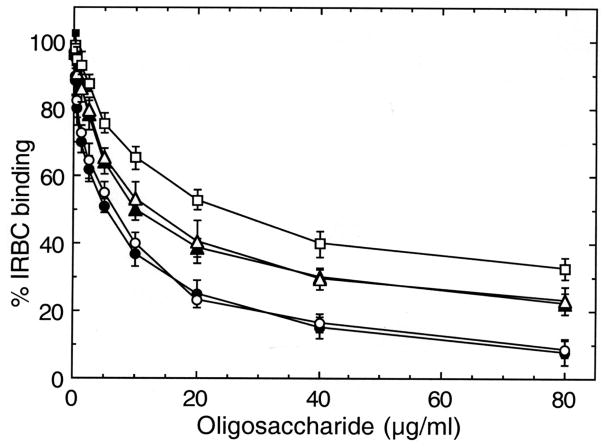
The 12 mers having different levels of 4-sulfated disaccharides were quantified based on GalNAc content as determined by hexosamine compositional analysis. The ability of oligosaccharides to inhibit adhesion of IRBCs from different parasite strains to placental CSPG was evaluated. In all parasite strains analyzed, 12 mers having two 4-sulfate groups could maximally inhibit IRBC binding to placental CSPG and, in each case, the inhibitory activity was comparable to that of C4S polymer (Figure 4 and not shown). The inhibitory activity of 12 mers with three or four 4-sulfate groups was significantly lower than 12 mers with two 4-sulfate groups. In contrast, 12 mers with one, five or six 4-sulfated disaccharide moiety were less inhibitory. These data demonstrate that two sulfate groups within the minimal 12 mer motif are sufficient for the maximal binding of IRBCs to CSPG. The results further indicate that at least two disaccharide moieties of the C4S 12 mers should be non-sulfated for effective IRBC binding, and that these structural requirements for maximal adhesion of IRBCs to CSPG are strain independent.
FIGURE 4.
Inhibition of P. falciparum IRBC binding to placental CSPG by C4S dodecasaccharides containing one to six 4-sulfate groups. Inhibition was performed with 80, 40, 20 or 10 μg/ml solutions of dodecasaccharides of varying sulfate contents or polysaccharide. The assays were performed two times each in duplicates. The extent of inhibition by oligosaccharides of varying sulfate content is expressed as IRBC binding relative to that of the control. Values are plotted as means + SEM. In the case of placental isolate, complete inhibition was observed at 120 μg/ml of 12 mer-2S and polysaccharide, whereas in laboratory strains, 80 μg/ml of 12 mer-2S and of polysaccharide could fully inhibit binding (not shown). NS, not significant, *, p <0.01.
To further evaluate the level of sulfate group requirement for IRBC adhesion, C4S 12 mers with different sulfate contents were prepared by enzymatic synthesis using hyaluronidase-catalyzed transglycosylation procedure developed by Takagaki and coworkers (23–26, 28). The approach involves transfer of C4S disaccharide moieties to acceptor oligosaccharides and purification by HPLC (Figure 5). Thus, five oligosaccharides containing one to five sulfate groups were prepared (Table 2), quantified by GalN content, and analyzed for their capacity to inhibit IRBC binding to placental CSPG (Figure 6). In agreement with the above results, 12 mer containing two sulfate groups could efficiently inhibit IRBC binding to placental CSPG. 12 mers having 3 and 4 sulfate groups could also inhibit binding, but their activity was 10–15% lower than 12 mer with two sulfate groups. The 12 mer with one or five sulfate groups showed substantially lower inhibitory capacity as compared to that with two sulfate groups. These results are consistent with those obtained from 12 mers prepared from testicular hyaluronidase hydrolysis of C4S.
FIGURE 5.
HPLC fractionation of C4S oligosaccharides prepared by testicular hyaluronidase-catalyzed transglycosylation reaction. The structures of the 12 mers are shown in bold in Table 2.
FIGURE 6.
Inhibition of P. falciparum IRBC binding to CSPG by dodecasaccharides prepared by transglycosylation reaction. The C4S 12 mers containing different 4-sulfate content, prepared by transglycosylation reaction followed by HPLC fractionation (see Figure 6 and Table 2), were analyzed for inhibition of IRBC binding to the placental CSPG. The analysis was done three times each in duplicate and the results are presented as mean + SEM. +, partially sulfated C4S with 40% 4-sulfated and 60% nonsulfated disaccharides; ○, oligosaccharide 4S-0S-0S-0S-0S-0S-PA; ●, 4S-4S-0S-0S-0S-0S-PA;Δ, 4S-4S-4S-0S-0S-0S-PA;σ, 4S-4S-4S-4S-0S-0S-PA; ■, 4S-4S-4S-0S-4S-4S-PA; ▪, 4S-4S-4S-4S-4S-4S.
The Carboxyl Group of Nonreducing End Glucuronic Acid in C4S Oligosaccharides Interacts With IRBCs
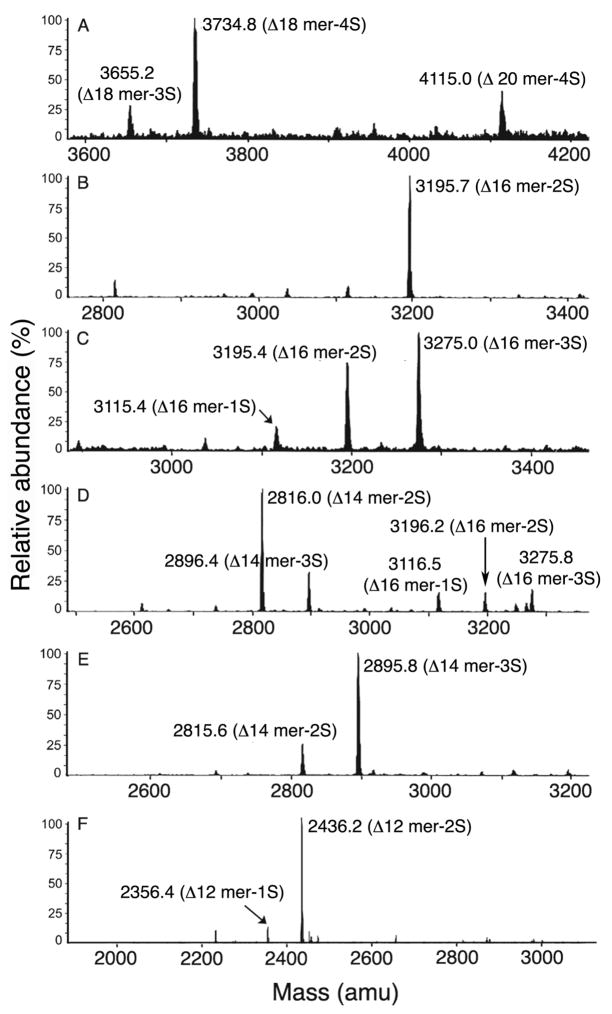
We previously showed that the carboxyl groups of C4S are critical for IRBC binding (32), but could not assess the contribution of individual group because of the practical difficulty in modifying specific carboxylic groups of C4S. However, in the present study, we aimed to determine the contribution of nonreducing end carboxyl group by preparing oligosaccharides with altered orientation of the terminal carboxyl group due to the introduction of double bond on GlcA. To achieve this goal and to further evaluate the inhibitory capacities of low sulfated C4S oligosaccharides, a C4S fraction consisting of 14% sulfated and 86% non-sulfated disaccharide moieties (Fractions III in Figure 2 and Table 1) was digested with S. dysgalactiae hyaluronidase. This is an endo-β-N-acetylhexosaminyl lyase that can degrade non-sulfated regions of C4S and produce oligosaccharides with 4,5-unsaturated GlcA residue at the nonreducing end (22). Bio-Gel P-6 chromatography of the enzyme digest furnished, in each case, mainly decasaccharides and higher oligosaccharides with ~30% 4-sulfate (fractions 22 to 29 in Figure S1 and Table S1 of the supporting information). The enzyme converted the non-sulfated regions of C4S polymers into di- and tetrasaccharides (fractions 45 to 52). HPLC of the selected Bio-Gel P-6 fractions yielded oligosaccharide fractions ranging in size from dodecasaccharides to eicosasaccharides (Figure S2 of the supporting information). The oligosaccharides were further characterized by mass spectrometry (Figure 7 and Table 3). The fractions were designated as Δ-oligosaccharides to indicate the presence of 4,5-unsaturated GlcA at the nonreducing end.
FIGURE 7.
Mass spectral analysis of C4S Δ-oligosaccharide subfractions obtained by HPLC of S. dysgalactiae hyaluronidase digestion products of C4S (see Figure S3 of the supporting information). Panel A, B, C, D, and E, respectively, represent mass spectrum of subfractions III-F25c, III-F26b, III-F26c, III-F28b, III-F28c, and III-F31b. The data is summarized in Table 2.
Table 3.
Compositions of C4S Δ-oligosaccharide subfractions determined by mass spectrometry
| Mass
|
||||
|---|---|---|---|---|
| Δ-Oligosaccharide subfractiona | Calculated | Found | Abundance (%) | Structure assignment |
| III-F25c | 3654.6 | 3655.4 | 16 | Δ18-mer 3Sb |
| 3734.6 | 3734.4 | 51 | Δ18-mer 4S | |
| 4114.0 | 4114.6 | 33 | Δ20-mer 4S | |
| III-F26b | 3195.2 | 3195.7 | 100 | Δ16-mer 2S |
| III-F26c | 3115.2 | 3115.4 | 10 | Δ16-mer 1S |
| 3195.2 | 3195.4 | 37 | Δ16-mer 2S | |
| 3275.2 | 3275.4 | 53 | Δ16-mer 3S | |
| III-F27b | 2815.8 | 2815.6 | 45 | Δ14-mer 2S |
| 3035.2 | 3036.0 | 12 | Δ16-mer 0S | |
| 3115.2 | 3116.8 | 16 | Δ16-mer 1S | |
| 3195.2 | 3196.2 | 27 | Δ16-mer 2S | |
| III-F27c | 2815.8 | 2815.6 | 12 | Δ14-mer 2S |
| 2895.8 | 2895.8 | 26 | Δ14-mer 3S | |
| 3115.2 | 3117.2 | 07 | Δ16-mer 1S | |
| 3195.2 | 3195.2 | 19 | Δ16-mer 2S | |
| 3275.2 | 3275.4 | 36 | Δ16-mer 3S | |
| III-F27d | 3275.2 | 3275.0 | 14 | Δ16-mer 3S |
| 3355.2 | 3355.6 | 31 | Δ16-mer 4S | |
| 3734.6 | 3734.8 | 55 | Δ18-mer 4S | |
| III-F28b | 2815.8 | 2816.0 | 49 | Δ14-mer 2S |
| 2895.8 | 2896.4 | 20 | Δ14-mer 3S | |
| 3115.2 | 3116.5 | 10 | Δ16-mer 1S | |
| 3195.2 | 3196.2 | 10 | Δ16-mer 2S | |
| 3275.2 | 3275.8 | 11 | Δ16-mer 3S | |
| III-F28c | 2815.8 | 2815.6 | 19 | Δ14-mer 2S |
| 2895.8 | 2895.8 | 81 | Δ14-mer 3S | |
| III-F28d | 2895.8 | 2895.6 | 13 | Δ14-mer 3S |
| 2975.8 | 2976.6 | 29 | Δ14-mer 4S | |
| 3195.2 | 3195.6 | 21 | Δ16-mer 2S | |
| 3355.2 | 3355.4 | 37 | Δ16-mer 4S | |
| III-F29c | 2436.4 | 2435.8 | 100 | Δ12-mer 2S |
Subfractions from HPLC of Δ-oligosaccharide fractions obtained are presented in Table S1, and Figures S1 and S2 of the supporting information.
1S, 2S, 3S, and 4S in oligosaccharide length abbreviations refer to number of 4-sulfated disaccharide moiety in oligosaccharides; the remainder is non-sulfated disaccharides.
In efforts to determine the contribution of carboxyl groups for IRBC binding, we analyzed the inhibitory activity of Δ-12 mers consisting of two 4-sulfated and four non-sulfated disaccharides (fraction III-F29c in Table 3). The activity of Δ-12 mers was substantially lower than that of 12 mer containing two 4-sulfated disaccharide moiety and unmodified GlcA at the nonreducing end (Figure 8). However, the activity of the Δ-12 mers was comparable to that of 10 mers with unmodified GlcA at the nonreducing end. Further, the activity of Δ-14 mers was comparable to that of C4S 12 mers with unmodified terminal GlcA. These data indicated that, in the case of Δ-oligosaccharides, the nonreducing end disaccharide moieties essentially do not interact with IRBCs and therefore, Δ-14 mers comprise the minimum C4S chain length required for complete inhibition of IRBC binding to C4S.
FIGURE 8.
Inhibition of P. falciparum IRBC binding to CSPG by Δ-oligosaccharide fractions. Δ-Oligosaccharide subfractions obtained by HPLC (Figure S2 of the supporting information) and characterized by mass spectrometry (Table 2) were analyzed for inhibition of IRBC binding to placental CSPG as outlined in Figure 1. Analysis was performed two times each in duplicates and the results are presented as mean + SEM. λ, partially sulfated C4S containing 35% 4-sulfated, 65% non-sulfated; ○, dodecasaccharide with unmodified GlcA at the nonreducing end (12 mer-2S in Figure 3); σ,Δ-dodecasaccharide (III-F29c);Δ, decasaccharide with unmodified GlcA at the nonreducing end (sample previously prepared from bovine tracheal C4S, Ref. 17); ■, Δ-decasaccharides obtained by S. dysgalactiae hyaluronidase digestion of C4S with 14% 4-sulfate followed by Bio-Gel P-6 chromatography.
Further Assessment of the Level of Sulfate Group Required for Optimal Binding of IRBCs to Placental CSPG
To confirm that two sulfate groups are sufficient for the effective binding of IRBC to placental CSPG, Δ-oligosaccharides ranging in size from tetradeca- to eicosasaccharides (Table 3) having two, three, or four 4-sulfated disaccharides were tested for inhibition of IRBC binding to CSPG. All oligosaccharides could efficiently inhibit IRBC binding and their inhibitory capacities were similar to each other (Figure 9). Fraction (III-F27b) containing appreciable amounts of non-sulfated and monosulfated oligosaccharides showed lower activity. Thus, these results conclusively show that oligosaccharides with two 4-sulfate groups per 12 mer could inhibit IRBC binding to CSPG as efficiently as that by C4S polysaccharide.
FIGURE 9.
Inhibition of P. falciparum IRBC binding to CSPG by C4S oligosaccharides containing different ratio of sulfated and non-sulfated disaccharides. Inhibition with oligosaccharides (see Figure 5 and Table 2)) was performed two times each in duplicate and the results are presented as mean + SEM. ●, partially sulfated C4S containing 35% 4-sulfated, 65% non-sulfated disaccharides; ○, oligosaccharide III-F26c; σ, oligosaccharide III-F27d; Δ, oligosaccharide III-F28c; ▪, oligosaccharide III-F27b; ■, oligosaccharide III-F26b; x, fully 4-sulfated C4S dodecasaccharide (12 mer-6S prepared by testicular hyaluronidase digestion of fully 4-sulfated CS). Fractions III-F25c, III-F27c, III-F28b and IIIF28d outlined in Table 2 were also analyzed and they exhibited maximum inhibitory activity (not shown). The inhibitory activity of 12 mer with 2S is similar to that of the partially 4-sulafted C4S shown in closed circle.
DISCUSSION
In this study, using chemically defined C4S oligosaccharides of varying sizes and sulfate contents, we investigated whether P. falciparum IRBC binding to human placental CSPG is structurally conserved among different parasite strains. The results show that C4S structural requirements for IRBC binding are strain independent. Adherent parasites from several P. falciparum strains and a placental isolate interacted maximally with C4S 12 mers and in all cases 12 mers with two sulfate groups could maximally inhibit IRBC binding to placental CSPG. Twelve mers with three or four 4-sulfated disaccharides could also effectively interact with IRBCs from various parasite strains, but their inhibitory activity was found to be significantly lower than that of 12 mers with 2 sulfate groups. In contrast, 12 mers containing one, five or six 4-sulfated disaccharides interacted with IRBCs to substantially lesser extents. Oligosaccharides obtained by three different procedures, including synthetic 12 mers of varying sulfate contents prepared by enzymatic transglycosylation reaction, showed similar inhibition pattern. Thus, our data conclusively demonstrate that dodecasaccharide is the minimum chain length and two sulfate groups are sufficient for IRBC binding to C4S. The C4S 12 mer containing two sulfated disaccharides can exist in 15 different isomeric forms. Further studies involving the synthesis of all possible 12 mer isomers having two sulfate groups at C-4 of GalNAc and evaluation of their inhibitory activity should help in understanding the precise structural interactions required for binding of parasite adhesive protein to C4S.
Our observation that the synthetic 12 mer containing two sulfate groups, one on each consecutive disaccharide moiety from the nonreducing end, can efficiently inhibit IRBC binding suggest that a sulfate groups close to the nonreducing end portion of C4S 12 mer motif is critical for binding. This is further supported by the observation that 12 mer containing sulfate group only on the nonreducing end disaccharide moiety also significantly inhibits IRBC binding. Since in solution C4S assumes a left-handed, single stranded helical conformation having three disaccharides per turn (33), a C4S 12 mer motif represents two turns of the helix. Therefore, the requirement of 12 mer chain length for maximal inhibitory activity suggests the involvement of a C4S conformational structure in IRBC binding to CSPG. It is possible that specific structural features from each turn of the two helical motifs of 12 mer are recognized in binding. Further, our results also show that the presence of two nonsulfated disaccharide moieties is critical for optimal IRBC binding. Therefore, it appears that one or more C-4 hydroxyl group of specific GalNAc residue(s) within the dodecasaccharide motif interact with IRBCs. Based on these observations, we predict that C4S 12 mer having a sulfate group on C-4 of GalNAc proximal to the nonreducing end helical turn presents a critical sulfate group and possibly a C-4 hydroxyl group in a conformationally favorable manner for interactions with parasite adhesive protein(s), although another sulfate group and a C-4 hydroxyl group at other locations within the 12 mer motif also likely interact.
Recently, it was reported that var2-CSA PfEMP1 binds to lipid-conjugated C4S 8 mers, containing unsaturated nonreducing end GlcA, but 10 mers maximally inhibited IRBC binding to placental tissue sections (20). Further, different parasite strains were reported to exhibit varied requirement for C4S sulfate content. This was attributed to variation in the PfEMP1 binding characteristics because of gene polymorphism (20). In contrast, our study shows that binding of IRBCs to CSPG is maximally inhibited by C4S 12 mer having two 4-sulfated disaccharide moieties irrespective of parasite strains. Since, the minimal structural requirement for effective inhibition does not vary with parasite strains, the binding domain(s) of parasite PfEMP1 is conserved among different parasite isolates.
Although polymorphism is a general feature of P. falciparum var genes, recent studies demonstrated that polymorphism in the var2-CSA PfEMP1 DBL3X, the domain that is implicated in C4S binding, confines to smaller discrete variable regions within long stretches of highly conserved regions (34–37). The PfEMP1 polymorphic regions could correspond to surface exposed variable regions, which seems to be the target of host immune recognition rather than involved in C4S binding. More recently, the crystal structure DBL3X-C4S oligosaccharide complex showed that a conformational cluster of positively charged amino acid residues in the subdomains 2 and 3 of DBL3X interacts with a sulfate group of C4S oligosaccharides and this conformational motif has been suggested to be involved in C4S binding (38, 39). The basic amino acid residues of DBL3X that are suggested to interact with the 4-sulfate group of C4S are present in the constant regions and are conserved in different P. falciparum strains, including many placental isolates (34). Further, we cloned and sequenced DBL3X from the parasite strains used in this study. Sequence alignment shows that all the key basic amino acid residues suggested to be involved in C4S binding are conserved in these P. falciparum strains (Figure S3 of the supporting information). The above data taken together suggest that the structural interactions involved in binding of IRBCs to C4S are conserved and PfEMP1 polymorphic regions do not alter IRBC binding specificity.
Another important finding of this study is that the nonreducing end disaccharide moiety of C4S 12 mers is involved in IRBC binding. This conclusion is supported by the observation that Δ-12 mers containing 4,5-unsaturated GlcA at the nonreducing end, obtained by the partial depolymerization of C4S with S. dysgalactiae hyaluronidase lyase, show considerably lower inhibitory activity as compared to 12 mers containing unmodified GlcA (17, 18). Actually, the activity of Δ-12 mers is similar to that of 10 mer with the terminal unmodified GlcA formed by treatment of C4S with testicular hyaluronidase. Further, 10 mers with terminal unmodified GlcA showed ~2-fold lower activity (based on IC50 value) compared to 12 mers, Δ-10 mers were ~4-fold less inhibitory (Figure 8). Thus, the results of this study clearly show that, in the case of Δ-oligosaccharides, nonreducing end disaccharide moiety does not interact with IRBCs, and that only tetradecasaccharides and higher oligosaccharides show activity comparable to that of the C4S polymer. This conclusion is consistent with the results of a previous study that Δ-12 mers obtained by partial depolymerization of C4S with chondroitinase ABC exhibit substantially lower inhibitory activity, whereas Δ-14 mers and higher Δ-oligosaccharides were as effective as C4S polymers (15). We have previously shown that modification of the reducing end GalNAc of C4S 12 mers by coupling bulky substituents at C1 does not affect the inhibitory activity (32). Thus, the nonreducing end GlcA residue, but not the reducing end GalNAc moiety of 12 mers, interacts with IRBCs.
Our data further demonstrate that the carboxyl group of terminal GlcA interacts with IRBCs. Since the C-4 hydroxyl group of GlcA residues in C4S are involved in glycosidic bond formation and because inhibitory activity of 12 mers containing unmodified GlcA are similar to that of C4S polymer, it is obvious that the C-4 hydroxyl group of GlcA in C4S oligosaccharides does not interact with IRBCs. Further, the fact that introduction of double bond between C-4 and C-5 perturbs the chair conformation of GlcA indicates that the carboxyl group of the nonreducing end disaccharide moiety and its spatial orientation are important for IRBC adherence.
The results of this study that C4S structural requirements for IRBC binding to placental CSPG is parasite strain-independent has important implications for the development and evaluation of C4S dodecasaccharide-based therapeutics and/or vaccine for placental malaria. Further, our results also have important implications for the development of C4S-oligosaccharide probes for complete understanding of structural interactions involved in IRBC binding to C4S.
Acknowledgments
Supporting Information Available: Figure data on the Bio-Gel P-6 HPLC chromatography of oligosaccharides formed by treatment of partially sulfated C4S with S. dysgalactiae hyaluronidase, and cDNA sequence of DBL-3X domain of P. falciparum strains. This material is available free of charge via the Internet at http://pubs.acs.org
Footnotes
This work was supported by the US Public Health Service grant AI45086 from the National Institute of Allergy and Infectious Diseases, NIH. Work in the laboratory of Dr. Takagaki was supported by Grants-in Aid (Nos. 15370041, 17591153 and 17770106) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Grants for Priority Research Designated by the President of Hirosaki University, and by the Fund for Cooperation for Innovative Technology and Advanced Research in Evolution Area (CITY AREA).
The abbreviations used are: IRBCs, infected red blood cells; PfEMP1, Plasmodium falciparum erythrocyte membrane protein 1; var, variant; GAG, glycosaminoglycan; CS, chondroitin sulfate; PG, proteoglycan; CSPG, chondroitin sulfate proteoglycan; C4S, chondroitin 4-sulfate; bCSA, bovine tracheal chondroitin sulfate A; BSA, bovine serum albumin; PA, polyamine; GalNAc, N-acetylgalactosamine; GlcA, glucuronic acid; 6 mer, 8 mer, 10 mer, 12 mer and 14 mers refer to C4S hexa-, octa- deca-, dodeca- and tetradecasaccharide, respectively.
References
- 1.Pasloske BL, Howard RJ. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Malaria and the red cell. Hematol Am Soc Hematol Educ Program. 2002:35–57. doi: 10.1182/asheducation-2002.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Schlichtherle M, Wahlgren M. Molecular aspects of severe malaria. Clinic Microbiol Rev. 2000;13:439–450. doi: 10.1128/cmr.13.3.439-450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman IW, Eda S, Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 5.Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Cooke B, Coppel R, Wahlgren M. Falciparum malaria: sticking up, standing out and out-standing. Parasitol Today. 2000;16:416–420. doi: 10.1016/s0169-4758(00)01753-1. [DOI] [PubMed] [Google Scholar]
- 7.Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, Chen Q, Barragan A, Wahlgren M, Marsh K. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect Immun. 2001;69:5849–5856. doi: 10.1128/IAI.69.9.5849-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birth weight and placental weight. Trans R Soc Trop Med Hyg. 1983;77:232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 9.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, Font F, Alonso PL. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 10.Beeson JG, Duffy PE. The immunology and pathogenesis of malaria during pregnancy. Curr Top Microbiol Immunol. 2005;297:187–227. doi: 10.1007/3-540-29967-x_6. [DOI] [PubMed] [Google Scholar]
- 11.Duffy PE, Fried M. Plasmodium falciparum adhesion in the placenta. Curr Opin Microbiol. 2003;6:371–376. doi: 10.1016/s1369-5274(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 12.Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- 13.Achur RN, Valiyaveettil M, Gowda DC. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J Biol Chem. 2003;278:11705–11713. doi: 10.1074/jbc.M211015200. [DOI] [PubMed] [Google Scholar]
- 14.Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am J Pathol. 2004;164:2013–2025. doi: 10.1016/S0002-9440(10)63761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeson JG, Chai W, Rogerson SJ, Lawson AM, Brown GV. Inhibition of binding of malaria-infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infect Immun. 1998;66:3397–3402. doi: 10.1128/iai.66.7.3397-3402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gysin J, Pouvelle B, Fievet N, Scherf A, Lepolard C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect Immun. 1999;67:6596–6602. doi: 10.1128/iai.67.12.6596-6602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- 18.Chai W, Beeson JG, Lawson AM. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J Biol Chem. 2002;277:22438–22446. doi: 10.1074/jbc.M111401200. [DOI] [PubMed] [Google Scholar]
- 19.Fried M, Lauder RM, Duffy PE. Plasmodium falciparum: adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Exp Parasitol. 2000;95:75–78. doi: 10.1006/expr.2000.4510. [DOI] [PubMed] [Google Scholar]
- 20.Beeson JG, Andrews KT, Boyle M, Duffy MF, Choong EK, Byrne TJ, Chesson JM, Lawson AM, Chai W. Structural basis for binding of Plasmodium falciparum erythrocyte membrane protein 1 to chondroitin sulfate and placental tissue and the influence of protein polymorphisms on binding specificity. J Biol Chem. 2007;282:22426–22436. doi: 10.1074/jbc.M700231200. [DOI] [PubMed] [Google Scholar]
- 21.Dische Z. A new specific color reaction of hexuronic acids. J Biol Chem. 1947;167:189–198. [PubMed] [Google Scholar]
- 22.Hamai A, Morikawa K, Horie K, Tokuyasu K. Purification and characterization of hyaluronidase from Streptococcus dysgalactiae. Agric Biol Chem. 1989;53:2163–2168. [Google Scholar]
- 23.Saitoh H, Takagaki K, Majima M, Nakamura T, Matsuki A, Kasai M, Narita H, Endo M. Enzymic reconstruction of glycosaminoglycan oligosaccharide chains using the transglycosylation reaction of bovine testicular hyaluronidase. J Biol Chem. 1995;270:3741–3747. doi: 10.1074/jbc.270.8.3741. [DOI] [PubMed] [Google Scholar]
- 24.Takagaki K, Munakata H, Majima M, Endo M. Enzymatic reconstruction of a hybrid glycosaminoglycan containing 6-sulfated, 4-sulfated, and unsulfated N-acetylgalactosamine. Biochem Biophys Res Commun. 1999;258:741–744. doi: 10.1006/bbrc.1999.0697. [DOI] [PubMed] [Google Scholar]
- 25.Takagaki K, Munakata H, Majima M, Kakizaki I, Endo M. Chimeric glycosaminoglycan oligosaccharides synthesized by enzymatic reconstruction and their use in substrate specificity determination of Streptococcus hyaluronidase. J Biochem. 2000;127:695–702. doi: 10.1093/oxfordjournals.jbchem.a022659. [DOI] [PubMed] [Google Scholar]
- 26.Takagaki K, Kakizaki I, Iwafune M, Ishido K, Endo M. Enzymatic reconstruction of glycosaminoglycan oligosaccharides. International Congress Series. 2001;1223:239–244. [Google Scholar]
- 27.Hardy MR, Townsend RR. High-pH anion-exchange chromatography of glycoprotein-derived carbohydrates. Methods Enzymol. 1994;230:208–225. doi: 10.1016/0076-6879(94)30014-3. [DOI] [PubMed] [Google Scholar]
- 28.Takagaki K, Nakamura T, Izumi J, Saitoh H, Endo M, Kojima K, Kato I, Majima M. Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass spectrometry. Biochemistry. 1994;33:6503–6517. doi: 10.1021/bi00187a017. [DOI] [PubMed] [Google Scholar]
- 29.Achur RN, Muthusamy A, Madhunapantula SV, Bhavanandan VP, Seudieu C, Gowda DC. Chondroitin sulfate proteoglycans of bovine cornea: structural characterization and assessment for the adherence of Plasmodium falciparum-infected erythrocytes. Biochim Biophys Acta. 2004;1701:109–119. doi: 10.1016/j.bbapap.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Yang HO, Gunay NS, Toida T, Kuberan B, Yu G, Kim YS, Linhardt RJ. Preparation and structural determination of dermatan sulfate-derived oligosaccharides. Glycobiol. 2000;10:1033–1039. doi: 10.1093/glycob/10.10.1033. [DOI] [PubMed] [Google Scholar]
- 31.Chai W, Kogelberg H, Lawson AM. Generation and Structural Characterization of a Range of Unmodified Chondroitin Sulfate Oligosaccharide Fragments. Anal Biochem. 1996;237:88–102. doi: 10.1006/abio.1996.0205. [DOI] [PubMed] [Google Scholar]
- 32.Gowda AS, Madhunapantula SV, Achur RN, Valiyaveettil M, Bhavanandan VP, Gowda DC. Structural basis for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate and design of novel photoactivable reagents for the identification of parasite adhesive proteins. J Biol Chem. 2007;282:916–928. doi: 10.1074/jbc.M604741200. [DOI] [PubMed] [Google Scholar]
- 33.Cael JJ, Winter WT, Arnott S. Calcium chondroitin 4-sulfate: modular conformation and organization of polysaccharide chains in proteoglycan. J Mol Biol. 1978;125:21–42. doi: 10.1016/0022-2836(78)90252-8. [DOI] [PubMed] [Google Scholar]
- 34.Dahlbäck M, Rask TS, Andersen PH, Nielsen MA, Ndam NT, Resende M, Turner L, Deloron P, Hviid L, Lund O, Pedersen AG, Theander TG, Salanti A. Epitope mapping and topographic analysis of VAR2CSA DBL3X involved in P. falciparum placental sequestration. PLoS Pathog. 2006;2:1069–1082. doi: 10.1371/journal.ppat.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avril M, Kulasekara BR, Gose SO, Rowe C, Dahlbäck M, Duffy PE, Fried M, Salanti A, Misher L, Narum DL, Smith JD. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect Immun. 2008;76:1791–800. doi: 10.1128/IAI.01470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlbäck M, Theander T, Lund O, Salanti A. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 2008;4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resende M, Nielsen MA, Dahlbäck M, Ditlev SB, Andersen P, Sander AF, Ndam NT, Theander TG, Salanti A. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar J. 2008;7:1–11. doi: 10.1186/1475-2875-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins M. The structure of a chondroitin sulfate-binding domain important in placental malaria. J Biol Chem. 2008;283:21842–21846. doi: 10.1074/jbc.C800086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3X domain of pregnancy-associated malaria protein VAR2CSA complexed with chondriotin sulfate A. Nat Struct Biol. 2008;15:932–938. doi: 10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]