Conspectus
The cores of many types of polymers, ligands, natural products, and pharmaceuticals contain biaryl or substituted aromatic structures, and efficient methods of synthesizing these structures are crucial to the work of a broad spectrum of organic chemists. Recently, Pd-catalyzed carbon-carbon bond-forming processes, particularly the Suzuki–Miyaura cross-coupling reaction (SMC), have risen in popularity for this purpose. The SMC has many advantages over other methods for constructing these moieties, including mild conditions, high tolerance toward functional groups, the commercial availability and stability of its reagents, and the ease of handling and separating byproducts from its reaction mixtures.
Until 1998, most catalysts for the SMC employed triarylphosphine ligands. More recently, new bulky and electron-rich phosphine ligands, which can dramatically improve the efficiency and selectivity of such cross-coupling reactions, have been introduced. In the course of our studies on carbon-nitrogen bond-forming reactions, we found that the use of electron-rich and bulky phosphines enhanced the rate of both the oxidative addition and reductive elimination processes; this was the beginning of our development of a new family of ligands, the dialkylbiarylphosphines L1–L12. These ligands can be used for a wide variety of palladium-catalyzed carbon–carbon, carbon–nitrogen, and carbon–oxygen bond-forming processes as well as serving as supporting ligands for a number of other reactions.
The enhanced reactivity of these catalysts has expanded the scope of cross-coupling partners that can be employed in the SMC. Using such dialkylbiarylphosphine ligands, the coupling of unactivated aryl chlorides, aryl tosylates, heteroaryl systems, and very hindered substrate combinations have become routine. The utility of these ligands has been successfully demonstrated in a wide number of synthetic applications, including industrially relevant processes.
In this account, we provide an overview of the use and impact of dialkylbiarylphosphine ligands in the SMC. We discuss our studies on the mechanistic framework of the reaction, which have allowed us to rationally modify the ligand structures in order to tune their properties. We also describe selected applications in the synthesis of natural products and new materials to illustrate the utility of these dialkylbiarylphosphine ligands in various “real-world” synthetic applications.
Introduction
The impact of the Suzuki-Miyaura reaction (SMC) on academic and industrial research as well as on production has been immense.1 Over the past two decades, it has become arguably one of the most efficient methods for the construction of biaryl or substituted aromatic moieties; compounds that contain these substructures constitute important building blocks of polymers,2 ligands,3 a wide range of natural products such as alkaloids, as well as in numerous biologically active pharmaceuticals.4 The key advantages of the SMC are the mild conditions under which these are conducted, the high tolerance towards functional groups that is observed, the commercial availability and stability of boronic acids to heat, oxygen and water, and the ease of handling and separation of boron-containing byproducts from the reaction mixtures.5 These desirable features make the SMC an important tool in medicinal chemistry as well as in the large-scale synthesis of pharmaceuticals and fine chemicals.6 In addition to aryl and heteroaryl boronic acids and esters, vinyl and alkyl derivatives are also commonly used in the SMC. In order to simplify the text of this account, we will focus most of the discussion on the use of the aryl and heteroaryl species.
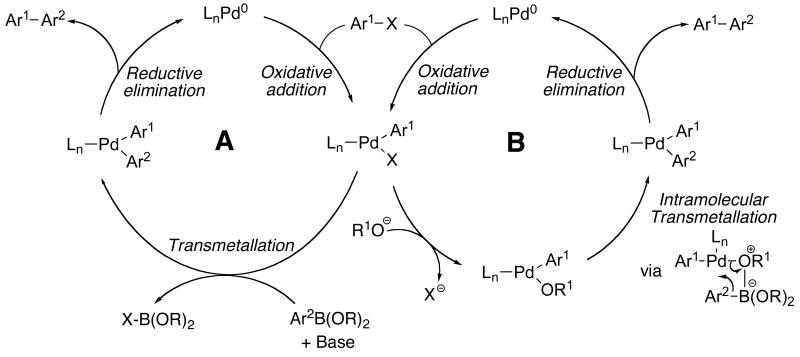
As in other cross-coupling reactions, the catalytic cycle of the palladium-catalyzed SMC is thought to follow a sequence involving the oxidative-addition of an aryl halide to a Pd(0) complex to form an arylpalladium(II) halide intermediate. Transmetallation with a boronic acid and reductive-elimination from the resulting diarylpalladium complex affords the corresponding biaryl and regenerates the Pd(0) complex (Figure 1).7 The bases we have most commonly employed for these processes are K3PO4 and K2CO3. Others including KOH or KF have also been used. At present, however, the choice of base is still empirical and no general rule for their selection has been established. The role of the base in these reactions is to facilitate the otherwise slow transmetalation of the boronic acid by forming a more reactive boronate species that can interact with the Pd center and transmetallate in an intramolecular fashion (path A).8 Alternatively, it has also been proposed that the base replaces the halide in the coordination sphere of the palladium complex and facilitates an intramolecular transmetallation (path B).9 While in most cases the exact nature of the actual catalyst remains ambiguous, recent reports with bulky ligands have provided circumstantial evidence of a mechanism involving highly reactive monoligated L1Pd species, where the the L:Pd ratio can play a large role in the catalytic performance.10
Figure 1.
General catalytic cycle for Suzuki-Miyaura couplings
Most early work in the SMC was conducted using triarylphosphines as supporting ligands. During the last ten years, the application of new ligands has dramatically improved the efficiency and selectivity attainable in such cross-coupling reactions. In the ever-growing catalogue of available ligands for cross-coupling reactions, bulky dialkylbiaryl11 and trialkylphosphines12 remain the most widely used, followed by N-heterocyclic carbenes (NHCs).13
The purpose of this review is to provide an overview of the use and impact of dialkylbiarylphosphine ligands in SMC. Selected applications in the synthesis of natural products and new materials that illustrate the utility of these ligands will also be discussed.
Background: Dialkylbiarylphosphines
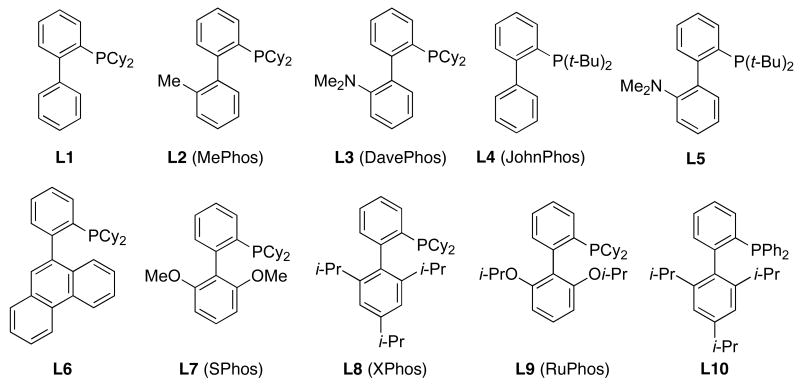
Since their introduction in 1998, monodentate, bulky and electron-rich dialkylbiaryl phosphines (Figure 2) have seen wide use as supporting ligands in a variety of transformations, especially in Pd-catalyzed carbon-carbon,14 carbon-nitrogen15 and carbon-oxygen bond-forming processes.16
Figure 2.
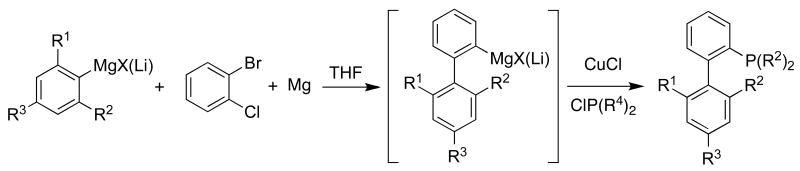
These ligands can be prepared in a direct one-pot protocol by addition of an aryl Grignard or an aryllithium reagent to an in-situ generated benzyne intermediate, followed by trapping the intermediate with an appropriate chlorophosphine (Scheme 1).3 These compounds have been prepared on a >10 kg scale and efforts to increase the scale of their production are underway. The ready availability of Grignard and organolithium reagents makes the route highly modular, thereby allowing the preparation of a variety of new and structurally diverse ligands.
Scheme 1.
In addition to the high reactivity of catalytic systems based upon biarylphosphines, these ligands possess a number of attributes that make them particularly attractive for organic synthesis: (a) they are crystalline materials, (b) they are air stable, even in solution,17 (c) they possess a high degree of thermal stability, (d) many of these ligands are commercially available from either Strem or Aldrich and (e) the processes that employ these ligands are operationally simple, not requiring the use of a glovebox.
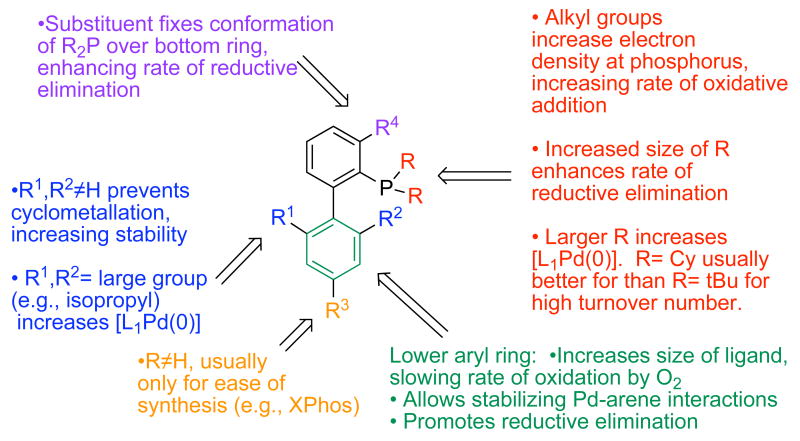
In Figure 3, we show the different structural features of the biarylphosphine ligands and how each of these contributes to the efficiency of the catalysts that are derived from them. The studies that we have carried out provide us with a mechanistic framework with which to rationally modify the ligand structures in order to tune their properties. This and the fact that their synthesis is short and modular, provides us with a mechanistically guided process for the evolution of ligands which confer enhanced properties on the catalysts derived from them.
Figure 3.
Structural Features of the Dialkylbiarylphosphines and Their Impact on the Efficacy of Catalysts Using These Ligands
As indicated, the outstanding activity of the catalysts derived from the biarylphosphine ligands has been attributed to a combination of electronic and steric properties that enhances the rates of oxidative addition, transmetallation and the reductive elimination steps in the catalytic cycle. This can be rationalized as follows: (a) The bulky and electron-donating character of these ligands is important for stabilizing the monoligated L1Pd intermediates, which are believed to be key species in the catalytic cycle (Figure 1).9 (b) Both with these and related ligands it has been shown that oxidative-addition of aryl halides is much faster with L1Pd(0) species than to more highly coordinated complexes.18 This is simply due to the smaller size of an L1Pd(0) complex compared to a L2Pd(0) one, allowing the substrate to approach the latter more closely and, hence, reacting at a faster rate. We presume that transmetallation to a L1Pd(Ar)X intermediate is faster, in general, than to a L2Pd(Ar)X complex for related reasons. (c) It is well-documented that the rate of reductive-elimination from LPd(Ar)R (R = aryl, NR2, OR) is faster than for the same process for an analogous L2Pd(Ar)R complex.19 (d) Finally, studies in our laboratories have demonstrated that the addition of ortho substituents on the bottom ring of the biarylphosphine (eg., SPhos (L7) and XPhos (L8)) lead to a significant increase in activity and stability by preventing palladacycle formation.20 Additionally, this ortho, ortho' substitution increases the size of the ligand relative to those with no ortho substituents (thus increasing the concentration of L1Pd species).
Development of General Catalysts for Suzuki-Miyaura Reactions
There has been an impetus to develop catalysts for the SMC that can efficiently couple hindered substrate combinations, utilize unactivated aryl chlorides21 and heteroaryl substrates, and operate at low catalyst loadings and/or at room temperature.
While evaluating the efficacy of dialkylbiaryl phosphine ligands in carbon-nitrogen bond-forming reactions, we found that the use of DavePhos (L3) provided a particularly active catalyst for SMC as well.22 We note that, at the same time, our colleagues in the Fu group developed their important chemistry for SMC using PtBu3 as a supporting ligand.23 Further studies revealed that catalysts supported by JohnPhos (L4) were substantially more reactive than those with DavePhos (L3) in SMC at room temperature (Table 1).24 These results indicated that the dimethylamino group in DavePhos (L3) was not necessary for effective catalysis. While JohnPhos (L4) provided the best results for room-temperature reactions, the use of the dicyclohexyl analogue (L1), provided a more active system when using low catalyst loadings or with more hindered substrate combinations.25 That JohnPhos (L4) gives a more active system at room-temperature than ligands with a dicyclohexyl phosphino group is almost certainly due to the greater concentration of L1Pd(0) and L1Pd(Ar)Cl intermediates (instead of the corresponding L2Pd complexes) with JohnPhos (L4) than with the latter class of ligands.
Table 1.
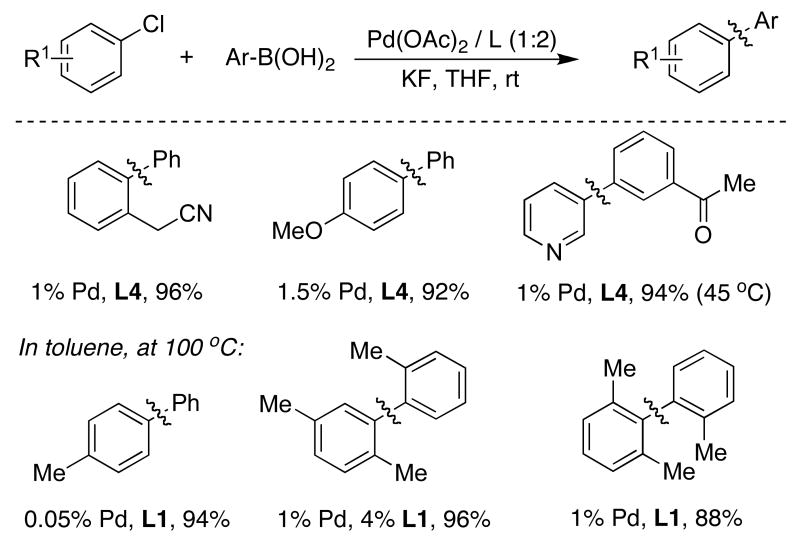
|
Reactions involving the cross-coupling of two hindered arenes where each reactant possessed two ortho substituents remained difficult. At the time of this work, the only example reported in the literature for the synthesis of unsymmetrical tetra-ortho substituted biaryls had been accomplished using a Negishi cross-coupling reaction.26 Our initial efforts utilized phenanthrene derivative L6, which proved to be an excellent ligand for the construction of tetra-ortho-substituted biaryls via SMC.27
We later found that SPhos (L7), which can be prepared in an experimentally convenient one-pot protocol from 1,3-dimethoxybenzene, was an outstanding ligand for this and other purposes. The reactions with catalysts based upon SPhos (L7) exhibited unprecedented scope, reaction rate and stability.28 The new catalytic system was also found to be remarkably efficient for the cross-coupling of unactivated aryl chlorides and bromides at catalyst loadings as low as 5×10-4 mol% Pd (Table 2).
Table 2.
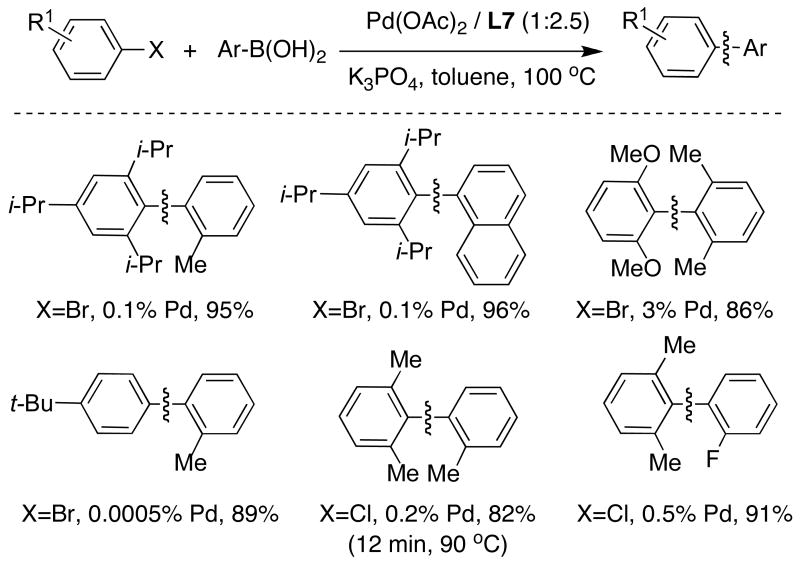
|
We attribute the longevity of catalysts based on SPhos (L7) to two main factors. First, the stabilization of Pd(0) intermediates by favorable interactions of the aromatic π system with the Pd center as supported by X-ray crystallography of the L7/Pd0(dba) complex. As shown in Figure 4, this complex possesses a Pd(0) η1-arene interaction with the ipso carbon.29 We also believe that the high activity of catalysts based on SPhos (L7) is due to the ability of this ligand to stabilize and maximize the concentration of the L1Pd intermediates with a relatively small ligand (compared to, e.g., XPhos (L8)). These intermediates would be expected to be particularly reactive in oxidative addition and transmetallation processes for the reasons previously discussed.
Figure 4.
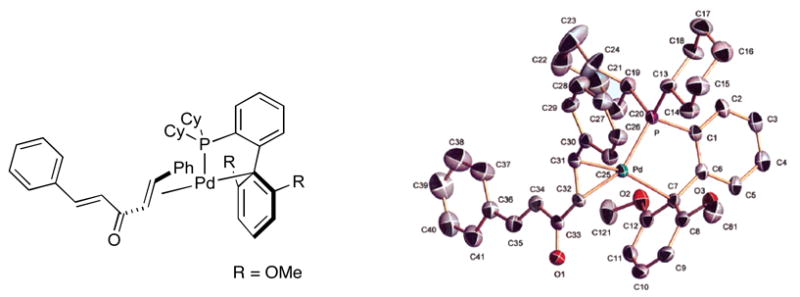
Reproduced with permission from J Am Chem Soc. 2005, 127, 4685-4696. Copyright 2005 American Chemical Society.
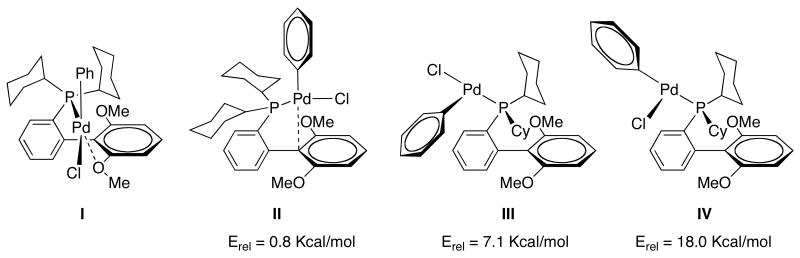
In order to probe the reasons for the high stability and/or activity of SPhos (L7) as a supporting ligand in SMC, we conducted ground-state energy optimizations on the corresponding oxidative addition complex (L7-Pd(Ph)Cl) by DFT computational studies and four minima were found (Figure 5).30
Figure 5.
Ground-state energy optimizations of L7-Pd(Ph)Cl
The most favored structures possess either a Pd-arene interaction with the ipso carbon (II) or a Pd-O interaction with an oxygen atom of the methoxy group of the bottom ring of the ligand (I). We believe that both the Pd-arene and Pd-O interactions contribute to the stability and hence to the efficiency of catalysts based on SPhos (L7). In particular, the existence of this extra Pd-O interaction relative to other dialkylbiarylphosphines likely further stabilizes the oxidative addition intermediate before the transmetallation step. As this is believed to be the rate-limiting step in SMC,31 this complex should be present in a relatively high concentration and hence its stabilization its of great importance. Supporting evidence consistent with these theoretical findings was found experimentally by NMR spectroscopy of the isolated oxidative addition product L7-Pd(Ph)Cl.32 While at 25 °C the 31P NMR spectra showed two main peaks with a relative ratio of 72:28, the two peaks rapidly and reversibly coalesce at about 45 °C (Figure 6). This experimental data is consistent with two rotameric species of L7-Pd(Ph)Cl, e.g., I and II, which were the two lowest energy conformers found by DFT calculations (Figure 5).
Figure 6.
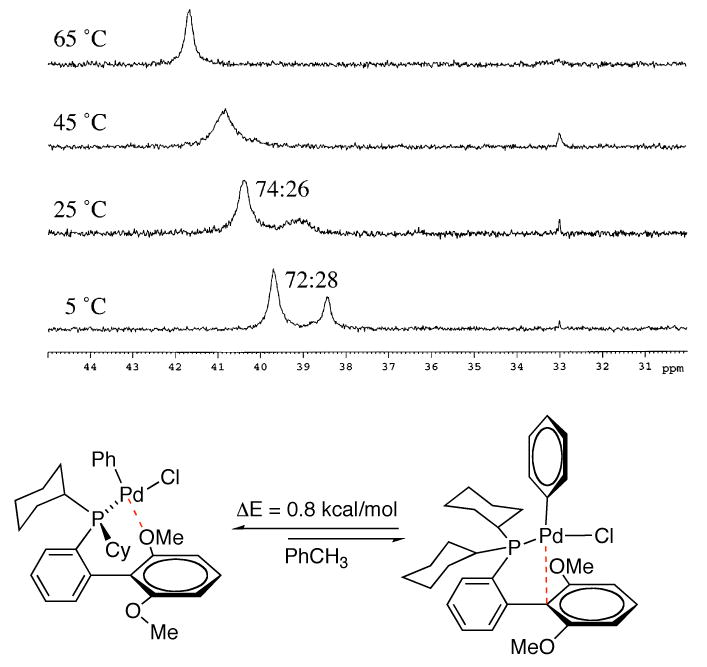
Reproduced with permission from Organometallics 2007, 26, 2183-2192. Copyright 2007 American Chemical Society.
Overall, these results shed light on the specific nature of the interactions accessible to Pd complexes based upon SPhos (L7) and are consistent with the notion that they stabilize the intermediate complexes and contribute to the long-lived nature of these catalysts.
The green character of water has prompted many groups to investigate the use of this reaction medium for cross-coupling reactions.32 We found that SPhos (L7) could be sulfonated on the bottom ring in quantitative yield by simple treatment with H2SO4.33 This water-soluble version of SPhos, (L11), allowed SMC to proceed in excellent yields with a wide variety of substrates, including highly functionalized aryl chlorides or heteroaryl halides with either aryl or alkyl boronic acids in aqueous media (Table 3).
Table 3.
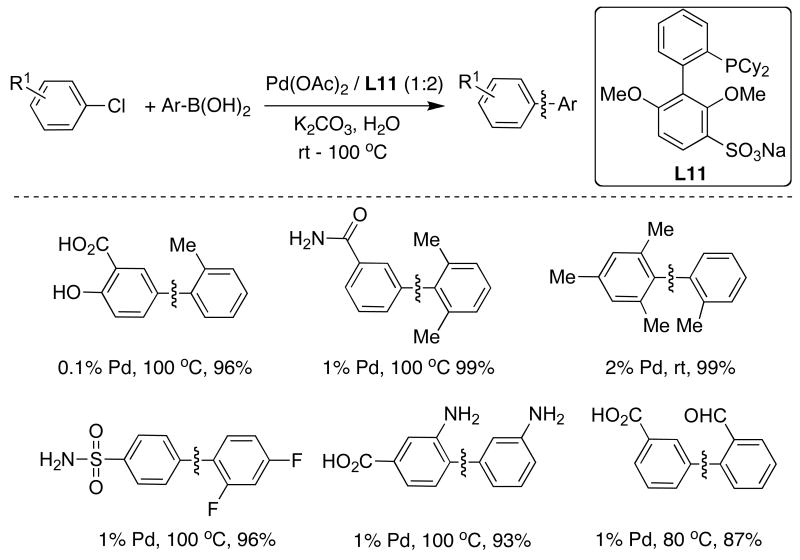
|
Nitrogen heterocycles are structural constituents of a wide variety of biologically active natural products, medicinally important compounds, and organic materials. Their construction and derivatization by cross-coupling processes is widespread.34 In general, however, cross-coupling reactions with heteroaryl substrates have proven significantly more challenging than those with all-carbon substrates.35 Consequently, the problems associated with these coupling processes have, to some extent, limited their application in drug development. As one example, chloroaminopyridines and chloroaminopyrimidines are quite difficult substrates to employ in cross-coupling processes. In some instances, chemists have resorted to protecting the free amino group in order to use these substrates.36 It has also been suggested that it is requisite to employ bidentate ligands in order to prevent binding of the substrate and/or product to Pd(II) intermediates which would result in catalyst deactivation.37 Given these reports, we decided to examine the SMC of heteroaryl compounds using SPhos (L7) and XPhos (L8) as the supporting ligands. We found, in contrast to what had been suggested for the earliest versions of dialkylbiarylphosphines,37 that catalysts based on SPhos (L7) were not inhibited by the presence of aminopyridines or aminopyrimidines (Table 4).38 In addition, electron-rich, electron-deficient as well as sterically hindered boronic acids reacted smoothly, producing the desired biaryls in high yields. An interesting example was the coupling of phenylboronic acid with 4-amino-2-chloropyridine, the most basic among the substrates used to provide the products shown in Table 4. While this coupling has been reported to proceed in low yields, even with bidentate ligands,37 with SPhos (L7) the product was efficiently formed.
Table 4.
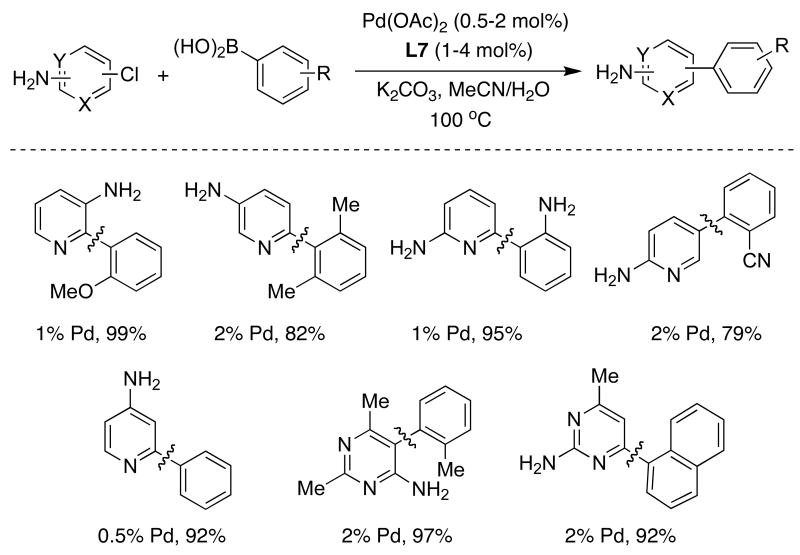
|
As part of our study of the SMC of heterocycles, we examined in detail the use of pyrrole-derived boronic acids and esters. A key to achieving success in this venture was when we studied the solvent dependence of the reaction of N-TIPS-3-pinacolboronatepyrrole (A) with heteroaryl bromides.39 The key to these coupling reactions was the use of alcoholic solvents, with n-butanol being the most efficient. The effect of alcohol solvents can be rationalized by Miyaura's results.9 We also discovered that the presence of water was critical to the success of the reactions, presumably due to the need for partial or complete hydrolysis of the boronate ester. In addition, its presence led to higher yields of product and less reduction of the aryl halide. A study was conducted in which it was determined that the optimum ratio of n-butanol:water was 2.5:1 with respect to the rate and efficiency of the reaction of A and 2-bromothiophene (Table 5).
Table 5.
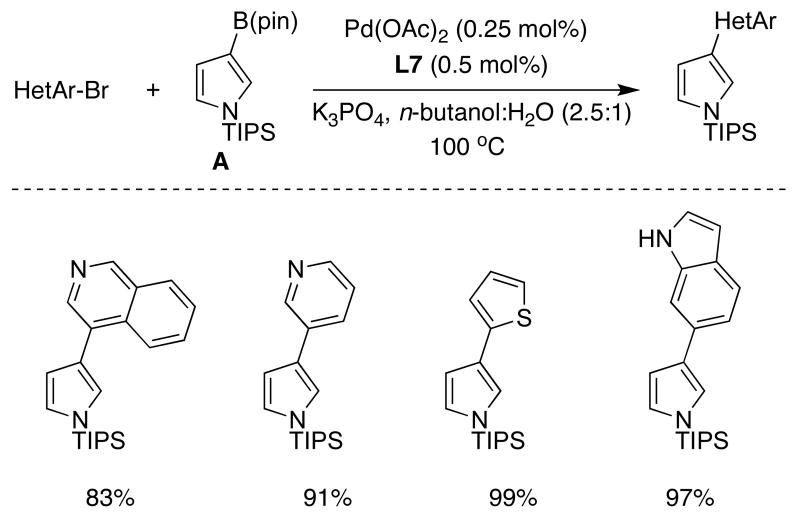
|
Similarly, n-butanol proved to be the ideal solvent for the SMC of N-Boc-pyrrole-2-boronic acid (B) with aryl and heteroaryl bromides possessing a variety of functional groups (Table 6);39 in this case, however, the addition of water resulted in poor yields due to an increased production of reduced aryl halide. In constrast to prior reports,40 significant homocoupling or protodeboronation of B was not detected using a catalyst system based upon SPhos (L7).
Table 6.
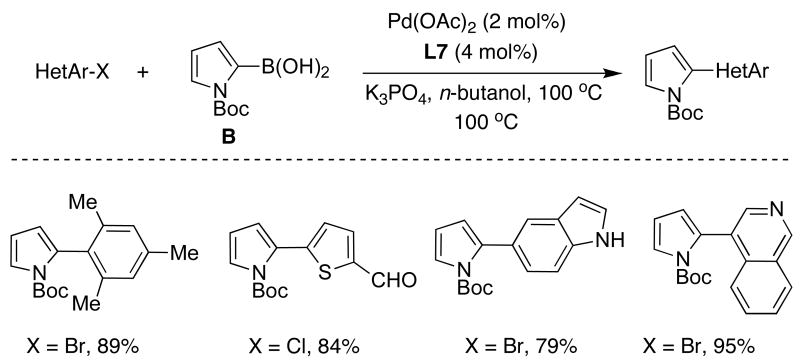
|
The use of XPhos (L8) as supporting ligand allowed an expansion of the scope of the SMC for the combination of thiophene and pyridylboronic acids with a wide range of activated and unactivated heteroaryl chlorides, even highly basic aminopyridines, with high efficiency and relatively low catalyst loadings (Table 7).39 These results are of particular significance due to the slow rate of transmetallation and the tendency of these coupling counterparts to undergo protodeboronation in polar solvents.
Table 7.
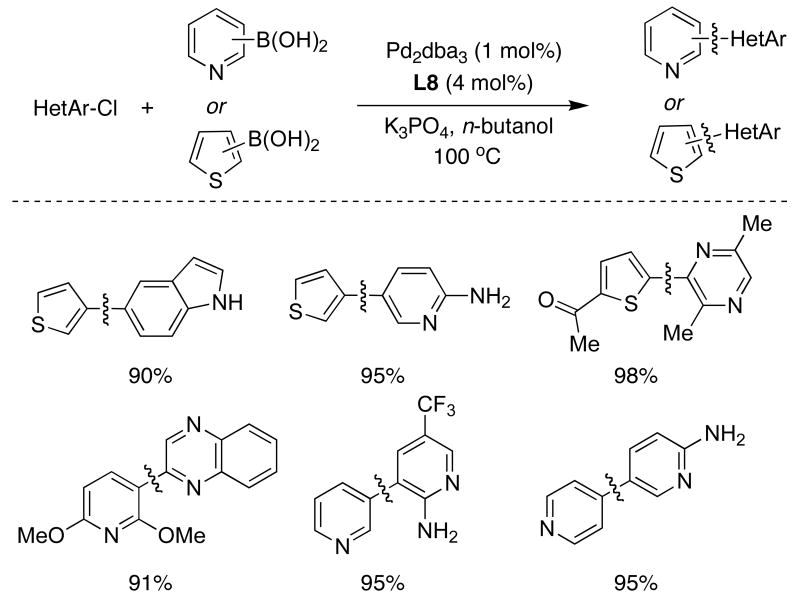
|
In recent years Pd-catalyzed methods have emerged for the preparation of aryl boronate esters from unactivated aryl chlorides.41 Unfortunately, they typically require high catalyst loadings, long reaction times and manifest a limited ability to utilize functionalized substrates and/or sterically encumbered substrates. We have recently demonstrated that SPhos (L7) or XPhos (L8) provide highly active catalysts for the borylation of aryl chlorides under mild reaction conditions (Table 8).42
Table 8.
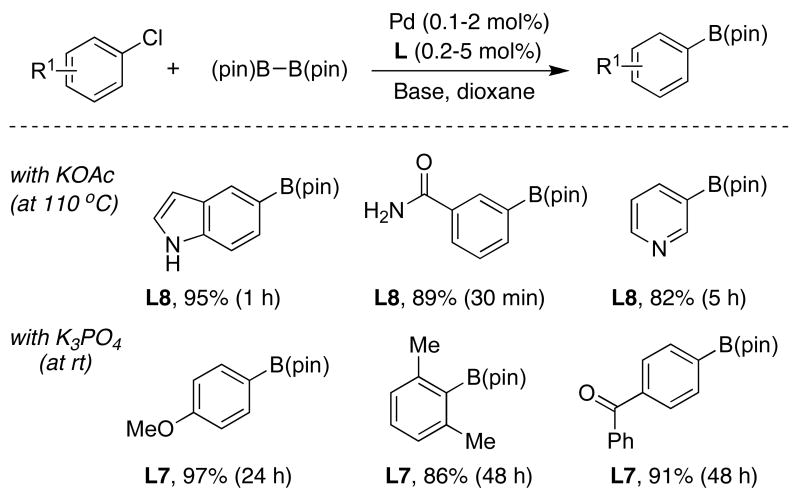
|
A catalyst system based on SPhos (L7) could be employed for the direct, one-pot synthesis of unsymmetrical biaryls from different aryl or heteroaryl chlorides. In this process, the substrates were subjected to the standard Pd-catalyzed borylation conditions with subsequent addition of the second aryl chloride and aqueous K3PO4, resulting in the clean formation of the desired biaryls (Table 9).42
Table 9.
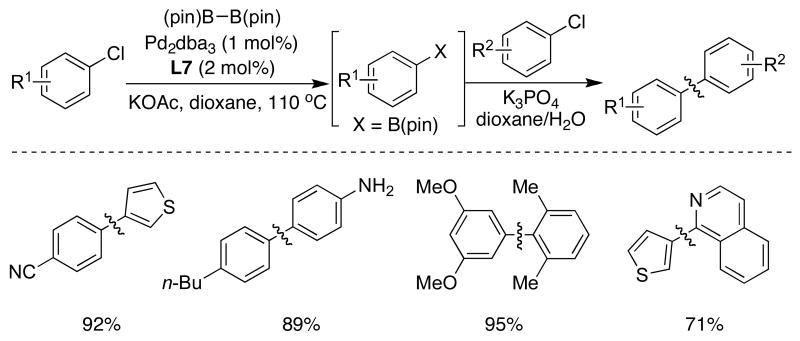
|
Selected Synthetic Applications
Once it became clear that catalysts derived from biarylphosphine ligands possessed a higher level of activity than previous systems, their application in target-oriented and other areas of organic synthesis began to appear rapidly. The examples discussed are illustrative of the utility of these catalysts in a variety of venues.
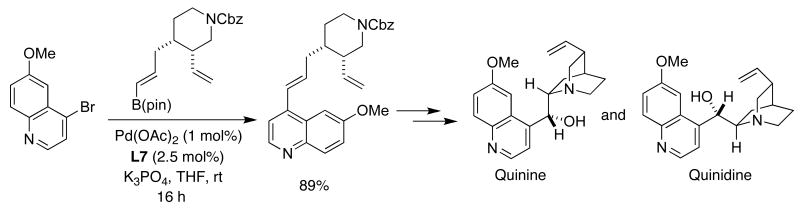
In early 2004, Jacobsen reported the first catalytic asymmetric synthesis of quinine and quinidine.43 Along the way, he needed to conduct the cross-coupling of an advanced boronate ester intermediate with the bromoquinoline compound. Attempts under standard SMC conditions proved unsuccessful. However, the use of SPhos (L7) at room temperature afforded the desired trans olefin in a selective fashion (Figure 7).
Figure 7.
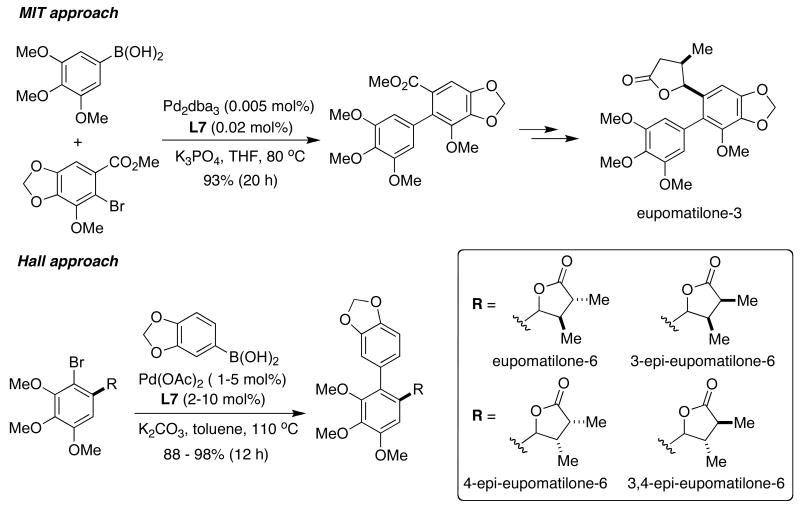
In 2005, the group of Hall and our research group reported the total syntheses of several members of the family of the eupomatilones.44 Both approaches relied upon a highly efficient SMC of different highly oxygenated aryl halides using SPhos (L7) as the ligand (Figure 8). In the MIT approach,44a a very low catalyst loading (0.005 mol% palladium) could be achieved for this transformation, in part because of the presence of an ortho carbomethoxy group. Hall's route had the advantage of being more convergent, but required a very challenging SMC.44b In his publication, he stated, “All other attempted conditions and catalysts failed in this notoriously difficult substitution pattern for a Suzuki-Miyaura biaryl cross-coupling.”
Figure 8.
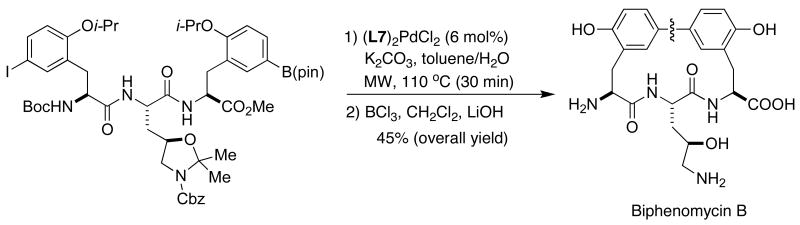
Biphenomycin B, a compound structurally related to the vancomycin glycopeptide antibiotics, displays potent activity against β-lactam-resistant bacteria. Recently, Zhu has disclosed a concise total synthesis of this important alkaloid by way of an intramolecular SMC (Figure 9).45 The synthetic challenges posed by the seemingly simple biaryl fragment should not be underestimated. A detailed survey of reaction conditions led to a process that demonstrated the superior activity of SPhos (L7), affording an intermediate containing the desired biaryl backbone. Global deprotection provided biphenomycin B in high overall yield.
Figure 9.
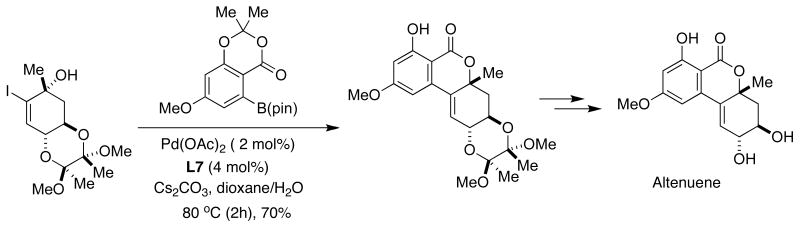
In 2006, Podlech reported the total synthesis of altenuene, a compound with activity towards HeLa cells.46 In his approach, two highly functionalized precursors were combined to give the intermediate shown below by means of SMC using SPhos (L7) as the ligand (Figure 10). In this work, the formation of the carbon-carbon bond precedes creation of the lactone unit which forms with liberation of the phenolic hydroxyl group previously protected as an acetal. Subsequent deprotection of the diol moiety in acidic media afforded altenuene in good overall yield.
Figure 10.
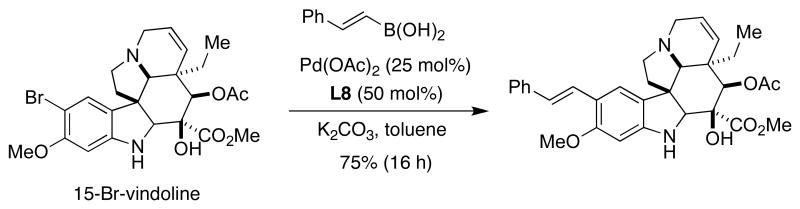
The molecular complexity of natural products such as vindoline, a biosynthetic precursor of the potent antitumor agent vinblastine, constitutes another example to test the use of the dialkylbiaryl phosphine ligands. As shown below, Rawal used the SMC of alkenyl boronic acids with C15-bromovindoline, a heavily functionalized coupling counterpart that proceeded in good yield using XPhos (L8) as a supporting ligand (Figure 11).47 It is worth mentioning that only XPhos allowed for an efficient coupling process with alkenyl boronic acids; other ligands tested by the authors gave the desired product in low yield. Despite the high catalyst loading required, this example illustrates the applicability of the method to the derivatization of highly functionalized molecules.
Figure 11.
Studies of metabotropic glutamate receptors have shown that they are involved in maladies such as anxiety, depression, mental retardation and pain.48 Recently, Newman has designed a series of heterobicyclic templates with essential features of the pharmacophores of mGluR5 antagonists.47 His approach relied on the SMC of heteroaryl halides and heteroarylboronic acids or esters. SPhos (L7) turned out to be the most efficient ligand, giving rise to the desired heterobicyclic cores in good yields (Figure 12).
Figure 12.
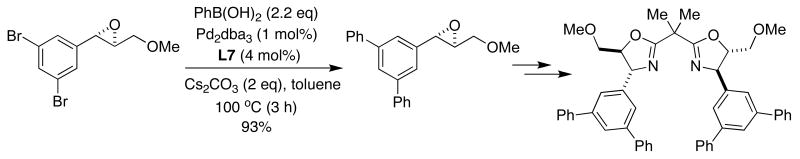
Although the tolerance of activated epoxides in transition-metal catalyzed cross-coupling reactions has little precedent,49 Pericàs showed that SPhos (L7) was the optimal supporting ligand in the SMC of enantiomerically-pure epoxides in high yield.50 The straightforward synthesis of these compounds allowed rapid access to chiral C2 symmetrical bis(oxazolines), potentially useful ligands for asymmetric catalysis (Figure 13).
Figure 13.
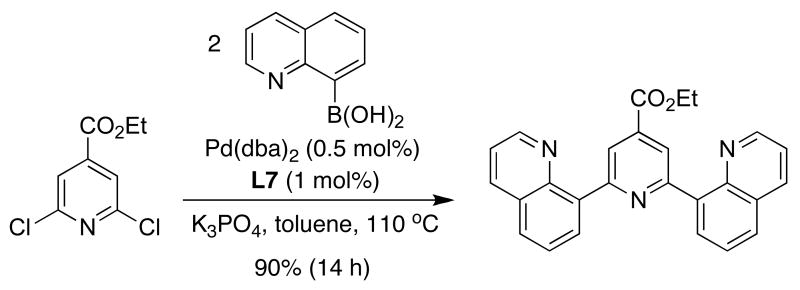
Another application of SPhos (L7) is the preparation of 2,6-di(quinolin-8-yl)-pyridine compounds, which have been reported to be excellent bistridentate ligands for RuII, providing complexes with microsecond luminescent lifetimes. Johansson demonstrated that these compounds can be efficiently prepared by the double SMC of an heteroarylboronic acid and halopyridine derivatives (Figure 14).51
Figure 14.
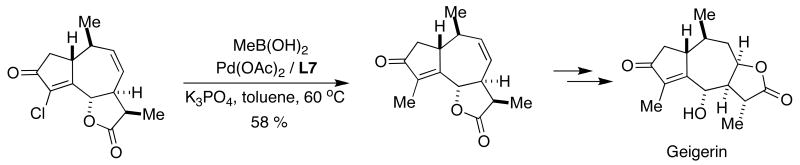
Deprés recently described the first total synthesis of naturally occurring geigerin, a member of the guaiane class of sesquiterpenes.52 This approach was accomplished in only 8 steps from the tropylium cation without the need for protecting groups. The installation of the C4 methyl group was achieved by SMC of an advanced synthetic intermediate using SPhos (L7) as the supporting ligand (Figure 15). This is one of the few examples of a SMC with alkylboronic acids that has been described in the total synthesis of a natural product.53
Figure 15.
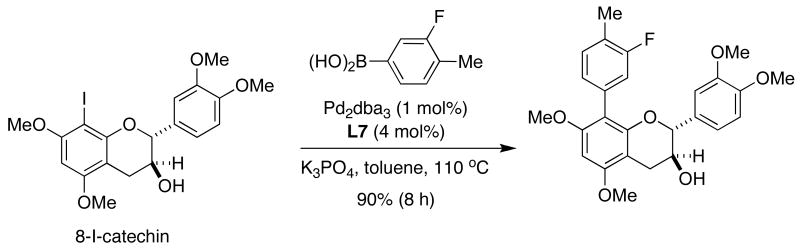
Cacchi has recently described the synthesis of derivatives of the catechins, biologically active compounds that have exhibited anticarcinogenic properties and the inhibition of platelet aggregation. In his report, a small library of 8-arylated analogues of the relatively hindered and highly electron-rich catechin core was prepared using the SMC (Figure 16).54 While most of the commonly-employed catalyst systems gave unsatisfactory results, the use of SPhos (L7) afforded the desired compounds in excellent yield.
Figure 16.
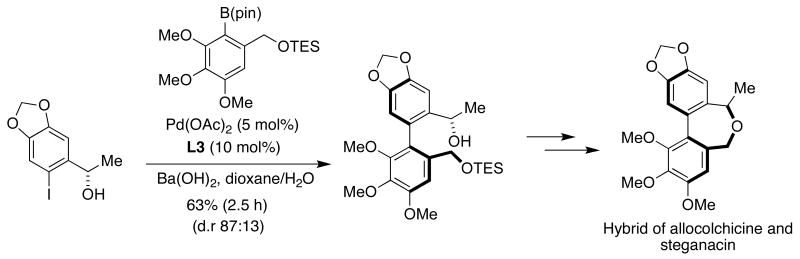
The unusual structural features of allocolchicine and steganacin have spurred a number of studies on their synthesis. Baudoin described the asymmetric synthesis of biaryl hybrids of these important molecules that featured an atropo-diastereoselective biaryl SMC in which a benzylic hydroxyl stereocenter was able to confer diastereoselectivity in the constitution of the biaryl axis (Figure 17).55 As shown below, the use of DavePhos (L3) as the ligand in the optimized reaction conditions afforded the best compromise of yield and diastereoselectivity.
Figure 17.
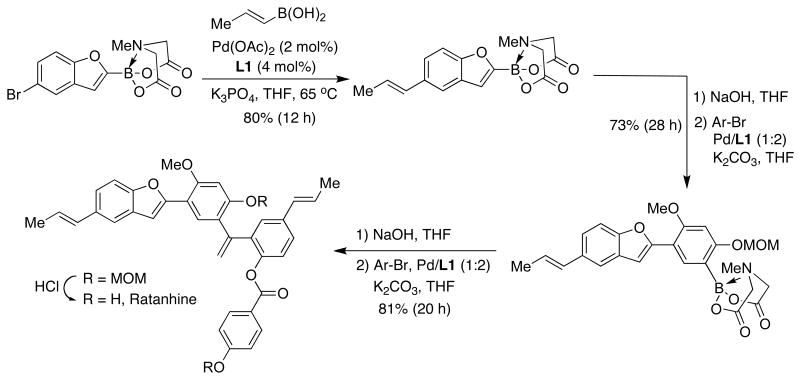
Ratanhine is a complex neolignan. Its total synthesis by Burke reflects the effect of the availability of general SMC methods in retrosynthetic planning (Figure 18).56 This route involves the clever assembly of coupling partners that contains both an aryl bromide and a protected (in an inactive form) boronic acid. It is worth mentioning that all of the carbon-carbon bond-forming reactions in the iterative route shown below are Pd-catalyzed SMC. In this particular case, the use of L1 proved to be optimal when using highly stable N-methyliminodiacetic acid protected organoboranes.
Figure 18.
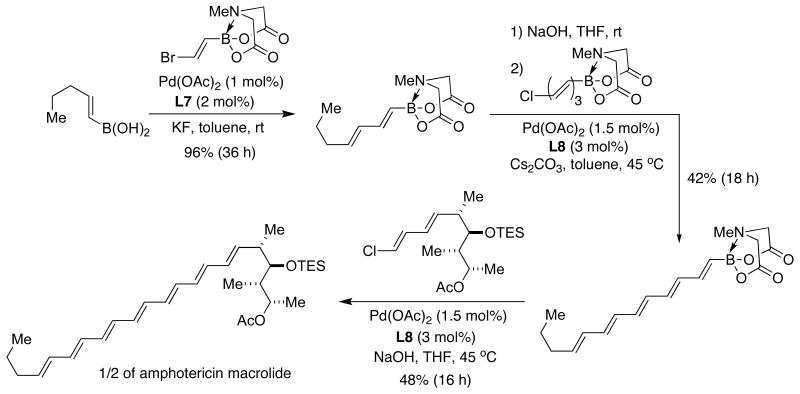
The same concept was used by Burke as an impressive illustration of the power of the iterative Suzuki-Miyaura approach in the synthesis of the amphotericin B skeleton.57 In this particular case, SPhos (L7) and XPhos (L8) proved to be necessary supporting ligands for the key SMC (Figure 19). In view of its high efficiency, the route depicted below holds promise for the application of B-protected haloalkenylboronic acids to the synthesis of a variety of complex polyene-containing natural products.
Figure 19.
Organic semiconductors and materials are important components of many optoelectronic and photonic applications, such as light-emitting diodes, photovoltaics, lasers or sensors. Most of these systems include a π-conjugated backbone, such as oligothiophene or indolo[3,2-b]carbazole skeletons. Yamaguchi58 and Leclerc59 have described highly modular routes to these interesting compounds with formation of several carbon-carbon bonds by SMC using SPhos (L7) as the ligand (Figure 20).
Figure 20.
The examples shown above illustrate the broad applicability of the SMC supported by dialkylbiaryl phosphine ligands. Given that SPhos (L7), perhaps the most versatile ligand for these reactions, was only reported in 2004, we are confident that a great number of important applications will be reported in the future.
Summary
Dialkylbiaryl phosphine ligands have been demonstrated to be widely applicable to a wide variety of Pd-catalyzed cross-coupling reactions.
The range of substrates for SMC based on these ligands include aryl bromides, aryl triflates, unactivated aryl chlorides, aryl tosylates, a variety of heteroaryl systems as well as very hindered substrate combinations. Such processes are highlighted by their broad scope and often can be carried out at room temperature and/or with low catalyst loadings. The properties of these ligands can be varied with respect to the steric and electronic effects associated with the substituents in the biaryl backbone due to the modular nature of their synthesis.
Based upon the use of these ligands in Pd-catalyzed C-C bond-forming processes in target-oriented synthesis to date, we strongly believe that the performance and the synthetic applicability of dialkylbiarylphosphine ligands in metal-catalyzed cross-coupling reactions will be utilized even further in future endeavors.
Acknowledgments
We sincerely thank all co-workers from the Buchwald laboratory and in particular those involved in the studies referred to in this article for their invaluable intellectual and experimental contributions. This research has been supported by the National Institutes of Health (GM-46059). Merck, Amgen, BASF (gifts of Pd compounds), Nippon Chemical and Boehringer Ingelheim are gratefully acknowledged for additional support.
Biographies
Stephen L. Buchwald is currently the Camille Dreyfus Professor of Chemistry at the Massachusetts Institute of Technology (MIT). He has been a member of the MIT faculty since 1984. He has received numerous awards including the American Chemical Society award in Organometallic Chemistry (2000) and for Creative Work in Synthetic Organic Chemistry (2006), the Bristol-Myers Squibb Award for Distinguished Achievement in Organic Synthesis (2005) and the Siegfried medal (2006). He has also been elected as a fellow of the American Academy of Arts and Sciences (2000) and as a member of the National Academy of Science (2008).
Ruben Martin received his Ph.D in 2003 at the University of Barcelona with Prof. Antoni Riera. In 2004, he moved to the Max-Planck-Institut für Kohlenforschung as a Humboldt postdoctoral fellow with Prof. Alois Fürstner, where he worked in the application of Fe-catalysts for cross-coupling and Alder-ene type reactions. He then undertook further postdoctoral studies at MIT with Prof. Stephen L. Buchwald where he is currently working on new synthetic strategies for metal-catalyzed C-C and C-N bond-forming reactions.
References
- 1.Leading reviews: Miyaura N. In: Metal-Catalyzed Cross-Coupling Reaction. Diederich F, de Meijere A, editors. Chapt 2 Wiley-VCH; New York: 2004. Bellina F, Carpita A, Rossi R. Palladium catalysts for the Suzuki cross-coupling reaction: an overview of recent advances. Synthesis. 2004;15:2419–2440.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev. 1995;95:2457–2483.
- 2.Kertesz M, Choi CH, Yang S. Conjugated polymers and aromaticity. Chem Rev. 2005;105:3448–3481. doi: 10.1021/cr990357p. [DOI] [PubMed] [Google Scholar]
- 3.Kaye S, Fox JM, Hicks FA, Buchwald SL. The use of catalytic amounts of CuCl and other improvements in the benzyne route to biphenyl-based phosphine ligands. Adv Synth Catal. 2001;343:789–794. [Google Scholar]
- 4.Kotha S, Lahiri K, Kashinath D. Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis. Tetrahedron. 2002;58:9633–9695. [Google Scholar]
- 5.Hall DG. In: Boronic Acids-Preparation, Applications in Organic Synthesis and Medicine. Hall DG, editor. Wiley-VCH; Weinheim: 2005. pp. 1–99. [Google Scholar]
- 6.Lipton MF, Mauragis MA, Maloney MT, Veley MF, VanderBor DW, Newby JJ, Appell RB, Daugs ED. The synthesis of OSU 6162: efficient, large-scale implementation of a Suzuki coupling. Org Process Res Dev. 2003;7:385–392. [Google Scholar]
- 7.Phan NTS, Van der Sluys M, Jones CW. On the nature of the active species in palladium-catalyzed Mizoroki-Heck and Suzuki-Miyaura couplings – homogeneous or heterogeneous catalysis, a critical review. Adv Synth Catal. 2006;348:609–679. [Google Scholar]
- 8.Braga AAC, Morgon NH, Ujaque G, Maseras F. Computational characterization of the role of the base in the Suzuki-Miyaura cross-coupling reaction. J Am Chem Soc. 2005;127:9298–9307. doi: 10.1021/ja050583i. [DOI] [PubMed] [Google Scholar]
- 9.Miyaura N. Cross-coupling reaction of organoboron compounds via base-assisted transmetalation to palladium(II) complexes. J Organomet Chem. 2002;653:54–57. [Google Scholar]
- 10.Christmann U, Vilar R. Monoligated palladium species as catalysts in cross-coupling reactions. Angew Chem Int Ed. 2005;44:366–374. doi: 10.1002/anie.200461189. [DOI] [PubMed] [Google Scholar]
- 11.For selected references of dialkylbiaryl-type ligands not developed at MIT, see: So CM, Lau CP, Kwong FY. Easily accessible and highly tunable indolyl phosphine ligands for Suzuki-Miyaura coupling of aryl chlorides. Org Lett. 2007;15:2795–2798. doi: 10.1021/ol070898y.Harkal S, Rataboul F, Zapf A, Fuhrmann C, Riermeier T, Monsees A, Beller M. Dialkylphosphinoimidazoles as new ligands for palladium-catalyzed coupling reactions of aryl chlorides. Adv Synth Catal. 2004;346:1742–1748.
- 12.Littke AF, Dai C, Fu GC. Versatile catalysts for the Suzuki cross-coupling of arylboronic acids with aryl and vinyl halides and triflates under mild conditions. J Am Chem Soc. 2000;122:4020–4028. [Google Scholar]
- 13.Kantchev EAB, O'Brien CJ, Organ MG. Palladium complexes of N-heterocyclic carbenes as catalysts for Cross-coupling reactions-A synthetic chemist's perspective. Angew Chem Int Ed. 2007;46:2768–2813. doi: 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]
- 14.Representative examples for Kumada-Corriu reactions: Martin R, Buchwald SL. Pd-catalyzed Kumada-Corriu cross-coupling reactions at low temperatures allow the use of knochel-type Grignard reagents. J Am Chem Soc. 2007;129:3844–3845. doi: 10.1021/ja070830d. Hiyama reactions: Denmark SE, Kallemeyn JM. Stereospecific palladium-catalyzed cross-coupling of (E)- and (Z)-alkenylsilanlates with aryl chlorides. J Am Chem Soc. 2006;128:15958–15959. doi: 10.1021/ja065988x. α-Arylation of carbonyl compounds: Martin R, Buchwald SL. A general method for the direct α-arylation of aldehydes with aryl bromides and chlorides. Angew Chem Int Ed. 2007;46:7236–7239. doi: 10.1002/anie.200703009.
- 15.Recent examples: Ikawa T, Barder TE, Biscoe MR, Buchwald SL. Pd-catalyzed amidations of aryl chlorides using monodentate biaryl phosphine ligands: a kinetic, computational and synthetic investigation. J Am Chem Soc. 2007;129:13001–13007. doi: 10.1021/ja0717414.Biscoe MR, Barder TE, Buchwald SL. Electronic effects on the selectivity of Pd-catalyzed C-N bond-forming reactions using biarylphosphine ligands: the competitive roles of amine binding and acidity. Angew Chem Int Ed. 2007;46:7232–7235. doi: 10.1002/anie.200702122.
- 16.Anderson KW, Ikawa T, Tundel RE, Buchwald SL. The selective reaction of aryl halides with KOH: synthesis of phenols aromatic ethers and benzofurans. J Am Chem Soc. 2006;128:10694–10695. doi: 10.1021/ja0639719. [DOI] [PubMed] [Google Scholar]
- 17.Barder TE, Buchwald SL. Rationale behind the resistance of dialkylbiaryl phosphines toward oxidation by molecular oxygen. J Am Chem Soc. 2007;129:5096–5101. doi: 10.1021/ja0683180. [DOI] [PubMed] [Google Scholar]
- 18.Leading references: Barrios-Landeros F, Hartwig JF. Distinct mechanisms for the oxidative addition of chloro-, bromo-, and iodoarenes to a bisphosphine palladium(0) complex with hindered ligands. J Am Chem Soc. 2005;127:6944–6945. doi: 10.1021/ja042959i.Hartwig JF, Paul F. Oxidative addition of aryl bromide after dissociation of phosphine from a two-coordinate palladium(0) complex, bis(tri-o-tolylphosphine)palladium(0) J Am Chem Soc. 1995;117:5373–5374.
- 19.Leading references: Hartwig JF. Electronic effects on reductive elimination to form carbon-carbon heteroatom bonds from palladium(II) complexes. Inorg Chem. 2007;46:1936–1947. doi: 10.1021/ic061926w.Barder TE, Buchwald SL. Insights into amine binding to biaryl phosphine palladium oxidative addition complexes and reductive elimination from biaryl phosphine arylpalladium amido complexes via density functional theory. J Am Chem Soc. 2007;129:12003–12010. doi: 10.1021/ja073747z.
- 20.Strieter ER, Buchwald SL. Evidence for the formation and structure of palladacycles during Pd-catalyzed C-N bond formation with catalysts derived from bulky monophosphinobiaryl ligands. Angew Chem Int Ed. 2006;45:925–928. doi: 10.1002/anie.200502927. [DOI] [PubMed] [Google Scholar]
- 21.Littke AF, Fu GC. Palladium-catalyzed coupling reactions of aryl chlorides. Angew Chem Int Ed. 2002;41:4176–4211. doi: 10.1002/1521-3773(20021115)41:22<4176::AID-ANIE4176>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Old DW, Wolfe JP, Buchwald SL. A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J Am Chem Soc. 1998;120:9722–9723. [Google Scholar]
- 23.Littke AF, Fu GC. A convenient and general method for Pd-catalyzed Suzuki cross-couplings of aryl chlorides and arylboronic acids. Angew Chem In Ed. 1998;37:3387–3388. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3387::AID-ANIE3387>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe JP, Buchwald SL. A highly active catalyst for the room-temperature amination and Suzuki coupling of aryl chlorides. Angew Chem Int Ed. 1999;38:2413–2416. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe JP, Singer RA, Yang BH, Buchwald SL. Highly active palladium catalysts for Suzuki coupling reactions. J Am Chem Soc. 1999;121:9550–9561. [Google Scholar]
- 26.Dai C, Fu GC. The First General Method for Palladium-Catalyzed Negishi Cross-Coupling of Aryl and Vinyl Chlorides: Use of Commercially Available Pd(P(t-Bu)3)2 as a Catalyst. J Am Chem Soc. 2001;123:2719–2724. doi: 10.1021/ja003954y. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, Rainka MP, Zhang XX, Buchwald SL. A highly active Suzuki catalyst for the synthesis of sterically hindered biaryls: novel ligand coordination. J Am Chem Soc. 2002;124:1162–1163. doi: 10.1021/ja017082r. [DOI] [PubMed] [Google Scholar]
- 28.(a) Walker SD, Barder TE, Martinelli JR, Buchwald SL. A rationally designed universal catalyst for Suzuki-Miyaura coupling processes. Angew Chem Int Ed. 2004;43:1871–1876. doi: 10.1002/anie.200353615. [DOI] [PubMed] [Google Scholar]; (b) Barder TE, Walker SD, Martinelli JR, Buchwald SL. Catalysts for Suzuki-Miyaura coupling processes: scope and studies of the effect of ligand structure. J Am Chem Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 29.(a) Hayashi T, Iwamura H, Naito M, Matsumoto Y, Uozumi Y. Catalytic asymmetric reduction of allylic esters with formic acid catalyzed by palladium-MOP complexes. J Am Chem Soc. 1994;116:775–776. [Google Scholar]; (b) Kočovoský P, Vyskočil S, Císařová I, Sejbal J, Tišlerová I, Smrčina M, Lloyd-Jones GC, Stephen SC, Butts CP, Murray M, Langer V. Palladium(II) Complexes of 2-dimethylamino-2′-diphenylphosphino-1,1′-binaphthyl (MAP) with unique P,Cσ-coordination and their catalytic activity in allylic substitution, Hartwig-Buchwald amination, and Suzuki coupling. J Am Chem Soc. 1999;121:7714–7715. [Google Scholar]
- 30.Barder TE, Biscoe MR, Buchwald SL. Structural insights into active catalyst structures and oxidative addition to (biaryl)phosphine-palladium complexes via density functional theory and experimental studies. Organometallics. 2007;26:2183–2192. [Google Scholar]
- 31.Braga AAC, Morgon NH, Ujaque G, Lledós A, Maseras F. Computational study of the transmetallation process in the Suzuki-Miyaura cross-coupling of aryls. J Organomet Chem. 2006;691:4459–4466. [Google Scholar]
- 32.Shaughnessy KH. Beyond TPPTS: New approaches to the development of efficient palladium-catalyzed aqueous-phase cross-coupling reactions. Eur J Org Chem. 2006:1827–1835. [Google Scholar]
- 33.Anderson KW, Buchwald SL. General catalysts for the Suzuki-Miyaura and Sonogashira coupling reactions of aryl chlorides and for the coupling of challenging substrate combinations in water. Angew Chem Int Ed. 2005;44:6173–6177. doi: 10.1002/anie.200502017. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura I, Yamamoto Y. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem Rev. 2004;104:2127–2198. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- 35.Tyrrell E, Brookes P. The synthesis and applications of heterocyclic boronic acids. Synthesis. 2003;4:469–483. [Google Scholar]
- 36.Caron S, Massett SS, Bogle DE, Castaldi MJ, Braish TF. An efficient and cost-effective synthesis of 2-phenyl-3-aminopyridine. Org Process Res Dev. 2001;5:254–256. [Google Scholar]
- 37.Itoh T, Mase T. Direct synthesis of hetero-biaryl compounds containing an unprotected NH2 group via Suzuki-Miyaura reaction. Tetrahedron Lett. 2005;46:3573–3577. [Google Scholar]
- 38.Billingsley K, Anderson KW, Buchwald SL. A Highly active catalyst for Suzuki-Miyaura cross-coupling reactions of heteroaryl compounds. Angew Chem Int Ed. 2006;45:3484–3488. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]
- 39.Billingsley K, Buchwald SL. Highly efficient monophosphine-based catalyst for the palladium-catalyzed Suzuki-Miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters. J Am Chem Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]
- 40.Johnson C, Stemp G, Anand N, Stephen S, Gallaguer T. Palladium(0)-Catalysed Arylations using Pyrrole and Indole 2-Boronic Acids. Synlett. 1998:1025–1027. [Google Scholar]
- 41.(a) Murata M, Sambommatsu T, Watanabe S, Masuda Y. An efficient catalyst system for palladium-catalyzed borylation of aryl halides with pinacolborane. Synlett. 2006;12:1867–1870. [Google Scholar]; (b) Ishiyama T, Ishida K, Miyaura N. Synthesis of pinacol arylboronates via cross-coupling reaction of bis(pinacolato)diboron with chloroarenes catalyzed by palladium(0)–tricyclohexylphosphine complexes. Tetrahedron. 2001;49:9813–9816. [Google Scholar]
- 42.Billingsley K, Barder TE, Buchwald SL. Palladium-catalyzed borylation of aryl chlorides: scope, applications and computational studies. Angew Chem Int Ed. 2007;46:5359–5363. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]
- 43.Raheem IT, Goodman SN, Jacobsen EN. Catalytic asymmetric total syntheses of Quinine and Quinidine. J Am Chem Soc. 2004;126:706–707. doi: 10.1021/ja039550y. [DOI] [PubMed] [Google Scholar]
- 44.(a) Rainka MP, Milne JE, Buchwald SL. Dynamic kinetic resolution of α,β-unsaturated lactones through asymmetric copper-catalyzed conjugate reduction: application to the total synthesis of Eupomatilone-3. Angew Chem Int Ed. 2005;44:6177–6180. doi: 10.1002/anie.200501890. [DOI] [PubMed] [Google Scholar]; (b) Yu SH, Ferguson MJ, McDonald R, Hall DG. Brønsted acid-catalyzed allylboration: short and stereodivergent synthesis of all four eupomatilone diastereomers with crystallographic assignments. J Am Chem Soc. 2005;127:12808–12809. doi: 10.1021/ja054171l. [DOI] [PubMed] [Google Scholar]
- 45.Lépine R, Zhu J. Microwave-assisted intramolecular Suzuki-Miyaura reaction to macrocycle, a concise asymmetric total synthesis of Biphenomycin B. Org Lett. 2005;14:2981–2984. doi: 10.1021/ol050949w. [DOI] [PubMed] [Google Scholar]
- 46.Altemöller M, Podlech J, Fenske D. Total synthesis of Altenuene and Isoaltenuene. Eur J Org Chem. 2006:1678–1684. [Google Scholar]
- 47.Johnson PD, Sohn JH, Rawal VH. Synthesis of C-15 Vindoline analogues by palladium-catalyzed cross-coupling reactions. J Org Chem. 2006;71:7899–7902. doi: 10.1021/jo061243y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni SS, Newman AH. Discovery of heterobicyclic templates for novel metabotropic glutamate receptor subtype 5 antagonists. Bioorg Med Chem Let. 2007;17:2987–2991. doi: 10.1016/j.bmcl.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.(a) Zou G, Reddy K, Falck JR. Ag(I)-promoted Suzuki-Miyaura cross-coupling of n-alkylboronic acids. Tetrahedron Lett. 2001;42:7213–7215. [Google Scholar]; (b) Falck JR, Kumar PS, Reddy YK, Zou G, Capdevila JH. Tetrahedron Lett. 2001;42:7211–7212. [Google Scholar]
- 50.Cattoën X, Pericàs MA. Suzuki cross-coupling on enantiomerically pure epoxides: efficient synthesis of diverse, modular amino alcohols from single enantiopure precursors. J Org Chem. 2007;72:3253. doi: 10.1021/jo062612t. [DOI] [PubMed] [Google Scholar]
- 51.Jager M, Eriksson L, Bergquist J, Johansson O. Synthesis and characterization of 2,6-di(quinolin-8-yl)pyridines. New ligands for bistridentate RuII complexes with microsecond luminescent lifetimes. J Org Chem. 2007;72:10227–10330. doi: 10.1021/jo7015373. [DOI] [PubMed] [Google Scholar]
- 52.Carret S, Deprés JP. Access to Guaianolides: highly efficient stereocontrolled total synthesis of (±)-Geigerin. Angew Chem Int Ed. 2007;46:6870–6873. doi: 10.1002/anie.200702031. [DOI] [PubMed] [Google Scholar]
- 53.(a) Nicolaou KC, Bulger PG, Sarlah D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew Chem Int Ed. 2005;44:4442–4449. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]; (b) Chemler SR, Trauner D, Danishefsky SJ. The B-alkyl Suzuki-Miyaura cross-coupling reaction: development, mechanistic study, and applications in natural product synthesis. Angew Chem Int Ed. 2001;40:4544–4568. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 54.Bernini R, Cacchi S, De Salve I, Fabrizi G. Synthesis of 8-arylated Catechin and Epicatechin derivatives via Suzuki cross-coupling. Tetrahedron Lett. 2007;48:4973–4976. [Google Scholar]
- 55.Joncour A, Décor A, Liu JM, Tran Huu Dau ME, Baudoin O. Asymmetric synthesis of antimicrotubule biaryl hybrids of Allocolchicine and Steganacin. Chem Eur J. 2007;13:5450–5465. doi: 10.1002/chem.200601764. [DOI] [PubMed] [Google Scholar]
- 56.Gillis EP, Burke MD. A simple and modular strategy for small molecule synthesis: iterative Suzuki-Miyaura coupling of B-protected haloboronic acid building blocks. J Am Chem Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 57.Lee SJ, Gray KC, Paek JS, Burke MD. Simple, efficient and modular syntheses of polyene natural products via iterative cross-coupling. J Am Chem Soc. 2008;130:466–468. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wakayima A, Mori K, Yamaguchi S. 3-Boryl-2,2′-bithiophene as a versatile core skeleton for full-color highly emissive organic solids. Angew Chem Int Ed. 2007;46:4273–4276. doi: 10.1002/anie.200604935. [DOI] [PubMed] [Google Scholar]
- 59.Boudreault PLT, Wakim S, Blouin N, Simard M, Tessier C, Tao Y, Leclerc M. Synthesis, characterization, and application of Indolo[3,2-b]carbazole semiconductors. J Am Chem Soc. 2007;129:9125–9136. doi: 10.1021/ja071923y. [DOI] [PubMed] [Google Scholar]