Abstract
A method was developed for the hydroboration of alkenyl-containing organotrifluoroborates to generate dibora intermediates. The reactivity differences between organotrifluoroborates and trialkylboranes facilitated the cross-coupling of the borane moiety of these intermediates in a highly chemoselective fashion with aryl halides, leaving the trifluoroborate intact for subsequent transformation. A one-pot hydroboration/two-directional cross-coupling sequence was also demonstrated, providing the fully elaborated products in good yields. These conditions were also amenable in the cross-coupling of trialkylboranes to halo-containing organotrifluoroborates. The stability of the trifluoroborate moiety to these conditions allows simple and efficient strategies for complex molecule construction.
Several different strategies utilizing two orthogonally reactive boron species have been used to increase complexity and diversity in organic substructures. Although assorted dibora-substituted organic molecules have been described and utilized in this context,1 for the most part this approach suffers from a few significant limitations. For example, the two boron species are often functionally identical and both are chemically processed in the same way.2 In other cases, the ultimate fate of the two organoborons takes advantage of inherent reactivity differences related to the reactive substituent on boron.3 Lastly, the two boron species may be symmetrically reactive, and reaction at one center leaves an organoboron that is unreactive under similar conditions.4
There are a few examples detailing the cross-coupling of electrophilic partners containing protected versions of boronic acids,5 but to date a single example can be found that generates a dibora compound and sequentially cross-couples the organoborons with selectivity based solely on the nature of the non-participating boron substituents.6
Herein we report the development of dibora-substituted linchpins allowing bidirectional syntheses of diverse organic substructures. Although cross-couplings of organotrifluoroborates with aryldiazoniums and diaryliodoniums can be carried out under anhydrous conditions,7 other electrophilic partners (e.g., halides, triflates) require aqueous or protic conditions.8 Owing to the reactivity of trialkylboranes in the absence of water, we explored their formation and selective cross-coupling in the presence of organotrifluoroborates.
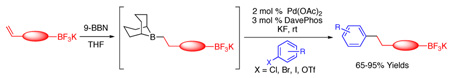
The hydroboration of alkenyl-containing organotrifluoroborates with 9-BBN generates intermediate dibora species. These linchpins undergo selective cross-coupling at the trialkylborane, providing pure, elaborated trifluoroborates. Subsequently, a further increase in molecular complexity can be achieved by cross-coupling the organotrifluoroborate.
Using 4-bromobenzonitrile as a model electrophile with potassium 4-(but-3-enyl)phenyltrifluoroborate, a brief screening of the cross-coupling step was carried out.11 Use of 2 mol % Pd(OAc)2, 3 mol % DavePhos,12 and KF (3 equiv), with stirring overnight at rt afforded the highest conversion and isolated yield (79%) of the corresponding cross-coupled product (Table 1, entry 1).
Table 1.
Hydroboration and Suzuki-Miyaura Cross-Coupling of Alkenyl-Containing Potassium Organotrifluoroboratesa
 | ||||
|---|---|---|---|---|
| entry | R | X | % isolated yield | |
| 1 | CN | Br | 79 | |
| 2 | CN | Cl | 82 | |
| 3 | CN | I | 69 | |
| 4 | CN | OTf | 72 | |
| 5 | pyrrole | Cl | 71 | |
| 6 | pyrrole | Cl | 70 | |
| 7 | CN | Br | 86 | |
| 8 | CN | Cl | 87 (87b) | |
| 9 | CN | Br | 67 | |
| 10 | CN | Cl | 80 | |
| 11 | pyrrole | Cl | 76 | |
| 12 | pyrrole | Cl | 95 | |
| 13 | pyrrole | Cl | 65 | |
| 14 | pyrrole | Cl | 65 | |
General conditions: RBF3K (1.0 equiv), 9-BBN (1.0 equiv) and THF (0.25 M) then Pd(OAc)2 (2 mol %), DavePhos (3 mol %), aryl electrophile (1.0 equiv), and KF (3 equiv), rt, overnight.
Reaction scaled to 5 mmol.
With conditions in hand, various alkenyl-containing aryltrifluoroborate substrates were hydroborated and selectively cross-coupled (Table 1, entries 1–8, and 14). Aryl bromides, chlorides, iodides, and triflates were found to cross-couple with complete selectivity at the borane moiety of the dibora intermediate, in each case providing the elaborated potassium aryltrifluoroborate product in good yields. The reaction also proved to be scalable to 5 mmol (Table 1, entry 8).
The reaction of potassium vinyltrifluoroborate also proceeded smoothly in the sequence, cleanly generating the cross-coupled product (Table 1, entries 9–11). Of particular note is the regioselectivity of the initial hydroboration step, creating a reversal in the pattern associated with tricoordinate alkenylborons. The resulting dibora species represents a 1,2-dianion equivalent that is markedly distinct from the 1,1-dibora intermediates traditionally formed when tricoordinate alkenylborons undergo hydroboration.13
Alkenyl-containing alkyltrifluoroborates were found to be suitable substrates in the reaction sequence (Table 1, entries 12–13). The limited solubility of potassium alkoxymethyltrifluoroborates14 in THF (Table 1, entry 13) required more dilute reaction conditions and significantly longer reaction time for the hydroboration step to occur.
With the ultimate goal of bidirectional functionalization, we turned our attention to a one-pot hydroboration/bidirectional cross-coupling sequence (Table 2). After subjecting the substrates to the first hydroboration/cross-coupling sequence, reaction with a second electrophile under conditions previously optimized for aryltrifluoroborates (entries 1–3),15 N,N-dialkylaminomethyltrifluoroborates (entry 4),17 and alkoxymethyltrifluoroborates (entry 5)16 generated the desired products.
Table 2.
One-Pot Hydroboration and Bidirectional Suzuki-Miyaura Cross-Coupling of Alkenyl-Containing Organotrifluoroboratesa
General Conditions: RBF3K (1.0 equiv), 9-BBN (1.0 equiv) and THF (0.25 M) then Pd(OAc)2 (2 mol %), DavePhos (3 mol %), aryl electrophile (1.0 equiv), and KF (3 equiv), rt, overnight.
RBF3K coupling: Pd(OAc)2 (0.5 mol %), electrophile (1.0 equiv), K2CO3 (3 equiv) and MeOH (0.125 M), 65 °C, 2 h.
RBF3K coupling: Pd(OAc)2 (3 mol %), XPhos9 (6 mol %), electrophile (1.0 equiv), Cs2CO3 (3 equiv) and 10:1 THF/H2O (0.25 M), 80 °C, 24 h.
RBF3K coupling: Pd(OAc)2 (3 mol %), RuPhos10 (6 mol %), electrophile (1.0 equiv), Cs2CO3 (3 equiv) and 10:1 dioxane/H2O (0.25 M), 100 °C, 24 h.
We then investigated the cross-coupling of trialkylborane reagents with electrophiles containing the organotrifluoroborate moiety. Treatment of allylbenzene with 9-BBN followed by exposure to the conditions previously described for the cross-coupling of dibora substrates provided the elaborated aryl- and alkyltrifluoroborate products in good yields (Table 3). This method can be extended to bidirectional functionalization as well. This one-pot sequence provided the fully elaborated product in excellent yield (eq 1).
Table 3.
Hydroboration of Allylbenzene and Suzuki-Miyaura Cross-Coupling with Halo-Containing Trifluoroboratesa
 | |||
|---|---|---|---|
| entry | product | % isolated yield | |
| 1 | X = Br; 91 X = Cl; 85 |
||
| 2 |  |
 |
81 |
| 3 |  |
71 | |
General conditions: 9-BBN (1.1 equiv), allylbenzene (1.1 equiv), and THF (0.25 M), then Pd(OAc)2 (2 mol %), DavePhos (3 mol %), RBF3K (1.0 equiv), and KF (3 equiv), rt, 4h.
 |
(1) |
Taking into consideration the reactivity differences between organotrifluoroborates and trialkylboranes, a method was developed to hydroborate alkenyl-containing organotrifluoroborates. Conditions were found for reaction of the borane moiety of these dibora-intermediates in a highly chemoselective fashion, leaving the trifluoroborate intact for subsequent transformation. The stability of the trifluoroborate moiety to these metal catalyzed reactions allows simple and efficient strategies for multicomponent complex molecule construction.
Supplementary Material
Experimental procedures, compound characterization data, and NMR spectral data of all compounds synthesized. This material is available free of charge via the Internet at http://pubs.acs.org
Acknowledgement
The authors thank the NIH (General Medical Sciences) and Merck Research Laboratories for their generous support of our program. We acknowledge Johnson Matthey for their donation of palladium catalysts and Frontier Scientific for their donation of boronic acids. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining HRMS data.
References
- 1.Dembitsky VM, Ali HA, Srebnik M. Appl. Organomet. Chem. 2003;17:327. [Google Scholar]
- 2.(a) (a) Wiesauer C, Weissensteiner W. Tetrahedron: Asymmetry. 1996;7:5. [Google Scholar]; (b) Morgan JB, Miller SP, Morken JP. J. Am. Chem. Soc. 2003;125:8207. doi: 10.1021/ja035851w. [DOI] [PubMed] [Google Scholar]; (c) Trudeau S, Morgan JB, Shrestha M, Morken JP. J. Org. Chem. 2005;70:9538. doi: 10.1021/jo051651m. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kalendra DM, Duenes RA, Morken JP. Synlett. 2005:1749. [Google Scholar]; (b) Miller SP, Morgan JB, Nepveaux FJV, Morken JP. Org. Lett. 2004;6:131. doi: 10.1021/ol036219a. [DOI] [PubMed] [Google Scholar]; (c) Desurmont G, Klein R, Uhlenbrock S, Laloë E, Deloux L, Giolando DM, Kim YW, Pereira S, Srebnik M. Organometallics. 1996;15:3323. [Google Scholar]; (d) Brown HC, Narla G. J. Org. Chem. 1995;60:4686. [Google Scholar]; (e) Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. J. Am. Chem. Soc. 2004;126:16328. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]; (f) Woodward AR, Burks HE, Chan LM, Morken JP. Org. Lett. 2005;7:5505. doi: 10.1021/ol052312i. [DOI] [PubMed] [Google Scholar]; (g) Sieber JD, Morken JP. J. Am. Chem. Soc. 2006;128:74. doi: 10.1021/ja057020r. [DOI] [PubMed] [Google Scholar]; (h) Pelz NF, Morken JP. Org. Lett. 2006;8:4557. doi: 10.1021/ol0616891. [DOI] [PubMed] [Google Scholar]; (i) Burks HE, Liu S, Morken JP. J. Am. Chem. Soc. 2007;129:8766. doi: 10.1021/ja070572k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Morgan JB, Morken JP. Org. Lett. 2003;5:2573. doi: 10.1021/ol034936z. [DOI] [PubMed] [Google Scholar]; (b) Ballard CE, Morken JP. Synthesis. 2004:1321. [Google Scholar]
- 5.(a) Gillis EP, Burke MD. J. Am. Chem. Soc. 2007;129:6716. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]; (b) Noguchi H, Hojo K, Suginome M. J. Am. Chem. Soc. 2007;129:758. doi: 10.1021/ja067975p. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Gray KC, Paek JS, Burke MD. J. Am. Chem. Soc. 2008;130:466. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genêt J-P, Darses S, Brayer J-L, Demoute J-P. Tetrahedron Lett. 1997;38:4393. [Google Scholar]
- 8.Molander GA, Biolatto B. J. Org. Chem. 2003;68:4302. doi: 10.1021/jo0342368. [DOI] [PubMed] [Google Scholar]; (b) Batey RA, Quach TD. Tetrahedron Lett. 2002;42:9099. [Google Scholar]; (c) Batey RA, Thadani AN. Org. Lett. 2002;4:3827. doi: 10.1021/ol026619i. [DOI] [PubMed] [Google Scholar]; (d) Hutton CA, Yuen AK. Tetrahedron Lett. 2005;46:7899. [Google Scholar]
- 9.2-Dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl.
- 10.2-Dicyclohexylphosphino-2',6'-diisopropoxy-1,1'-biphenyl.
- 11.(a) Old DW, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1998;120:9772. [Google Scholar]; (b) Wolfe JP, Singer RA, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:9550. [Google Scholar]
- 12.(2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl.
- 13.(a) Colberg JC, Rane A, Vaquer J, Soderquist JA. J. Am. Chem. Soc. 1993;115:6065. [Google Scholar]; (b) Wrackmeyer B, Schanz H-J. Collect. Czech. Chem. Commun. 1997;62:1254. [Google Scholar]
- 14.Molander GA, Canturk B. Org. Lett. 2008;10:2135. doi: 10.1021/ol800532p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molander GA, Biolatto B. Org. Lett. 2002;4:1867. doi: 10.1021/ol025845p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, compound characterization data, and NMR spectral data of all compounds synthesized. This material is available free of charge via the Internet at http://pubs.acs.org