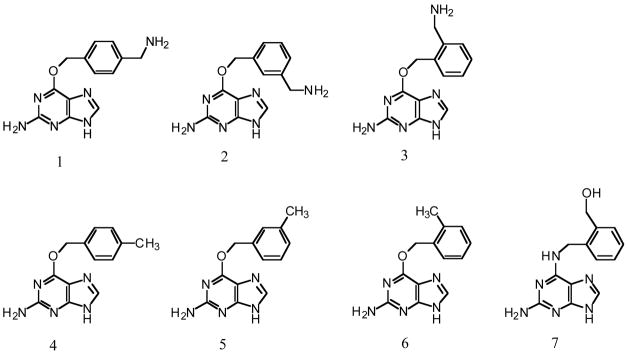
Abstract
O6-Benzylguanine is an irreversible inactivator of O6-alkylguanine-DNA alkyltransferase currently in clinical trials to overcome alkyltransferase-mediated resistance to certain cancer chemotherapeutic alkylating agents. In order to produce more soluble alkyltransferase inhibitors, we have synthesized three aminomethyl-substituted O6-benzylguanines and found that the substitution at the meta- position greatly enhances inactivation of alkyltransferase whereas para- substitution has little effect and ortho- substitution virtually eliminates activity. Molecular modeling of their interactions with alkyltransferase provided a molecular explanation for these results. The square of the correlation coefficient (R2) obtained between E-model scores (obtained from GLIDE XP/QPLD docking calculations) vs. log(ED50) values via a linear regression analysis was 0.96. The models indicate that the ortho- substitution causes a steric clash interfering with binding whereas the meta- aminomethyl substitution allows an interaction of the amino group to generate an additional hydrogen bond with the protein.
Introduction
O6-Alkylguanine-DNA alkyltransferase (alkyltransferasee) is a unique DNA repair protein that acts in a single step to restore DNA with O6-alkylguanine adducts by transferring the alkyl group to an acceptor site, which in human alkyltransferase is located at Cys145 1–3. Alkyltransferase activity in tumors is an important source of resistance to therapeutic alkylating agents such as dacarbazine, temozolomide, 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) or 1-(2-chloroethyl)-3-(trans-4- methylcyclohexyl)-1-nitrosourea 1, 2, 4–8. O6-Benzylguanine (BG) is a potent inhibitor of human alkyltransferase, which acts as a pseudosubstrate. After binding in the active site, it leads to the formation of S-benzylcysteine at the Cys145 acceptor site irreversibly inactivating the protein 9–11. A related compound O6-(4-bromothenyl)guanine was found to be a slightly more potent inactivator of alkyltransferase 12 and is thought to act in the same way. Both compounds are undergoing clinical trials 4, 7, 8, 13–18. Although there have been some responses in these trials providing proof of concept for the target, it is clear that improved inhibitors will be needed.
Since the original development of BG, structural and biochemical studies have provided a much greater understanding of the repair reaction catalyzed by alkyltransferase and the binding and reaction of this inhibitor. After binding the substrate DNA via the minor groove using a helix-turn-helix motif, the alkyltransferase protein brings about a change in the DNA structure that rotates the alkylated guanine deoxynucleoside from the base stack into an active site pocket which contains the Cys145 acceptor residue 3, 19. This places the O6-alkylguanine in the correct position for repair in a hydrophobic cleft where the Cys145 and Val148 carbonyls accept hydrogen bonds from the exocyclic amine of the guanine, the N of Ser159 donates a hydrogen bond to the substrate guanine-O6, and the hydroxyl of Tyr114 donates a hydrogen bond to guanine-N3. These interactions exactly position the alkyl group for attack by Cys145. This residue is highly reactive by virtue of its activation to a thiolate anion by a Glu172:His146:water:Cys145 hydrogen bond network 3, 11, 20. BG binds much more weakly at the active site pocket since all of the interactions with the alkyltransferase DNA-binding domain are lost but it is held in a position that allows attack by Cys145 via the interactions with the guanine moiety described above and the interaction of the benzyl group with the side-chain of Pro140 3, 11. Mutation of this Pro residue profoundly reduces the ability of BG to inactivate human alkyltransferase 21, 22. Despite the weak binding, the reactivity of benzyl in bimolecular displacement reactions such as that occurring in the alkyltransferase active site facilitates the inactivation reaction. However, the rate constant for the reaction with BG free base is only c. 600 M−1. sec−1 10, which is >10,000 times less than that for the repair of O6-methylguanine in DNA or of oligodeoxyribonucleotides containing BG, which are much more potent inactivators 23, 24. Such oligodeoxyribonucleotides are not ideal for clinical use and other attempts to improve the binding of low M.W. pseudosubstrates are needed.
Another serious problem with BG is its poor solubility. In an attempt to make more soluble derivatives we have now tested O6-[(aminomethyl)benzyl]guanines (compounds 1– 3 shown in Figure 1) for the ability to inactivate human alkyltransferase. It was found that the nature and position of the substitution profoundly affected inhibition and that compound 2, the substitution with an aminomethyl group at the meta- position, provides a more potent and much more soluble inhibitor than BG. Comparison with O6-(methylbenzyl)guanines (compounds 4–6) was used to determine if the improved activity was due to stabilization of the transition state by a positional effect of substitution on the benzyl ring or to the ability of the meta-aminomethyl group to bind more tightly to the active site. Compounds 4–6 did not show improvement over BG suggesting that the latter was the case. Molecular modeling studies using one of the crystal structures available for the protein that involved its interaction with compounds 1–6 and BG were carried out and provide a plausible explanation for these results and show that the meta-substituent on 2 provides additional interactions with the active site pocket that increase the affinity for binding to the alkyltransferase protein.
Figure 1.
Substituted BGs and an N6-[(hydroxymethyl)benzyl]-2-aminoadenine (7) resulting from a rearrangement of 3.
Results and Discussion
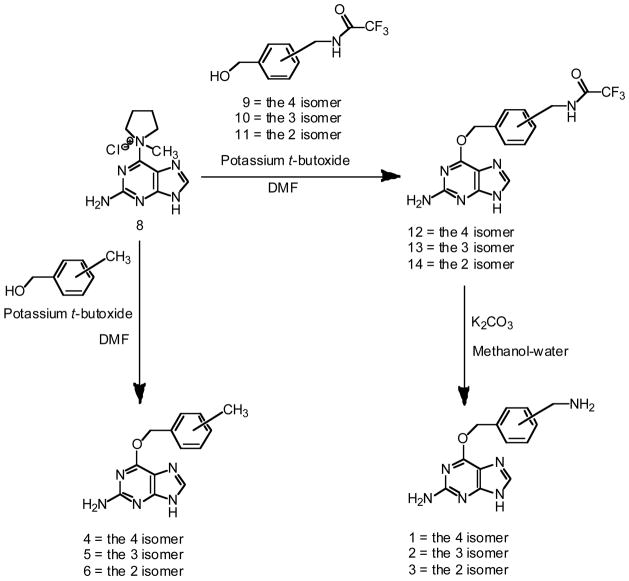
Synthesis of methyl- and aminomethyl- substituted BGs
Preparation of the three isomeric O6-[(aminomethyl)benzyl]guanines (1–3), was adapted from the method reported by Keppler et al. 25 to make compound 1 as shown in Scheme 1. 2-Amino-6-chloropurine was converted to 1-(2-Amino-9H-purin-6-yl)-1-methyl-pyrrolidinium chloride (8). The amine groups of the three isomers of (aminomethyl)benzyl alcohol were protected as the corresponding trifluoroacetamides (9, 10 and 11). Treatment of compound 8 in DMF with one of the three protected (aminomethyl)benzyl alcohols in the presence of potassium t-butoxide gave the corresponding trifluoroacetyl protected O6-(aminomethyl)benzylguanines (12, 13 and 14). Removal of the trifluoroacetate protecting groups with potassium carbonate in methanol-water then gave the three O6-(aminomethyl)benzylguanines (1, 2 and 3). Similarly, the treatment of 8 with one of the three isomers of methylbenzyl alcohol in the presence of potassium t-butoxide gave the three methylbenzylguanines 4, 5 and 6 (Scheme 1).
Scheme 1.
Synthesis of O6-[(aminomethyl)benzyl]guanines and O6-(methylbenzyl)guanines.
While attempting to recrystallize a sample of O6-[2-(aminomethyl)benzyl]guanine (3) from boiling water, an insoluble precipitate formed. This precipitate was recovered by filtration. The proton NMR spectrum of the precipitated material was not consistent with the original sample of compound 3, even though MS analysis of the original sample and the precipitate gave similar molecular ions with m/z=271.1 [M+H]+. A sample of compound 3 was dissolved in water and heated at 65°C for one week, the precipitate was then recovered for further analysis by NMR. The NMR spectrum of the precipitated material is consistent with N6-[2-(hydroxymethyl)benzyl]-2-aminoadenine (7 in Figure 1), which we propose is formed by intramolecular attack of the benzylic amine at the 6-position of the purine, displacing the oxygen. This rearrangement was not observed with 1 and 2.
Inactivation of purified human alkyltransferase in vitro
Compound 1, the para- substituted aminomethyl derivative of BG, was only slightly more active than BG itself but the meta- substituted derivative 2 was considerably more active with an ED50 of approx. 4 nM compared to 100 nM for BG when assayed in the presence of DNA and 17 nM compared to 300 nM when assayed in the absence of DNA (Table 1). The inactivation of the S159A mutant alkyltransferase by compounds 1 and 2 differed only slightly from the inactivation of wild type (Table 1). This rules out the possibility that there is an interaction between the side chain of this serine residue in alkyltransferase and the aminomethyl group of these inhibitors.
Table 1.
Inactivation of purified human alkyltransferase in vitro
| Alkyltransferase Tested | Inhibitor | ED50 (μM)* | |
|---|---|---|---|
| No DNA | + DNA | ||
| Wild type | BG | 0.30 | 0.10 |
| Wild type | 2 | 0.017 | 0.004 |
| Wild type | 1 | 0.23 | 0.053 |
| Wild type | 3 | 80 | 45 |
| Wild type | 5 | 0.25 | 0.16 |
| Wild type | 4 | 0.32 | 0.20 |
| Wild type | 6 | 185 | 90 |
| Wild type | 7 | >1000 | >1000 |
| S159A mutant | 2 | 0.013 | 0.003 |
| S159A mutant | 1 | 0.16 | 0.028 |
Results shown are the mean values for 3–5 assays with different preparations of recombinant alkyltransferase, which agreed within ±20%.
The ortho-substituted derivative 3 was much less active than BG or the other substituted BGs with an ED50 of 45 μM when assayed in the presence of DNA and 80 μM when assayed in the absence of DNA (Table 1). This derivative was assayed immediately after a solution was made up since as described above it is unstable in aqueous solution rearranging to form 7 with a half life of c. 50 h. at 37°C. This compound was also tested and was inactive as an alkyltransferase inhibitor (less than 10% inhibition at 1 mM).
The improved activity of an aminomethyl meta- substituent is not mimicked by a simple methyl group. There was no difference between BG itself and compounds 4 or 5, which had ED50 values of c. 0.3 μM in the absence of DNA and c. 0.15μM in the presence of DNA (Table 1). The ortho-methyl substituted compound 6 was similar to 3 in leading to a large loss of inhibitory potency (ED50 values of 90 μM and 185 μM in the presence and absence of DNA) (Table 1). These results suggest that the improved activity of 2 was not caused by stabilization of the transition state due simply to a substitution on the benzyl ring at the meta- position.
In contrast to studies with folate ester derivatives of BG where the ED50 values were affected by the presence of a (His)6 tag on the alkyltransferase protein 26, there was no difference between assays with untagged alkyltransferase protein, and assays using either a C-terminal or N-terminal (His)6-tagged alkyltransferase with any of the compounds listed in Table 1. Some of these folate derivatives are less potent inactivators of a polymorphic form of human alkyltransferase in which Ile143 is changed to a Val and Lys178 to an Arg (I143V/K178R) 26. This variant was only slightly less susceptible than wild type to compound 2 (results not shown).
Molecular docking of inhibitors to human alkyltransferase
Computational docking studies were performed using the GLIDE program (version 4.5, Schrödinger, LLC, New York, NY, 2007). The docked structures were chosen for comparison with experimentally determined ED50 values using either the Glide Score or E-model scoring function. For enhanced docking accuracy, the best docked structures using GLIDE extra precision (XP) mode were used to calculate ligand partial charges in the protein environment and then redocked with XP using Schrödinger’s QPLD (Quantum Polarized Ligand Docking) method 27. The Maestro user interface, (version 8.0, Schrödinger, LLC, New York, NY, 2007) was employed to set up the GLIDE docking studies and for visualization of the results. The alkyltransferase X-ray crystal structure chosen for our modeling studies was human alkyltransferase bound to DNA containing O6-methylguanine and Cys145 mutated to Ser to prevent the alkylation reaction from taking place 19. In our modeling studies, Ser145 was mutated back to Cys. To validate the docking approach, self-docking was performed with XP/QPLD using the partial native ligand O6-methylguanine. The RMS of the docked pose when compared to the crystallographically observed position of the O6-methylguanine moiety was 0.14 Å, and thus GLIDE produced a docking mode that closely resembled the X-ray crystal structure. Thus, our hypothesis was that GLIDE would be capable of producing docking poses for the compounds studied that are similar to the position, orientation and conformation adopted by the ligands prior to nucleophilic attack by Cys145 and that the docking scores obtained would correlate well with the experimentally observed ED50 values for enzyme inactivation. Although there is a crystal structure available for human alkyltransferase benzylated at Cys145 11, this structure was not employed to model the BG analogs described in this study since the GLIDE program is unsuitable for modeling covalent bonds formed between the ligand and protein.
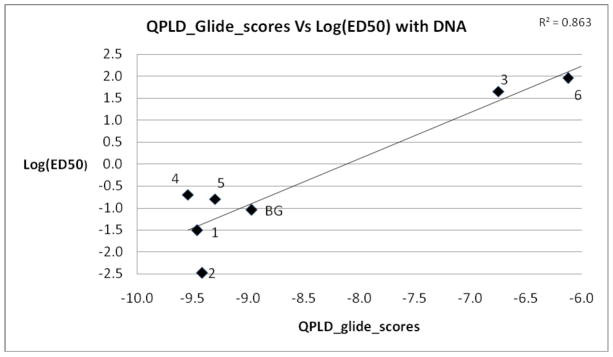
In addition to O6-methylguanine, compounds 1–6 and BG were docked to human alkyltransferase using the GLIDE XP. Each pose of these compounds was redocked with XP using the QPLD method. The coefficient of determination (i.e., the square of the correlation coefficient, R2) was calculated between QPLD Glide Scores vs. log(ED50) values, determined in the presence of DNA, as part of a linear regression analysis. An acceptable R2 value of 0.86 between log(ED50) values and XP/QPLD Glide Scores was obtained (Figure 2).
Figure 2.
Plot of log(ED50) values versus QPLD_Glide_scores. Numbering corresponds to compounds shown in Figure 1. The ED50 values determined in the presence of DNA from Table 1 were used.
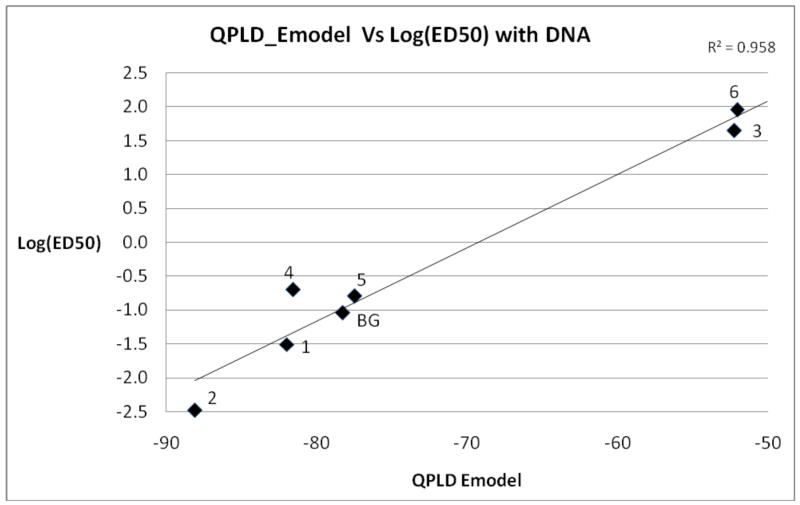
E-model scores were also used for correlation studies. It had been previously observed by Bytheway and Cochran 28 that E-model scores correlated better with log(ED50) values than Glide Scores in their particular study. In our study, the coefficient of determination obtained between E-model scores (obtained from XP/QPLD calculations) vs. log(ED50) values via a linear regression analysis gave an exceptional R2 of 0.96, shown in Figure 3. It is noteworthy that E-model is a composite scoring function, derived from a combination of the Glide Score itself coupled with Coulombic energy, van der Waals energy, and ligand strain energy terms, and is used by GLIDE to select the best docking pose for each individual inhibitor regardless of the scoring function subsequently used and can be used to rank order the inhibitors as well.
Figure 3.
Plot of log(ED50) values versus E-model scores. Numbering corresponds to compounds shown in Figure 1. The ED50 values determined in the presence of DNA from Table 1 were used.
From examination of the QPLD based docking modes, one can conclude that the binding affinities correlate quite well with the number of hydrogen bonds and good van der Waals contacts formed between the inhibitor and the alkyltransferase binding site. There is insufficient space to adequately accommodate the ortho-substituted benzylguanines. Thus, due to fewer favorable van der Waals contacts and due to their fewer hydrogen bonds, compounds 3 and 6 are the least potent of the inhibitors we tested in the modeling studies. On the other hand, compounds 1 and 2 exhibit three and four hydrogen bonds respectively and both form good van der Waals contacts with the alkyltransferase protein. All the values of these docking based binding interactions are summarized in Table 2.
Table 2.
Binding interactions derived from docking compounds 1–6, BG and O6-methylguanine to human alkyltransferase.
| Compound | E-model Score | Glide Score | H-bonds | Good vdw* Contacts | Bad vdw* Contacts |
|---|---|---|---|---|---|
| 2 | −88.1 | −9.42 | 4 | 297 | 8 |
| 1 | −82.0 | −9.46 | 3 | 312 | 8 |
| 4 | −81.5 | −9.54 | 3 | 306 | 7 |
| BG | −78.2 | −8.79 | 3 | 287 | 4 |
| 5 | −77.5 | −9.30 | 3 | 289 | 8 |
| O6-methylguanine | −56.6 | −7.16 | 4 | 177 | 3 |
| 3 | −52.2 | −6.75 | 2 | 260 | 5 |
| 6 | −52.0 | −6.12 | 1 | 180 | 1 |
vdw, Van der Waals
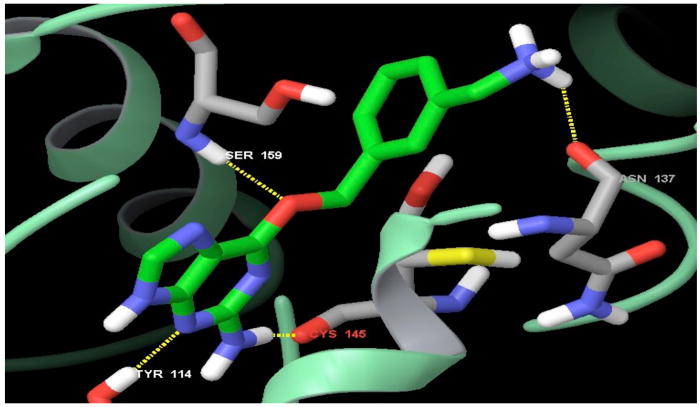
Figure 4 shows the key hydrogen bonding interactions of compound 2 with human alkyltransferase. Interactions with residues Tyr114, Cys145, Ser159 and Asn137 are depicted. All except for the interaction with Tyr114 involve interaction with protein backbone rather than the amino acid side chains.
Figure 4.
The key hydrogen bonding interactions of compound 2 (O6-[3-(aminomethyl)benzyl]guanine) with the protein residues represented in stick model. The inhibitor is represented in green for carbon, blue for nitrogen and red for oxygen. The rest of the protein as pale green ribbon cartoon.
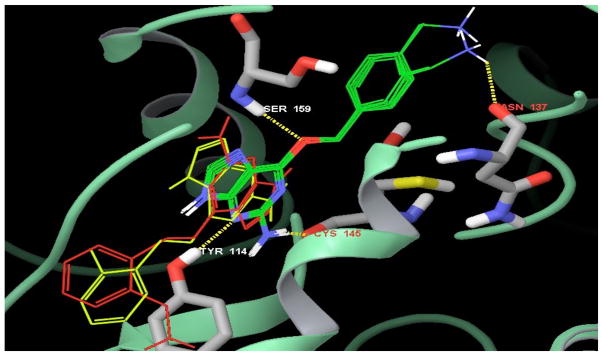
Figure 5 shows an overlay of all of the inhibitor poses in the active site with O6-methylguanine. It is clearly apparent that compounds 3 and 6 are not oriented in the catalytic site of human alkyltransferase in same way as the other inhibitors.
Figure 5.
Overlay of the position of all of the docked compounds in the human alkyltransferase active site with the native ligand O6-methylguanine and parent inhibitor BG. All of the potent inhibitor poses in green (compounds 1, 2, 4, 5 and BG) are oriented in the same way as the native ligand O6-methylguanine. The ineffective inhibitors O6-[2-(aminomethyl)benzyl]guanine (3) in red and O6-[2-(methyl)benzyl]guanine (6) in yellow are not oriented in the same way as O6-methylguanine. The rest of the color code is the same as Figure 4.
Inactivation of alkyltransferase in HT29 cells in culture
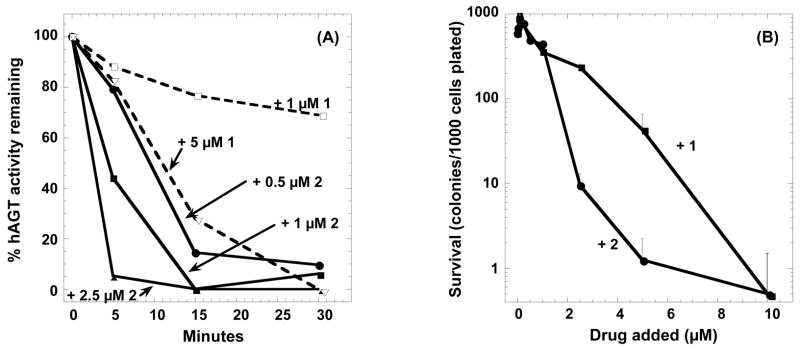
The striking difference between 1 and 2 in the inactivation of alkyltransferase was also seen when the compounds were added to the culture medium of HT29 cells (Figure 6). When cell extracts were prepared from these cells and residual alkyltransferase activity measured, it was found that within 30 min or less of exposure to 0.5 to 2.5 μM of compound 2, the alkyltransferase was almost completely reduced. Compound 1 was clearly less effective; the 1 μM dose only produced 20% loss of activity in 30 min (Figure 6A). This difference was also seen when the ability of 1 and 2 to sensitize HT29 cells to BCNU was examined (Figure 6B). Alkyltransferase activity provides resistance to BCNU and alkyltransferase inhibitors overcome this resistance 9, 12, 29–31. Compound 2 was much more effective than compound 1 in sensitizing HT29 cells to the chloroethylating agent (Figure 6B). Previously published studies have shown that BG passes very readily through cell membranes and very rapidly inactivates alkyltransferase in HT29 cells 9, 29, 32. Even though 2 produces a comparable degree of alkyltransferase inactivation, the rate of inactivation by 2 seen in Figure 6A is significantly slower than with BG. This is likely to be due to a reduced uptake of 2 due to its positive charge.
Figure 6.
Inactivation of alkyltransferase in HT29 cells. Panel A shows the loss of alkyltransferase (hAGT) activity as a function of time after addition of the compound indicated to cultures of HT29 cells. Panel B shows the effect of exposing HT29 cells to the compound indicated for 2 h prior to the addition of 40 μM BCNU. Cell survival was then measured using a colony forming assay as described in the Experimental Section.
Conclusions
The addition of a meta-aminomethyl group to BG forming compound 2 not only greatly increases the solubility but results in an approximately 20-fold improvement in the ability to inactivate purified human alkyltransferase. This improvement is seen in assays conducted with or without added DNA. It is noteworthy that the molecular modeling studies showed a similar trend and an R2 of 0.90 (data not shown) for a linear regression analysis of log(ED50), determined in the absence of DNA, vs. XP/QPLD Glide Scores and an R2 of 0.98 (data not shown) for log(ED50), determined in the absence of DNA, vs. E-model scores were obtained. As previously noted, inactivation of alkyltransferase by BG33 is enhanced 3–4 –fold by the presence of DNA, which stimulates the rate of alkyl transfer 34. In contrast, some BG derivatives with bulky substituents are much less potent inhibitors in the presence of DNA since they cannot be accommodated in the active site when DNA is bound there 34, 35. The inactivation of alkyltransferase by compounds 1 and 2 was increased by the presence of DNA indicating that these compounds, like BG, do not compete with DNA for access to the active site. However compound 1, the para-aminomethyl derivative, did not show any improvement in inhibitory potency over BG itself. These results are very well explained by the molecular modeling studies, which indicate the formation of an additional hydrogen bond when compound 2 is bound in the active site. The aminomethyl group from 2 but not 1 is able to interact with Asn137. This also explains why a simple methyl substitution (compound 5) was ineffective since it cannot form this hydrogen bond. The modeling studies also show clearly why the ortho- substituted BG derivatives (3 and 6) are much less effective (> 200-fold) than BG since, due to steric clashes, they cannot be positioned in the same way as the parent compound.
Our results not only demonstrate that 2 may be a valuable alkyltransferase inhibitor but they also show clearly the value of molecular modeling for the design of improved alkyltransferase inhibitors. Even though it has not yet been possible to obtain actual crystal structures of BG bound to wild type alkyltransferase or its C145S mutant, the remarkable R2 values obtained using XP/QPLD with E-model scoring for ranking the compounds tested here shows that the structure of human alkyltransferase bound to DNA containing O6-methylguanine and Cys145 mutated to Ser can be used as a starting structure for this process. The mutation of Ser145 back to Cys, as is appropriate for the wild type enzyme, and refinement of the structure obtained with MacroModel was essential in order to obtain the R2 values we report. The model we have generated should be highly useful in the design of more potent inhibitors by performing additional modeling studies on BG analogs and by virtually screening databases of commercially available compounds to identify potential new lead compounds for further elaboration.
A major advantage of compound 2 is that it is much more soluble (approx. 1000 times at physiological pH) than BG. The limited solubility of BG requires formulation in a diluent containing polyethylene glycol. Although adequate blood levels for alkyltransferase inactivation in a variety of tumors can be achieved with this route of administration 4, 13–17, the potentiation of the myelotoxicity of the alkylnitrosourea-based and methylating chemotherapies due to inactivation of alkyltransferase in the bone marrow limits effectiveness of BG. Regional delivery is an obvious way to attempt to circumvent this limitation, but, in many cases, only a small volume can be administered and the poor solubility of BG prevents its use in this way. This could be avoided by using 2.
In particular, 2 is suitable for testing for protocols involving administration by convection-enhanced therapy to treat brain tumors. Convection-enhanced therapy uses a hydrostatic pressure gradient-dependent direct intracerebral infusion to establish a bulk flow interstitial current that produces a relatively uniform spatial distribution of drugs at concentrations nearer the infused concentration at significant distances from the point of delivery. It is currently under development for use with a variety of agents directed against brain tumors 36–38. The hydrophilicity of the protonated 2 is also likely to prevent back-loss across the blood-brain barrier. The slower uptake of 2 compared to BG is not likely to prove a significantly obstacle to its use since total alkyltransferase inactivation is easily obtained within 30 min of exposure to 1 μM levels. Obviously, the success of the compound in clinical use will depend on its biological stability and bioavailability.
Experimental Section
Chemistry
Chemicals were obtained from Aldrich (Milwaukee, WI) and were used without further purification. UV spectra were determined on a Beckman Coulter DU 7400 spectrophotometer. Molar extinction coefficients for compounds 1–3 were determined by simply dissolving approximately 10 mg of the material in 1.00 L of 0.01 M phosphate buffer, pH=7.0 and recording the UV absorbance spectrum. Compounds 4–7 did not dissolve completely, even at this low concentration. As a result, these four compounds were predissolved in 1 mL of DMSO and then diluted to 1.00 L with phosphate buffer prior to recording their UV absorbance spectra. 1H NMR spectra were recorded with a Varian INOVA 400 MHz spectrometer. Chemical shifts are reported as d values in parts per million relative to TMS as an internal standard. Splitting pattern abbreviations are as follows: s = singlet, d = doublet, t = triplet and m = multiplet. Coupling constants are in hertz. Mass spectra were collected on a Thermo Finnigan TSQ Quantum spectrometer in positive ion electrospray mode scanning m/z=100 to 1500 in one second. The electrospray voltage was 3.5 kV, the transfer tube was at 350°C. Elemental analysis was performed by Atlantic Microlab, Inc. (Norcross, GA). All silica gel chromatography was carried out using Davisil, grade 633, 200–425 mesh 60 Å. 4-(Aminomethyl)benzyl alcohol was made according to the procedure reported by Keppler et al. 25 The NMR spectrum of this material was in agreement with the published data. 2-(Aminomethyl)benzyl alcohol was made as described by McCalmont et al. 39 and the NMR spectrum agreed with the reported data.
3-(Aminomethyl)benzyl alcohol
A sample of 3-cyanobenzoic acid (20 g, 136 mmol) was suspended in 100 mL of dry tetrahydrofuran (THF). Borane-THF complex (400 mL of 1.0 M solution in THF) was added dropwise over one h and the mixture was stirred for an additional 3 h. The reaction was quenched with 220 mL of concentrated HCl:water (1:1 v/v) to cleave the borate ester complex. The THF was then removed under vacuum. Solid NaOH pellets, 70 g total, were added slowly to neutralize the acid and bring the pH of the solution to 12. The aqueous phase was then extracted 4 times with 500 mL portions of ether. The ether extract was dried over MgSO4 and concentrated under vacuum to give 18 g (131 mmol, 96%) of 3-(aminomethyl)benzyl alcohol as a light colored oil. 1H NMR (CDCl3/TMS) δ = 1.95 (broad s, 3H, -OH and –NH2, exchangeable with D2O), 3.86 (s, 2H, -CH2-NH2), 4.66 (s, 2H, -CH2-OH), 7.18–7.35 (m, 4H, Ar) ppm in agreement with data published by Lee et al. 40 who made this compound by a different route.
1-(2-Amino-9H-purin-6-yl)-1-methyl-pyrrolidinium chloride (8)
This compound was prepared from 2-amino-6-chloropurine as described 25. The 1H NMR spectrum of this product was in agreement with the data in that publication.
General synthesis of 2,2,2-triflouro-N-[(hydroxymethyl)benzyl]acetamides
Protection of the amine groups of the three aminomethylbenzyl alcohols as trifluoroacetamides was performed according to the procedure used by Keppler et al. to make 2,2,2-trifluoro-N-[(4-(hydroxymethyl)benzyl]acetamide (9) 25.
2,2,2-Trifluoro-N-[(4-(hydroxymethyl)benzyl]acetamide (9)
The yield of this reaction was 89%, giving a white solid with a melting point of 91–92°C. 1H NMR (CDCl3/TMS) δ = 1.77 (s, 1H, -OH, exchanges with D2O), 4.52 (d, J=6.0, 2H, -CH2-NH-), 4.70 (s, 2H, -CH2-OH), 6.63 (broad s, 1H, -NH-CO-, exchanges with D2O), 7.26–7.39 (m, 4H, Ar) ppm, in agreement with the published data25.
2,2,2-Trifluoro-N-[(3-(hydroxymethyl)benzyl]acetamide (10)
The yield of this reaction was 91%, giving a white solid with a melting point of 73–74°C. 1H NMR (CDCl3/TMS) δ = 1.84 (s, 1H, -OH, exchanges with D2O), 4.52 (d, J=5.6, 2H, -CH2-NH-), 4.70 (s, 2H, -CH2-OH), 6.65 (broad s, 1H, -NH-CO-, exchanges with D2O), 7.20–7.4 (m, 4H, Ar) ppm.
2,2,2-Trifluoro-N-[2-(hydroxymethyl)benzyl]acetamide (11)
The yield of this reaction was 76%, giving a white solid with a melting point of 75–76°C. 1H NMR (CDCl3/TMS) δ = 2.20 (s, 1H, -OH, exchanges with D2O), 4.61 (d, J=5.6, 2H, -CH2-NH-), 4.78 (s, 2H, -CH2-OH), 7.30–7.44 (m, 4H, aromatic), 7.76 (broad s, 1H, -NH-CO-, exchanges with D2O) ppm.
General synthesis of the N-[(2-amino-9H-purin-6-yloxymethyl)benzyl]-2,2,2-trifluoro-acetamides (12, 13 and 14)
The procedure used by Keppler et al25 to make N-[4-(2-amino-9H-purin-6-yloxymethyl)benzyl]-2,2,2-trifluoro-acetamide (12) was used. Compound 8 was suspended in 35 to 40 mL/g of DMF. To this was added between 1.4 and 2.0 equivalents of one of the three protected aminomethyl benzyl alcohols (9–11) together with 2 equivalents, with respect to the benzyl alcohol, of potassium t-butoxide. After 3 to 5 h the reactions were quenched with acetic acid in water and the solvents were evaporated. The products were then purified by silica gel chromatography eluting with methanol:dichloromethane (1:50) followed by the same solvents (1:10).
N-[4-(2-amino-9H-purin-6-yloxymethyl)benzyl]-2,2,2-trifluoro-acetamide (12)
This reaction gave a white solid with a melting range of 213–215°C in 34% yield based on the purine. 1H NMR (DMSO-d6/TMS) δ = 4.40 (s, 2H, -CH2-NH-), 5.47 (s, 2H, -O-CH2-), 6.27 (s, 2H, guanine-NH2, exchanges with D2O), 7.27–7.52 (m, 4H, Ar), 7.83 (s, 1H, guanine H8), 10.02 (broad s, 1H, -NH-CO- exchanges with D2O), 12.44 (broad s, 1H, guanine H9, exchanges with D2O) ppm. Keppler et al.25 report the signal at 4.40 ppm as a doublet.
N-[3-(2-Amino-9H-purin-6-yloxymethyl)benzyl]-2,2,2-trifluoro-acetamide (13)
This reaction gave a white solid which decomposed above 210°C in 35% yield based on the purine. 1H NMR (DMSO-d6/TMS) δ = 4.42 (s, 2H, -CH2-NH-), 5.48 (s, 2H, -O-CH2-), 6.27 (s, 2H, guanine-NH2, exchanges with D2O), 7.23–7.45 (m, 4H, Ar), 7.83 (s, 1H, guanine H8), 10.03 (broad s, 1H, -NH-CO- exchanges with D2O), 12.45 (broad s, 1H, guanine H9, exchanges with D2O) ppm.
N-[2-(2-Amino-9H-purin-6-yloxymethyl)benzyl]-2,2,2-trifluoro-acetamide (14)
This reaction gave a white solid which decomposed slowly above 180°C in 25% yield based on the purine. 1H NMR (DMSO-d6/TMS) δ = 4.58 (d, J=5.6, 2H, -CH2-NH-), 5.57 (s, 2H, -O-CH2-), 6.29 (s, 2H, guanine-NH2, exchanges with D2O), 7.26–7.60 (m, 4H, Ar), 7.81 (s, 1H, guanine H8), 10.00 (t, J=5.6, 1H, -NH-CO- exchanges with D2O), 12.43 (broad s, 1H, guanine H9, exchanges with D2O) ppm.
General method for the removal of 2,2,2-trifluoroacetyl protecting groups
Trifluoroacetyl protecting groups were removed from compounds (9–11) using the procedure described by Keppler et al.25 to make O6-[4-(aminomethyl)benzyl]guanine (1),
O6-[4-(Aminomethyl)benzyl]guanine (1)
Recovered as a white solid which decomposed above 185°C in 76% yield. UV (0.01 M sodium phosphate pH=7.0), λmax= 241 nm (ε=7,400), λmax=282 nm (ε=8,500). 1H NMR (DMSO-d6/TMS) δ = 3.72 (s, 2H, -CH2-NH2), 5.45 (s, 2H, -O-CH2-) 6.26 (s, 2H, guanine-NH2, exchanges with D2O), 7.31–7.47 (m, 4H, Ar), 7.82 (s, 1H, guanine H8) ppm in agreement with the data published by Keppler et al.25. Neither the guanine H9 proton nor the benzylic amine protons are observed25. A sample of this material was recrystallized from water for elemental analysis. Analysis calculated for C13H14N6O1· 0.2 H2O (C, H, N). The material was converted to the hydrochloride salt by suspending it in water and adding one equivalent of HCl. The material was then filtered and lyophilized. Analysis of the hydrochloride salt was calculated for C13H15N6O1Cl1·0.5 H2O (C, H, N, Cl).
O6-[3-(Aminomethyl)benzyl]guanine (2)
Recovered as a white solid with a melting range of 158–160°C in 68% yield. UV (0.01 M sodium phosphate pH=7.0), λmax= 241 nm (ε=7,500), λmax=282 nm (ε=8,600). 1H NMR (DMSO-d6/TMS) δ = 4.02 (s, 2H, -CH2-NH2), 5.48 (s, 2H, -O-CH2-) 6.30 (s, 2H, guanine-NH2, exchanges with D2O), 7.29–7.48 (m, 4H, Ar), 7.83 (s, 1H, guanine H8) ppm. As with compound 1, neither the guanine H9 proton nor the benzylic amine protons are observed. A sample of this material was recrystallized from water for elemental analysis. Analysis calculated for C13H14N6O1 (C, H, N). The material was converted to the hydrochloride salt by suspending it in water and adding one equivalent of HCl. The material was then filtered and lyophilized. Analysis of the hydrochloride salt was calculated for C13H15N6O1Cl1 as the monohydrate (C, H, N, Cl).
O6-[2-(Aminomethyl)benzyl]guanine (3)
Recovered as a white solid which decomposed above 170°C in 74% yield. UV (0.01 M sodium phosphate pH=7.0), λmax= 241 nm (ε=7,400), λmax=282 nm (ε=8,400). 1H NMR (DMSO-d6/TMS) δ = 3.82 (s, 2H, -CH2-NH2), 5.55 (s, 2H, -O-CH2-) 6.27 (s, 2H, guanine-NH2, exchanges with D2O), 7.22–7.50 (m, 4H, Ar), 7.83 (s, 1H, guanine H8) ppm. As with compound 1, neither the guanine H9 proton nor the benzylic amine protons are observed. Elemental analysis calculated for C13H14N6O1 (C, H, N). This material was not converted to a hydrochloride salt because of the rearrangement observed below.
N6-[2-(Hydroxymethyl)benzyl]-2-aminoadenine (7)
Upon attempting to recrystallize O6-[2-(aminomethyl)benzyl)]guanine (3) from water, it was observed that the material came out of solution upon heating. The NMR spectrum (1H) of the recovered solid material indicated decomposition or rearrangement. The mass spectrum of the original sample of compound 3 and the precipitate contained similar molecular ions at m/z=271.1 [M+H]+.
A sample of O6-[2-(aminomethyl)benzyl]guanine (3), was dissolved in water and heated to 65°C for one week, the insoluble precipitate was then filtered, washed with water and dried under vacuum. This material with a melting range of 219–221°C was identified as N6-[(2-hydroxymethyl)benzyl]-2-aminoadenine (7) based on the NMR spectrum. UV (0.01 M sodium phosphate with 0.1% v/v DMSO, pH=7.0), λshoulder= 250 nm (ε=8,000), λmax=284 nm (ε=12,800). 1H NMR (DMSO-d6/TMS) δ = 4.64 (singlet with a broad shoulder down field, 4H, -CH2-NH- and -CH2-OH), 5.30 (s, 1H, -CH2-OH exchanges with D2O), 5.67 (s, 2H, purine-NH2, exchanges with D2O), 7.15–7.40 (m, 4H, Ar), 7.47 (broad d, J=7.2, 1H, benzylic NH, exchanges with D2O) 7.65 (s, 1H, purine H8), 12.06 (broad s, 1H, purine H9, exchanges with D2O) ppm. Elemental analysis calculated for C13H14N6O1·0.5 H2O (C, H, N). The conversion of a very dilute solution of compound 3 to compound 7 was followed by UV spectroscopy in 0.1 M sodium phosphate buffer at pH 7.4 and 37°C and was found to proceed with a half life of 51 h with first order kinetics.
General synthesis of O6-[(methyl)benzyl]guanines (4–6)
To a solution of one of the three isomers of methylbenzyl alcohol (2.5 g, 20 mmol) in 100 mL of dry DMF, potassium t-butoxide (4.5 g, 80 mmol) was added. After 30 min. compound 8 (2.54 g, 10 mmol) was added. The reaction stirred for 18 h and then the solvent was evaporated under vacuum. The resulting material was purified over silica gel loading with dichloromethane:methanol (25:1) and eluting with the same solvents (10:1). The three compounds produced in this way were each recrystallized from ethanol:water (1:1).
O6-[4-(Methyl)benzyl]guanine (4)
The above procedure gave 2.2g (8.6 mmol, 43%) of (4) as a white solid with a melting point of 205–207°C. UV (0.01 M sodium phosphate with 0.1% v/v DMSO, pH=7.0), λmax=241 nm (ε=7,600), λmax=282 nm (ε=8,700). 1H NMR (DMSO-d6/TMS) δ = 2.31 (s, 3H, -CH3), 5.45 (s, 2H, -O-CH2-), 6.27 (s, 2H, guanine-NH2, exchanges with D2O), 7.16–7.44 (m, 4H, Ar), 7.81 (s, 1H, guanine H8), 12.41 (broad s, 1H, guanine H9, exchanges with D2O) in agreement with the data published by Dolan et al. 9 who made this compound by a different route. Elemental analysis for C13H13N5O1 (C, H, N).
O6-[3-(Methyl)benzyl]guanine (5)
The above procedure gave 2.2g (8.6 mmol, 43%) of (5) as a white solid with a melting point of 201–203°C. UV (0.01 M sodium phosphate with 0.1% v/v DMSO, pH=7.0), λmax 241 nm (ε=7,500), λmax=282 nm (ε=8,700). 1H NMR (DMSO-d6/TMS) δ = 2.32 (s, 3H, -CH3), 5.44 (s, 2H, -O-CH2-), 6.27 (s, 2H, guanine-NH2, exchanges with D2O), 7.13–7.37 (m, 4H, Ar), 7.81 (s, 1H, guanine H8), 12.41 (broad s, 1H, guanine H9, exchanges with D2O). Elemental analysis for C13H13N5O1 (C, H, N).
O6-[2-(Methyl)benzyl]guanine (6)
The above procedure gave 1.8g (7.0 mmol, 35%) of (6) as a white solid with a melting point of 211–213°C. UV (0.01 M sodium phosphate with 0.1% v/v DMSO, pH=7.0), λmax 241 nm (ε=7,600), λmax=282 nm (ε=8,600). 1H NMR (DMSO-d6/TMS) δ = 2.35 (s, 3H, -CH3), 5.48 (s, 2H, -O-CH2-), 6.28 (s, 2H, guanine-NH2, exchanges with D2O), 7.18–7.49 (m, 4H, Ar), 7.82 (s, 1H, guanine H8), 12.43 (broad s, 1H, guanine H9, exchanges with D2O). Elemental analysis for C13H13N5O1 · 0.25 H2O (C, H, N).
Inactivation of purified recombinant human alkyltransferase
Human alkyltransferase proteins with and without (His)6 tags were expressed in E. coli and purified to homogeneity as previously described 26. ED50 values for the inactivation of purified human alkyltransferase in vitro were obtained essentially as previously described 34, 35. Briefly, purified recombinant human alkyltransferase was incubated with different concentrations of potential inhibitors in 0.5 mL of reaction buffer (50 mM Tris-HCl, pH 7.6, 0.1 mM EDTA, 5.0 mM dithiothreitol) containing 50 μg of hemocyanin for 30 min at 37°C. For assay in the presence of DNA, 10 μg calf thymus DNA was added to the 0.5 mL of reaction buffer. The remaining alkyltransferase activity was then determined after incubation with [3H]methylated calf thymus DNA substrate for 30 min at 37°C by measuring the [3H]methylated protein formed, which was collected on nitrocellulose filters. A graph of the percentage of the alkyltransferase activity remaining against inhibitor concentration was then plotted and the ED50 values representing the amount of inhibitor needed to produce a 50% loss of activity was calculated from the equation for the best fit exponential curve (KaleidaGraph, Synergy Software, Reading, PA 19606).
Cell culture and inactivation of cellular alkyltransferase
HT29 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and maintained by seeding at 1 × 105 cells/25-cm2 flask at weekly intervals. Inactivation of cellular alkyltransferase by potential inhibitors was determined in cells that reached about 80% confluence. After different times of exposure to the compounds, cells were harvested by trypsinization and cell pellets were washed with PBS. Cell extracts were prepared as previously described by sonication on ice and centrifugation at 15,000 × g, and supernatant extracts were stored at −70°C until assayed. Crude cell protein was determined using Bio-Rad protein assay dye (Bio-Rad, Hercules, CA). The remaining alkyltransferase activity was measured as removal of O6-[3H]methylguanine from [3H]methylated calf thymus DNA 9. Cell extracts were incubated with [3H]methylated DNA substrate for 30 min at 370C in 1 ml of reaction buffer (50 mM Tris-HCl, pH 7.6, 0.1 mM EDTA, 5.0 mM dithiothreitol). The DNA was precipitated, hydrolysed and modified bases were separated by reverse-phase HPLC. The results were expressed as the percentage of the alkyltransferase activity present in cultures to which no compound was added.
Sensitization of cells to killing by BCNU
The effect of alkyltransferase inactivators on the sensitivity of cells to BCNU was determined using a colony-forming assay. Cells were plated at a density of 106 in 25 cm2 flasks and 24 h later were exposed to different concentrations of potential inhibitors for 2 h as indicated before exposure to 40 μM BCNU for 2 h as previously described 35, 41. After 2 h, the medium was replaced with fresh medium containing the alkyltransferase inhibitor but no BCNU and the cells were left to grow for an additional 16–18 h. The cells were then replated at densities of 250–1000 cells per 25 cm2 flask and grown for 8 days until discrete colonies had formed. The colonies were washed with 0.9% saline solution, stained with 0.5% crystal violet in ethanol, and counted.
Molecular Docking Studies
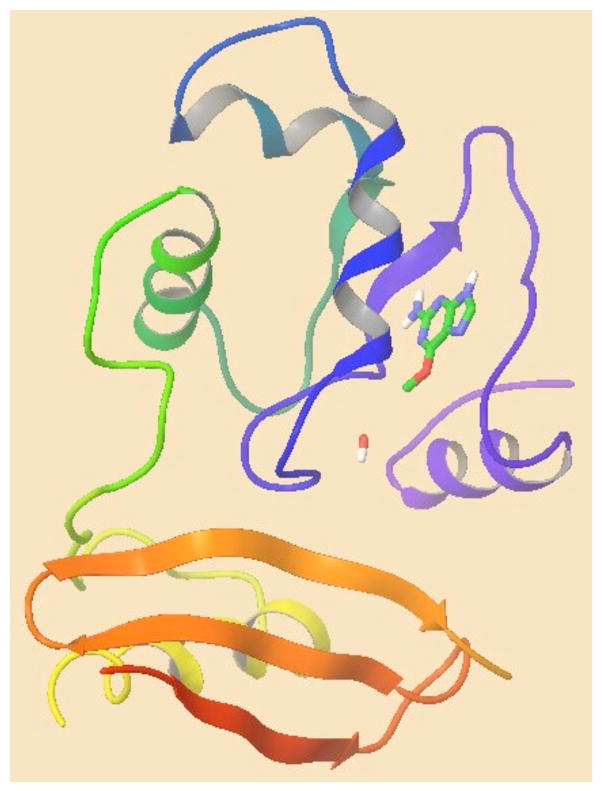
The computational modeling studies relied upon the GLIDE (Grid-based Ligand Docking from Energetics) program (Glide, version 4.5, Schrödinger, LLC New York, NY 2007)42–44 for the docking simulations. These simulations were performed using the X-ray crystal structure of the human alkyltransferase bound to a DNA oligonucleotide and containing O6-methylguanine determined at 3.2 Å resolution (PDB ID:1T38) 19, 45. For our studies, DNA was removed except for O6-methylguanine, see Figure 7. Solvent molecules in the protein crystal structure were deleted, except for a water molecule in the active site (WAT 1), and the protein was then prepared for the docking studies by processing it using Schrödinger’s protein preparation facility. This procedure minimizes the protein to 0.30 Å RMSD using the OPLS-2001 force field. Subsequent refinement of the protein/ligand complex was performed using the mixed torsional/Low-mode conformational sampling method within Schrödinger’s MacroModel program. The OPLS2001 force field was used and a distance dependent dielectric “constant” further attenuated by a factor of 2 was employed. A 5Å shell of residues surrounding the partial native ligand (O6--methylguanine), the ligand itself and a nearby water molecule (WAT 1) were fully flexible during the conformational sampling/energy minimization phase of the conformational search procedure. The rest of protein was frozen at its crystallographically determined position including residues Asn157 and Arg135, which were within the 5Å shell of mobile residues. The lowest energy conformer that retained the native ligand pose in the X-ray crystal structure of PDB 1T38 was used for subsequent studies. Structures of O6-methylguanine, BG and compounds 1–3 and 4–6 were prepared using Schrödinger’s LigPrep facility.
Figure 7.
Prepared protein of the human alkyltransferase without DNA. O6-Methylguanine and a bound water (WAT1) are also shown.
The initial docking studies were done with GLIDE (version 4.5, Schrödinger, LLC, New York, NY, 2007) operating in either SP or XP mode 42–44. Maestro, version 8.0, Schrödinger Suite 2007, LLC New York, NY 2007) was employed as the graphical user interface and for generation of the graphics used in the Figures. The best docked structure were chosen using the Glide_gscore function (Glide Score For enhanced docking accuracy). The best docked structures from XP were used to calculate the ligand partial charges in the environment of the protein and then redocked using Schrödinger’s QPLD (Quantum Polarized Ligand Docking) method 27. In order to validate the docking approach, self docking was performed using the partial native ligand O6-methylguanine using XP/QPLD. The RMS of the docked pose when compared to the crystallographically observed position was 0.14 Å, and thus GLIDE produced a docking mode that closely resembled the X-ray crystal structure.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Work in AEP’s laboratory was supported by grants CA-018137, CA-097209 and CA-071976 from the National Cancer Institute, National Institutes of Health, USA.
Footnotes
Abbreviations: alkyltransferase, O6-alkylguanine-DNA alkyltransferase; BG, O6- benzylguanine; BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea; DMSO, dimethylsulfoxide; DMF, dimethylformamide.
Supporting Information Available. Elemental analyses for compounds 1–7. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutation Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 2.Margison G, Povey AC, Kaina B, Santibáñez-Koref MF. Variability and regulation of O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 2003;24:625–635. doi: 10.1093/carcin/bgg005. [DOI] [PubMed] [Google Scholar]
- 3.Tubbs JL, Pegg AE, Tainer JA. DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy. DNA Repair (Amst) 2007;6:1100–1115. doi: 10.1016/j.dnarep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 5.Pegg AE, Dolan ME. Overcoming resistance to alkylating agents by inhibitors of O6-alkylguanine-DNA alkyltransferase. In: Panasci LC, Alaoui-Jamali M, editors. DNA Repair in Cancer Therapy. Humana Press, NJ: 2004. pp. 143–177. [Google Scholar]
- 6.Dolan ME, Pegg AE. O6-Benzylguanine and its role in chemotherapy. Clinical Cancer Res. 1997;3:837–847. [PubMed] [Google Scholar]
- 7.Middleton MR, Margison GP. Improvement of chemotherapy efficacy by inactivation of a DNA-repair pathway. Lancet Oncol. 2003;4:37–44. doi: 10.1016/s1470-2045(03)00959-8. [DOI] [PubMed] [Google Scholar]
- 8.Khan O, Middleton MR. The therapeutic potential of O6-alkylguanine-DNA alkyltransferase inhibitors. Expert Opin Investig Drugs. 2007;16:1573–84. doi: 10.1517/13543784.16.10.1573. [DOI] [PubMed] [Google Scholar]
- 9.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990;87:5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegg AE, Boosalis M, Samson L, Moschel RC, Byers TL, Swenn K, Dolan ME. Mechanism of inactivation of human O6-alkylguanine-DNA alkyltransferase by O6-benzylguanine. Biochemistry. 1993;32:11998–12006. doi: 10.1021/bi00096a009. [DOI] [PubMed] [Google Scholar]
- 11.Daniels DS, Mol CD, Arvai AS, Kanugula S, Pegg AE, Tainer JA. Active and alkylated human AGT structures: a novel zinc site, inhibitor and extrahelical binding. EMBO J. 2000;19:1719–1730. doi: 10.1093/emboj/19.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElhinney RS, Donnelly DJ, McCormick JE, Kelly J, Watson AJ, Rafferty JA, Elder RH, Middleton MR, Willington MA, McMurry TBH, Margison GP. Inactivation of O6-alkylguanine-DNA alkyltransferase 1. Novel O6-(hetarylmethyl)guanines having basic rings in the side chain. J Med Chem. 1998;41:5265–5271. doi: 10.1021/jm9708644. [DOI] [PubMed] [Google Scholar]
- 13.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE, 2nd, Bigner DD, Friedman HS. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178–87. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 14.Warren KE, Aikin AA, Libucha M, Widemann BC, Fox E, Packer RJ, Balis FM. Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol. 2005;23:7646–53. doi: 10.1200/JCO.2005.02.0024. [DOI] [PubMed] [Google Scholar]
- 15.Broniscer A, Gururangan S, MacDonald TJ, Goldman S, Packer RJ, Stewart CF, Wallace D, Danks MK, Friedman HS, Poussaint TY, Kun LE, Boyett JM, Gajjar A. Phase I trial of single-dose temozolomide and continuous administration of O6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin Cancer Res. 2007;13:6712–8. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 16.Batts ED, Maisel C, Kane D, Liu L, Fu P, O’Brien T, Remick S, Bahlis N, Gerson SL. O6-Benzylguanine and BCNU in multiple myeloma: a phase II trial. Cancer Chemother Pharmacol. 2007;60:415–21. doi: 10.1007/s00280-007-0442-7. [DOI] [PubMed] [Google Scholar]
- 17.Gajewski TF, Sosman J, Gerson SL, Liu L, Dolan E, Lin S, Vokes EE. Phase II trial of the O6-alkylguanine-DNA alkyltransferase inhibitor O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea in advanced melanoma. Clin Cancer Res. 2005;11:7861–5. doi: 10.1158/1078-0432.CCR-05-0060. [DOI] [PubMed] [Google Scholar]
- 18.Ranson M, Middleton MR, Bridgewater J, Lee SM, Dawson M, Jowle D, Halbert G, Waller S, McGrath H, Gumbrell L, McElhinney RS, Donnelly D, McMurry TB, Margison GP. Lomeguatrib, a potent inhibitor of O6-alkylguanine-DNA alkyltransferase: phase I safety, pharmacodynamic, and pharmacokinetic trial and evaluation in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2006;12:1577–84. doi: 10.1158/1078-0432.CCR-05-2198. [DOI] [PubMed] [Google Scholar]
- 19.Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA. Novel modes of DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat Struct Mol Biol. 2004;11:714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 20.Guengerich FP, Fang Q, Liu L, Hachey DL, Pegg AE. O6-Alkylguanine-DNA alkyltransferase: Low pKa and reactivity of cysteine 145. Biochemistry. 2003;42:10965–10970. doi: 10.1021/bi034937z. [DOI] [PubMed] [Google Scholar]
- 21.Xu-Welliver M, Pegg AE. Point mutations at multiple sites including highly conserved amino acids maintain activity but render O6-alkylguanine-DNA alkyltransferase insensitive to O6-benzylguanine. Biochem J. 2000;347:519–526. doi: 10.1042/0264-6021:3470519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegg AE, Xu-Welliver M, Loktionova NA. The DNA repair protein O6-alkylguanine-DNA alkyltransferase as a target for cancer chemotherapy. In: Ehrlich ME, editor. DNA alterations in cancer: genetic and epigenetic changes. Eaton Publishing; Natick, MA: 2000. pp. 471–488. [Google Scholar]
- 23.Pegg AE, Goodtzova K, Loktionova NA, Kanugula S, Pauly GT, Moschel RC. Inactivation of human O6-alkylguanine-DNA alkyltransferase by modified oligodeoxyribonucleotides containing O6-benzylguanine. J Pharm Exp Ther. 2001;296:958–965. [PubMed] [Google Scholar]
- 24.Luu KX, Kanugula S, Pegg AE, Pauly GT, Moschel RC. Repair of oligodeoxyribonucleotides by O6-alkylguanine-DNA alkyltransferase. Biochemistry. 2002;41:8689–8697. doi: 10.1021/bi025857i. [DOI] [PubMed] [Google Scholar]
- 25.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–9. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 26.Fang Q, Loktionova NA, Moschel RC, Javanmard S, Pauly GT, Pegg AE. Differential inactivation of polymorphic variants of human O6-alkylguanine-DNA alkyltransferase. Biochem Pharmacol. 2008;75:618–626. doi: 10.1016/j.bcp.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho AE, Guallar V, Berne B, Friesner RA. Importance of accurate charges in molecular docking: quantum mechanical/molecular mechanical (QM/MM) approach. J Comput Chem. 2005;26:915–931. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bytheway I, Cochran S. Validation of molecular docking calculations involving FGF-1 and FGF-2. J Med Chem. 2004;47:1683–1693. doi: 10.1021/jm030447t. [DOI] [PubMed] [Google Scholar]
- 29.Dolan ME, Mitchell RB, Mummert C, Moschel RC, Pegg AE. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991;51:3367–3372. [PubMed] [Google Scholar]
- 30.Kreklau EL, Kurpad C, Williams DA, Erickson LC. Prolonged inhibition of O6-methylguanine-DNA methyltransferase in human tumor cells by O6-benzylguanine in vitro and in vivo. J Pharmacol Exper Ther. 1999;291:1269–1275. [PubMed] [Google Scholar]
- 31.Griffin RJ, Arris CE, Bleasdale C, Boyle FT, Calvert AH, Curtin NJ, Dalby C, Kanugula S, Lembicz NK, Newell DR, Pegg AE, Golding BJ. Resistance-Modifying Agents. 7. Inhibition of O6-alkylguanine-DNA alkyltransferase by O6-alkenyl-, O6-cycloalkenyl- and O6-(2-oxoalkyl)-guanines, and potentiation of temozolomide cytotoxicity in vitro by O6-(1-cyclopentenylmethyl)guanine. J Med Chem. 2000;43:4071–4083. doi: 10.1021/jm000961o. [DOI] [PubMed] [Google Scholar]
- 32.Moschel RC, McDougall MG, Dolan ME, Stine L, Pegg AE. Structural features of substituted purine derivatives compatible with depletion of human O6-alkylguanine-DNA alkyltransferase. J Med Chem. 1992;35:4486–4491. doi: 10.1021/jm00101a028. [DOI] [PubMed] [Google Scholar]
- 33.Goodtzova K, Crone T, Pegg AE. Activation of human O6-alkylguanine-DNA alkyltransferase by DNA. Biochemistry. 1994;33:8385–8390. doi: 10.1021/bi00194a001. [DOI] [PubMed] [Google Scholar]
- 34.Pegg AE, Chung L, Moschel RC. Effect of DNA on the inactivation of O6-alkylguanine-DNA alkyltransferase by 9-substituted O6-benzylguanine derivatives. Biochem Pharmacol. 1997;53:1559–1564. doi: 10.1016/s0006-2952(97)00060-9. [DOI] [PubMed] [Google Scholar]
- 35.Nelson ME, Loktionova NA, Pegg AE, Moschel RC. 2-Amino-O4-benzylpteridine derivatives: potent inactivators of O6-alkylguanine-DNA alkyltransferase. J Med Chem. 2004;47:3887–3891. doi: 10.1021/jm049758+. [DOI] [PubMed] [Google Scholar]
- 36.Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JE, 2nd, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–9. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson S, Lesniak MS. Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Delivery. 2007;4:169–80. doi: 10.2174/156720107780362302. [DOI] [PubMed] [Google Scholar]
- 38.Lopez KA, Waziri AE, Canoll PD, Bruce JN. Convection-enhanced delivery in the treatment of malignant glioma. Neurol Res. 2006;28:542–8. doi: 10.1179/016164106X116836. [DOI] [PubMed] [Google Scholar]
- 39.McCalmont WF, Patterson JR, Lindenmuth MA, Heady TN, Haverstick DM, Gray LS, Macdonald TL. Investigation into the structure-activity relationship of novel concentration dependent, dual action T-type calcium channel agonists/antagonists. Bioorg Med Chem. 2005;13:3821–3839. doi: 10.1016/j.bmc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Lee TR, Niu J, Lawrence DS. The extraordinary active site substrate specifity of pp60c-src a multiple specificity protein kinase. J Biol Chem. 1995;270:5375–5380. doi: 10.1074/jbc.270.10.5375. [DOI] [PubMed] [Google Scholar]
- 41.Savanmard S, Loktionova NA, Fang Q, Pauly GT, Pegg AE, Moschel RC. Inactivation of O6-alkylguanine-DNA alkyltransferase by folate esters of O6-benzyl-2′-deoxyguanosine and of O6-[4-(hydroxymethyl)benzyl]guanine. J Med Chem. 2007 doi: 10.1021/jm0705859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 43.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47:1750–9. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 44.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–96. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 45.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]