Abstract
The intracellular processing of epidermal growth factor receptor (EGFR) induced by epidermal growth factor (EGF) and transforming growth factor-α (TGF-α has been studied meticulously, with the former resulting in EGFR degradation and the latter in EGFR recycling to the plasma membrane. However, little is known about how other EGF family growth factors affect the trafficking of the EGFR. Additionally, although both EGF and TGF-α have been shown to effectively induce initial c-Cbl (ubiquitin ligase)-mediated ubiquitination of the EGFR, limited information is available regarding the role of c-Cbl in the trafficking and signaling of recycling EGFR. Thus, in the present study we investigated the roles of c-Cbl in endogenous EGFR trafficking and signaling after stimulation with amphiregulin (AR). We demonstrated that a physiological concentration of AR induced recycling of endogenous EGFR to the plasma membrane, which correlated closely with transient association of EGFR with c-Cbl and transient EGFR ubiquitination. Most importantly, we used c-Cbl small interfering RNA (siRNA) duplexes and a c-Cbl dominant negative mutant to show that c-Cbl is critical for the efficient transition of EGFR from early endosomes to a recycling pathway, and that c-Cbl regulates the duration of extracellular-signal-regulated kinase 1/2 mitogen-activated protein kinase (ERK1/2 MAPK) phosphorylation. These data support novel functions of c-Cbl in mediating recycling of EGF receptors to the plasma membrane, as well as in mediating the duration of activation (transient vs. sustained) of ERK1/2 MAPK phosphorylation.
Keywords: Amphiregulin, EGFR recycling, c-Cbl, ERK1/2 MAPK
The epidermal growth factor receptor (EGFR) belongs to a family of cell surface receptor tyrosine kinases, which includes four ErbB members, i.e. EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4 [reviewed in (1)]. Many different growth factors can serve as ligands for the EGFR, and these include epidermal growth factor (EGF), transforming growth factor-α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), betacellulin (BTC), amphiregulin (AR), epiregulin (EPR), and epigen (EPG). All EGFR ligands are synthesized as membrane proteins and are subsequently released from the cell surface by regulated proteolysis. However, little is known about what dictates the cleavage and shedding of different EGFR ligands, or most importantly, about the physiological and pathological relevance of the different cognate ligands. To date, it still remains enigmatic how different EGFR ligands could serve distinct functions despite their shared interactions with the same receptor.
Ligand binding to the EGFRs causes the formation of homo- and hetero-dimers, a process, which subsequently induces autophosphorylation through activation of the EGFR tyrosine kinase activity. Following activation, the EGFR undergoes internalization and endocytic trafficking. After endocytosis, some receptors recycle from endosomes back to the plasma membrane, whereas others enter the degradative pathway to late endosomes and lysosomes, a process that results in receptor downregulation. In that regard, it is well established that EGF, but not TGF-α, triggers efficient degradation of the EGF receptors (2, 3). A recent report (4) also demonstrated that AR does not induce significant EGFR degradation.
There have been significant advances in the understanding of how receptor trafficking and signaling are functionally interrelated (5), yet this relationship still remains obscure. The signaling of activated EGFR involves numerous downstream pathways including mitogen-activated protein kinases, phosphotidylinositol-3 kinase, c-Src, and phospholipase C γ/protein kinase C. These complex signal transduction cascades modulate cell proliferation, differentiation, adhesion, migration, survival and death. Whereas EGFR signaling is crucial for many normal cellular processes, aberrant EGFR activation has been implicated in the pathophysiology of hyperproliferative diseases such as cancer.
The mammalian Cbl proteins constitute a highly conserved family of three ubiquitin ligases, known as c-Cbl, Cbl-b and Cbl-c [reviewed in (6)]. In recent years, Cbl has emerged as a critical player in regulating EGFR endocytic trafficking (7, 8). Numerous studies have provided direct evidence for the role of EGF-induced, Cbl-mediated, sustained EGFR ubiquitination in receptor targeting to lysosomes (3, 9–15). Importantly, although TGF-α has been shown to induce transient EGFR association with Cbl and receptor ubiquitination (3), there have been no reports so far in the literature addressing possible roles of Cbl in receptor recycling.
The role of c-Cbl as a regulator of signal transduction, and consequently cell function and development, is now well established (16). Evidence suggests that dysregulation and/or disruption of the function of c-Cbl contributes to the development of many pathological conditions, including immunological and malignant diseases. The role of c-Cbl in signaling is thought to be based largely on its ubiquitin ligase activity, but, many cellular events are dependent on its function as an adaptor molecule (16).
Others previously have shown that EGF and TGF-α induce differential fates of the internalized EGFR, with the former resulting in EGFR degradation and the latter in EGFR recycling. However, although c-Cbl has been implicated in the regulation of EGFR degradation, possible roles for c-Cbl in EGFR recycling have not yet been addressed. Therefore, in the present study we examined the roles of c-Cbl in ligand-specific EGFR trafficking and signaling. Concentrating on two members of EGFR ligand family, i.e. EGF and AR, we show that AR and EGF induced similar patterns of short term EGFR and c-Cbl phosphorylation, physical association of c-Cbl with EGFR, and EGFR ubiquitination; however, as previously reported for TGF-α (3), the effects of AR were much more transient than those of EGF. Most importantly, our new data implicate c-Cbl in the active sorting of the EGFR to recycling endosomes. We also show that c-Cbl regulates the duration of AR-induced extracellular-signal-regulated kinase 1/2 mitogen-activated protein kinase (ERK1/2 MAPK) activation. Taken together, our results shed some light on the new, unexplored aspects of specialized endocytic sorting to recycling pathways.
EXPERIMENTAL PROCEDURES
Materials
Human embryonic kidney (HEK293) cells were purchased from American Type Culture Collection (Manassas, VA). Human recombinant EGF, human recombinant AR, chloroquine, monensin and all other biochemical reagents were purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies were from Invitrogen (Invitrogen, Carlsbad, CA). Sheep polyclonal anti-EGFR and anti-phosphotyrosine (clone 4G10) antibodies were from Upstate Millipore (Billerica, MA). Rabbit polyclonal anti-Rab11 was from Zymed Laboratories (South San Francisco, CA). Mouse monoclonal anti-early endosome antigen 1 (EEA1) (clone 14) and mouse monoclonal anti-c-Cbl (clone 17) were from BD Transduction Laboratories (Franklin Lakes, NJ). Mouse monoclonal anti-lysosome-associated membrane protein (LAMP) (clone H4A3) was from BD Pharmingen (San Diego, CA). Mouse monoclonal anti-Cbl-b (clone G-1) was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-ubiquitin (clone FK2) was from Biomol (Plymouth Meeting, PA). Rabbit anti-phospho-ERK1/2, mouse anti-ERK1/2, and rabbit anti-phospho-EGFR (Tyr-1173) were from Cell Signaling Technologies (Danvers, MA). Mouse monoclonal anti-β-actin (clone AC-15) antibody was from Sigma-Aldrich (St. Louis, MO). Peroxidase-labeled secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA) and Rockland Immunochemicals (Gilbertsville, PA). SDS-PAGE molecular weight markers were from Bio-Rad (Hercules, CA).
Cell Culture, RNA Interference Experiments and DNA Transfection
HEK293 cells were grown in Eagle’s minimum essential medium (MEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen Gibco, Carlsbad, CA) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were grown to ~75% confluence following which they were placed in serum-deprived binding media (MEM supplemented with 0.1% bovine serum albumin) for 24 h prior to treatments. For synchronized ligand pulse experiments, cells at 4°C were incubated with agonists for 45 min in serum-deprived binding medium containing 20 mM HEPES, pH 7.4. Then, the cells were rinsed with ice-cold PBS to remove unbound ligand, following which the bound ligand stimulated EGFR upon exposure to pre-warmed ligand-free medium at 37°C.
A mixture of four SMARTselection-designed siRNAs targeting one gene (Thermo Fisher Scientific Dharmacon, Inc., Lafayette, CO) were transfected using oligofectamine (Invitrogen, Carlsbad, CA) reagent according to the manufacturer’s instructions. A pool of 4 siGenome non-targeting siRNAs, designated as scrambled (SCR) siRNA, was used as control. 72 h following transfection, cell lysates were assayed for silencing effectiveness by Western blotting and immunofluorescence staining.
The expression constructs pcDNA3GFP-Cbl-wt and pcDNA3GFP-Cbl-N have been described previously, and were kindly provided by Dr. Hamid Band (17). HEK293 cells were transiently transfected with the above constructs using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Western Blotting
After treatments, cells were rinsed briefly with PBS and extracted with RIPA buffer (150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1% NP-40, 0.1% SDS in PBS) containing protease inhibitors. Cells subsequently were sonicated, and protein concentrations were determined by the BCA assay (Pierce, Rockford, IL). Equal amounts of proteins were separated by SDS-PAGE on 4–12% polyacrylamide gels (Invitrogen, Carlsbad, CA), transferred to PVDF membranes and blocked with 5% milk in PBS for 1 h at room temperature. Following several washes with PBS containing 0.1% Tween, the membranes were incubated with the appropriate dilutions of primary and peroxidase-conjugated secondary antibodies (as directed by the manufacturer) in blocking solution. Immunoblotted proteins were detected using ECL reagents (GE Healthcare Amersham Biosciences, Piscataway, NJ).
Biotinylation of Cell Surface Proteins
HEK293 cells grown on 100 mm dishes were washed one time with ice-cold PBS and incubated with 0.5 mg/ml sulfo-NHS-biotin (Pierce, Rockford, IL) for 30 min at 4°C to label surface proteins. Cells then were washed with 15 mM glycine to quench excess, unreacted biotin. After the indicated treatments, cells were rinsed briefly with ice-cold PBS and extracted with Triton lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA) supplemented with protease inhibitor cocktail III (EMD Calbiochem, San Diego, CA), 1 mM PMSF, and phosphatase inhibitors (HALT phosphatase inhibitor cocktail, Pierce, Rockford, IL). Equal amounts of proteins (0.5 mg) were precleared by incubation for 30 min at 4°C with 30 μl of protein A/G Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). After a brief centrifugation, the supernatants were removed and incubated overnight at 4°C with 50 μl of streptavidin-agarose beads (Novagen, Madison, WI). The samples then were centrifuged and washed 3 times with 1 ml of Triton lysis buffer. Proteins were eluted from the beads using Laemmli sample buffer. Samples subsequently were analyzed by SDS-PAGE and Western blotting.
Immunoprecipitation
After the indicated treatments, HEK293 cells grown in 150 mm dishes were scraped into ice-cold PBS and centrifuged at 200 × g. Pellets were lysed in 1 ml of Triton lysis buffer supplemented with protease and phosphatase inhibitors, as described above. Equal amounts of proteins (1.5 mg) were precleared by incubation for 30 min at 4°C with 30 μl of protein A/G Sepharose beads. After a brief centrifugation, the supernatants were removed and incubated overnight at 4°C with either 8 μg of anti-EGFR or 13 μg of anti-Cbl antibodies. Immunoprecipitates were captured with 50 μl of protein A/G beads at 4°C for 1 h. The samples were then centrifuged and washed 3 times with 1 ml of Triton lysis buffer. Proteins were eluted from the beads using Laemmli sample buffer. Samples were subsequently analyzed by SDS-PAGE and Western blotting.
Immunofluorescence Staining and Confocal Microscopy
Cells were grown on 35 mm lysine-coated, glass-bottom culture dishes (MatTek Corporation, Ashland, MA). After treatments, cells were fixed with freshly prepared 3.7% paraformaldehyde in PBS for 15 min at room temperature. Subsequently, cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS for 5 min, following which nonspecific binding sites were blocked with 3% normal serum (Santa Cruz, Santa Cruz Biotechnologies, Santa Cruz, CA) in PBS for 1 h. Incubations with the appropriate dilutions of primary and Alexa Fluor-conjugated secondary antibodies (as directed by the manufacturer) were performed in blocking solution. Confocal microscopy was performed using a Zeiss LSM 510 META laser-scanning microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 60x objective, using the following laser wavelengths: excitation 488 nm, emission 505–530 nm; excitation 543 nm, emission 560–615 nm. Quantifications of the colocalization coefficients, derived from measured pixel overlaps between EGFR and EEA1, Rab11 and LAMP, were performed using Zeiss LSM 510 colocalization analysis software. The mean values were averaged from at least three independent single cell images.
Receptor Recycling Assay
HEK293 cells grown in 6-well plates were incubated with 100 ng/ml AR at 4°C for 45 min in serum-deprived binding medium containing 20 mM HEPES, pH 7.4. Then, the cells were rinsed with ice-cold PBS to remove unbound ligand, following which cells were incubated for 0, 15, 30 and 60 min in pre-warmed ligand-free medium at 37°C. The cells were then placed on ice and rinsed twice with ice-cold PBS, followed by a 7 minute incubation with a low pH stripping buffer (150 mM acetic acid, pH 2.7, containing 150 mM NaCl) to remove surface-bound ligands. The numbers of available cell surface ligand-binding sites were determined by incubating cells for 90 min on ice with [125I]-EGF (PerkinElmer, Boston, MA), as previously described (18). The specific activity of [125I]-EGF was approximately 5 ×105 cpm/ng. The cells then were washed 3 times with ice-cold PBS, solubilized with 1 ml of 0.1N NaOH containing 0.1% SDS, and the bound radioactivity was measured in a gamma counter. Nonspecific binding was determined in the presence of excess unlabeled HB-EGF.
Statistical Analyses
Statistical significance was determined using paired two-tailed t-test and analysis of variance (ANOVA) with Dunnett’s or Bonferroni post-test to correct for multiple comparisons (GraphPad Prism, version 4). p values < 0.05 were considered to be statistically significant.
RESULTS
Differential Fates of EGFR Induced by EGFR Ligands
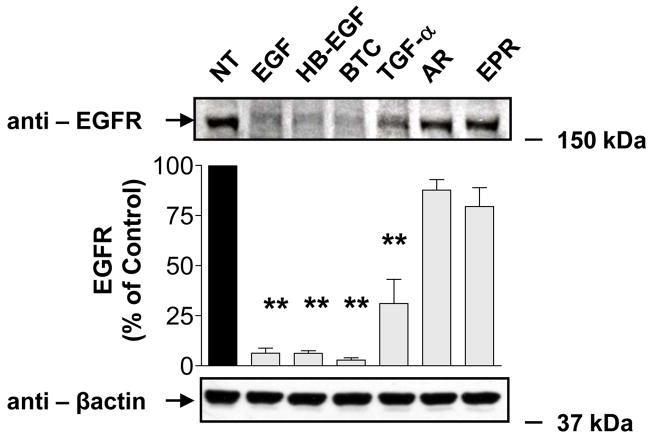
EGFR intracellular trafficking has been intensely studied, yet the overwhelming majority of studies have focused primarily on one ligand, namely EGF. Although, the effects of some other EGFR ligands on EGFR also have been investigated (3, 4, 18, 19), little is known about the effects of most of those other cognate EGFR ligands. Hence, we examined the effects of six known EGFR ligands, i.e. EGF, HB-EGF, BTC, TGF-α, AR and EPR, on HEK293 cells. We analyzed the fate of EGFR by Western blotting following treatment with a saturating concentration (100 ng/ml) of individual ligands for 180 min. As seen in Fig. 1, stimulation with HB-EGF or BTC resulted in degradation of EGFR similar in magnitude to that caused by EGF. In contrast, degradation in response to TGF-α treatment was significantly reduced as compared with EGF. AR- or EPR-stimulated cells did not show any significant loss of EGFR immunoreactivity. Because saturating concentrations of AR and EPR do not induce degradation of EGFR, we reasoned that these ligands might induce recycling of EGFR. To address the possibility that c-Cbl affects trafficking and signaling of recycling EGFRs, we chose to compare for the rest of our studies the effects of AR with well characterized EGF.
FIGURE 1. Differential fates of EGFR induced by EGFR ligands.
Serum-deprived HEK293 cells were treated at 37°C with vehicle (NT), 100 ng/ml EGF, HB-EGF, BTC, TGF-α, AR or EPR for 180 min, extracted with RIPA buffer and subsequently immunoblotted with anti-EGFR and anti-β-actin antibodies. Data shown are representative of three independent experiments. Results are mean ± S.E. (n=3), **, p <0.01 versus vehicle (NT).
Effects of AR and EGF on EGFR Autophosphorylation
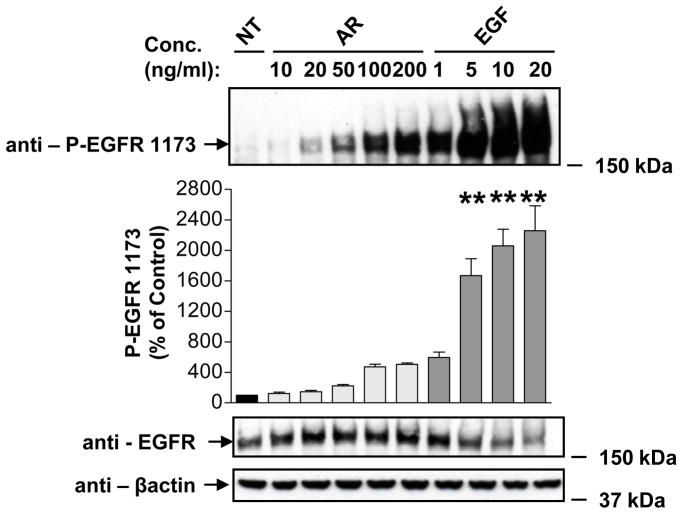
Due to the fact that AR has a lower affinity for EGFR than does EGF (20), we varied the concentrations of EGF and AR in order to find optimal concentrations that would bring about similar extents of short-term EGFR phosphorylation. This approach enabled us to characterize trafficking of EGFRs that were autophosphorylated/activated to a similar degree by the two ligands. We examined the effects of various concentrations of AR and EGF on phosphorylation of EGFR at Tyr-845 (c-Src-mediated), as well as Tyr-1068 and Tyr-1173 (major autophosphorylation sites). Phosphorylation of Tyr-845 is believed to stabilize the receptor activation loop and is required for the mitogenic function of the receptor. Phosphorylated Tyr-1068 is involved in receptor trafficking, whereas phosphorylated Tyr-1173 plays an important role in MAPK activation. Our concentration-response studies demonstrated that treatment of HEK293 cells with physiological concentrations of ligand −100 ng/ml (9 nM) AR or 1 ng/ml EGF (0.17 nM) - for 2 min caused comparable levels of phosphorylation of Tyr-1173 (Fig. 2). We had similar findings for EGFR Tyr-845 and Tyr-1068 (data not shown). Accordingly, we used 100 ng/ml AR or 1 ng/ml EGF for the remainder of our studies.
FIGURE 2. Effects of AR and EGF on EGFR autophosphorylation.
Serum-deprived HEK293 cells were treated at 37°C with vehicle (NT), 10, 20, 50, 100 and 200 ng/ml AR, or 1, 5, 10 and 20 ng/ml EGF for 2 min. After washing with ice-cold PBS, cells were extracted with RIPA buffer and cell lysates were immunoblotted with anti-phospho-EGFR Tyr-1173 antibody. Blots were then stripped and reprobed for total EGFR and β-actin to normalize for loading. Data shown are representative of three independent experiments. Results are mean ± S.E. (n=3), **, p <0.01 versus vehicle (NT).
Differential trafficking of EGFR induced by EGF and AR
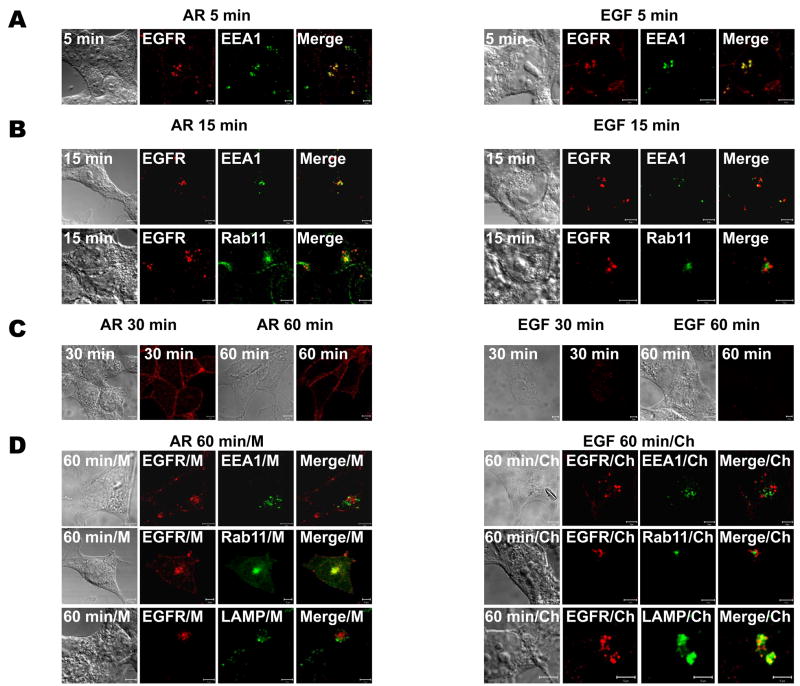
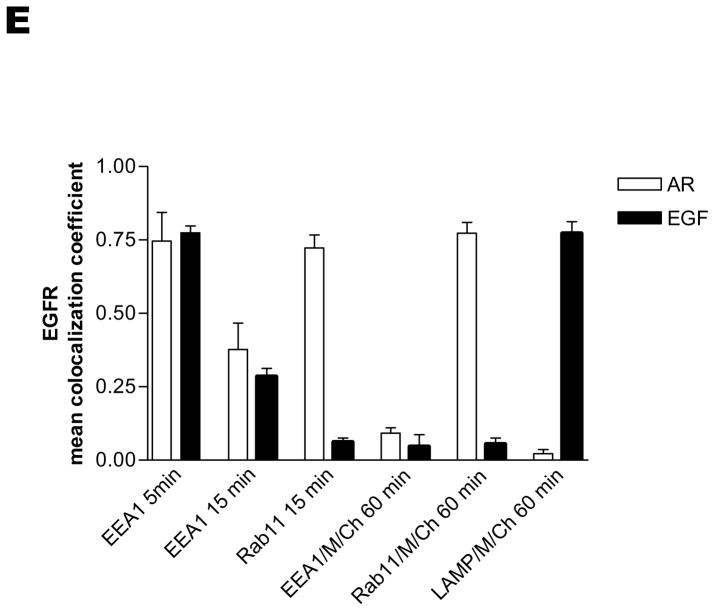
To further examine the intracellular localization of AR- or EGF-treated EGFR, we performed experiments in the presence or absence of chloroquine or monensin, agents that block lysosomal degradation and interfere with protein recycling (21), respectively. To circumvent low endogenous EGFR expression, and most importantly, the effects of simultaneous EGFR processing, i.e. receptor endocytosis, sorting, recycling and degradation, we used a widely employed synchronization approach (3, 13, 22–25). Briefly, following pre-binding of the ligand at 4°C, the cells were washed to remove unbound ligand and then shifted back to 37°C, which enabled initiation of synchronous, ligand-induced EGFR processing. We employed various markers of subcellular organelles, i.e. EEA1 for early endosomes, Rab11 for the perinuclear slow recycling compartment, and LAMP for lysosomes (Fig. 3). Five minutes following re-warming of the cells pretreated with either AR or EGF, EGFRs extensively co-localized with EEA1, suggesting that EGFRs enter early endosomes after stimulation with either AR or EGF (Fig. 3A, 5 min). In contrast, after 15 min, the AR-stimulated EGFRs partially localized to both early (EEA1-positive) and recycling (Rab11-positive) endosomes, whereas EGF-activated EGFRs only partially co-localized with EEA1 and did not appear to co-localize with Rab11 (Fig. 3B, 15 min). Thirty minutes after rewarming, the AR-stimulated EGFRs appeared predominantly on the plasma membrane; the EGFRs were fully recycled after 60 min. On the other hand, the paucity of staining for EGFRs treated with EGF after 30 and 60 minutes of re-warming was indicative of EGFR degradation (Fig. 3C). Importantly, our studies in the presence of monensin or chloroquine demonstrated clear, predominant co-localization of AR-stimulated EGFRs in Rab11-positive vesicles, and EGF-stimulated EGFR in LAMP-positive lysosomes, respectively (Fig. 3D, AR 60 min/M and EGF 60 min/Ch). It should be noted that consistent with previous studies (21, 26, 27), pretreatment with monensin also blocked EGF-induced EGFR degradation, whereas chloroquine had negligible effects on AR-induced EGFR recycling (Supplemental Figure 1). To provide numerical support for our colocalization observations, the colocalization coefficients, which measure pixel overlap between EGFR and EEA1, Rab11 and LAMP in Figs. 3A, B, D, were quantified using Zeiss LSM 510 colocalization analysis software (Fig. 3E). Taken together, the results presented in Fig. 3 provide unequivocal evidence that under synchronized conditions, AR triggers normal EGFR endocytosis, as previously has been observed for EGF (3, 13, 22–25). More importantly, we demonstrate that although both ligands induce similar levels of early EGFR autophosphorylation, EGF and AR cause differential trafficking of EGFRs.
FIGURE 3. Differential trafficking of EGFR induced by EGF and AR.
Serum-deprived HEK293 cells were pre-treated without (panels A–C) or with 100 μM chloroquine (Ch) or 100 μM monensin (M) for 15 min (panel D), incubated on ice with 1 ng/ml EGF or 100 ng/ml AR for 45 min, washed free of unbound ligand, warmed, and exposed to pre-warmed ligand-free medium at 37°C for 5 (A), 15 (B), or 30 and 60 (C–D) minutes. The cells then were fixed, stained with anti-EGFR antibody (visualized with Alexa Fluor 568-conjugated secondary antibody; red) and anti-EEA1, -Rab11 or -LAMP antibodies (visualized with Alexa Fluor 488-conjugated secondary antibody; green), and analyzed by confocal microscopy. Data shown are representative of three independent experiments. Yellow indicates co-localization. Bar, 5 μm. (E) The colocalizations between EGFR and EEA1, Rab11 or LAMP observed in panels A, B, and D were quantified using Zeiss LSM 510 META colocalization analysis software. The mean colocalization coefficients, averaged from at least three independent single cell images, represent pixel overlap between EGFR and EEA1, Rab11 or LAMP. The coefficients vary from 0 to 1, with 0 corresponding to non-overlapping images and 1 corresponding to 100% co-localization.
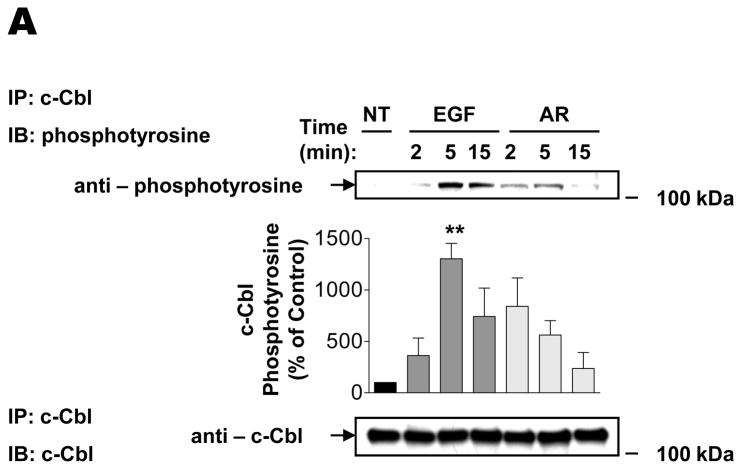
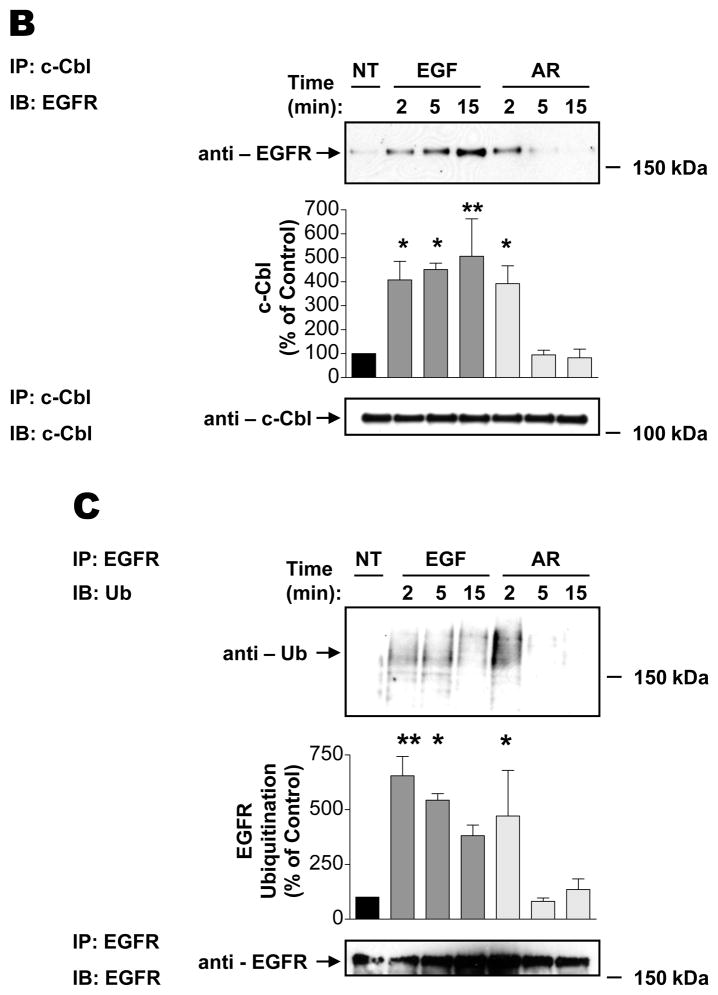
Effects of AR and EGF on EGFR Ubiquitination and Association with c-Cbl
It was previously shown that c-Cbl-mediated ubiquitination plays a central role in the lysosomal sorting and degradation of EGFR (3, 9–15). Thus, we hypothesized that the observed differences in the intracellular trafficking of the EGFR upon stimulation with EGF or AR, might result from differential EGFR association with c-Cbl and/or differential receptor ubiquitination by c-Cbl, as previously has been observed for TGF-α (3). To study these possibilities, we first examined the activation of c-Cbl after various periods of EGF and AR treatment in HEK293 cells. As demonstrated in Fig. 4A, although both ligands induced considerable tyrosine phosphorylation of c-Cbl (a surrogate of its activity), EGF evoked more robust and sustained responses than AR. Fig. 4B shows results from co-immunoprecipitation experiments in which c-Cbl was immunoprecipitated from HEK293 cells, following which Western blotting of EGFR was performed. Those results show similar amounts of EGFR in c-Cbl immunoprecipitates after a 2 minute treatment with either ligand. However, the association between c-Cbl and EGFR was transient in cells treated with AR in that there was virtually no co-immunoprecipitation after 5 and 15 min. In marked contrast, the association between c-Cbl and EGFR was more stable in cells treated with EGF, persisting without diminishing for 15 min. The pattern of EGFR co-immunoprecipitation with c-Cbl in Fig. 4B was closely recapitulated when we probed ubiquitination of EGFR at various time points after stimulation with EGR or AR (Fig. 4C). The fact that AR-induced c-Cbl phosphorylation lasted longer (Fig. 4A) than AR-induced association between c-Cbl and EGFR or EGFR ubiquitination (Figs. 4B and C, respectively), suggests that deubiquitinating enzymes acted on EGFR prior to dephosphorylation and inactivation of c-Cbl.
FIGURE 4. Effects of AR and EGF on EGFR ubiquitination and association with c-Cbl.
Serum-deprived HEK293 cells were treated at 37°C with vehicle (NT), 1 ng/ml EGF or 100 ng/ml AR for 2, 5, 15 min. After washing with ice-cold PBS, cell lysates were (A–B) subjected to immunoprecipitation with an antibody to c-Cbl, followed by immunoblotting with an antibody to phosphotyrosine or EGFR, or (C) subjected to immunoprecipitation with an antibody to EGFR, followed by immunoblotting with an antibody to ubiquitin. Blots were then stripped and reprobed for total c-Cbl or EGFR. Insets shown are representative of three independent experiments. Results are mean ± S.E. (n=3), *, p <0.05, **, p <0.01 versus vehicle (NT).
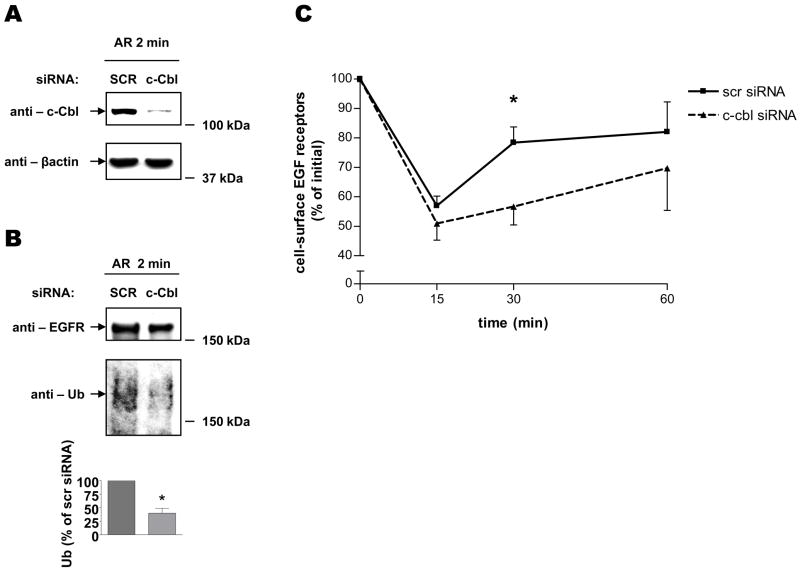
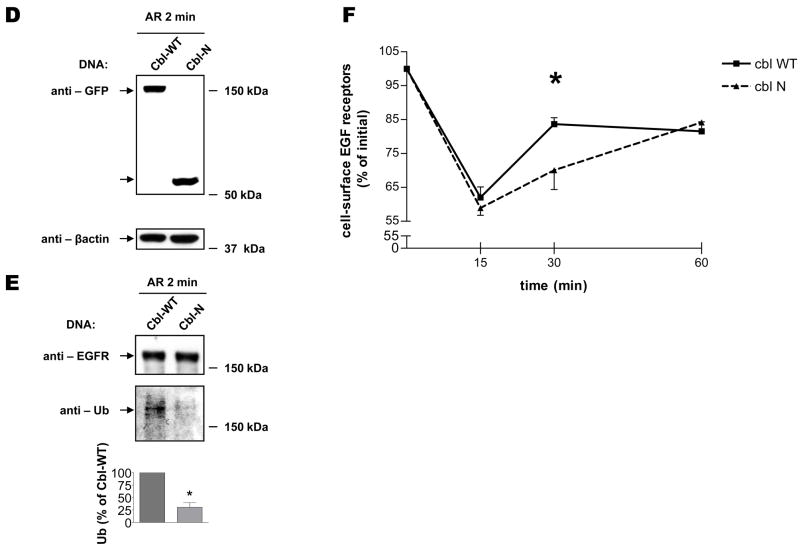
Role of c-Cbl in AR-induced EGFR Trafficking
To examine the potential importance of c-Cbl in recycling of EGFR, we used an siRNA knockdown approach, in which a pool of 4 different siRNA duplexes was utilized to selectively deplete endogenous c-Cbl. We analyzed the effectiveness of siRNA against c-Cbl by means of Western blotting analysis, and confirmed that c-Cbl protein expression could be suppressed considerably (Fig. 5A). We then assessed the ability of AR to induce EGFR ubiquitination under control and c-Cbl-knockdown conditions (Fig. 5B). Consistent with previous reports (13), Cbl deficiency significantly reduced EGFR ubiquitination. To test the possibility that c-Cbl is involved in AR-induced EGFR trafficking, we exposed control and c-Cbl-knockdown cells to AR, and determined the extent of receptor recycling by means of radioligand binding (Fig. 5C). These experiments demonstrate that similar levels of EGFR were endocytosed 15 minutes after a synchronous pulse with AR in control and c-Cbl-knockdown cells. Interestingly, however, receptor recycling in c-Cbl-depleted cells was significantly delayed compared with control cells (Fig. 5C, c-Cbl siRNA, 30 min). Importantly, it should be noted that similar observations were made in HEK293 cells, in which both c-Cbl and Cbl-b were simultaneously depleted (Supplemental Figure S2). To confirm the requirement for c-Cbl ubiquitin ligase activity in AR-induced receptor recycling, we used a dominant negative mutant of c-Cbl, named Cbl-N, which lacks regions required for c-Cbl-mediated EGFR ubiquitination (17). Consistent with our c-Cbl knockdown results, we demonstrated that overexpression of the Cbl-N mutant inhibited ubiquitination of AR-stimulated EGFR (Fig. 5E), and delayed AR-induced EGFR recycling (Fig. 5F). These results suggest that the ubiquitin ligase activity of c-Cbl is important for recycling of AR-stimulated EGFR.
FIGURE 5. Role of c-Cbl in AR-induced EGFR recycling.
Serum-deprived HEK293 cells, which had been transiently transfected with scrambled (SCR) or c-Cbl siRNA for 72 h (A–C), or which had been transiently transfected with GFP-c-Cbl-WT or GFP-c-Cbl-N for 24 h (D–F), were (A and D) treated with 100 ng/ml AR for 2 min, extracted with RIPA buffer, following which cell lysates were immunoblotted with an anti-c-Cbl, -GFP or –β-actin antibodies; (B and E) pre-incubated with sulfo-NHS-biotin for 30 min to label cell surface proteins and subsequently treated with 100 ng/ml AR for 2 min. After washing with ice-cold PBS, biotinylated proteins were analyzed by SDS-PAGE followed by immunoblotting with antibodies to EGFR and Ub; (C and F) incubated on ice with 100 ng/ml AR for 45 min, and subsequently incubated in pre-warmed ligand-free medium at 37°C for 0, 15, 30 and 60 min. The cells were then rinsed with ice-cold binding buffer, followed by a 7 minute long incubation with a low pH stripping buffer. The specific binding was determined by incubating cells for 90 min on ice with [125I]-EGF. The results are expressed as percentage of the original binding sites measured at 0 min. Data shown in panels A, B D, and E are representative of three independent experiments. Results in panel C and F are mean ± S.E. of three separate experiments, *, p < 0.05 versus scrambled siRNA.
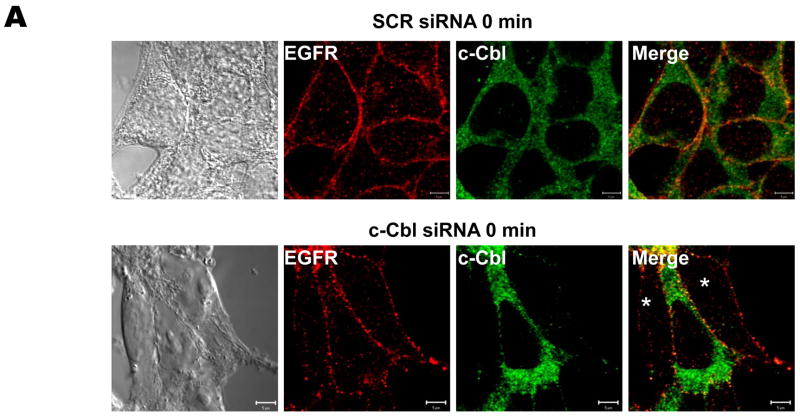
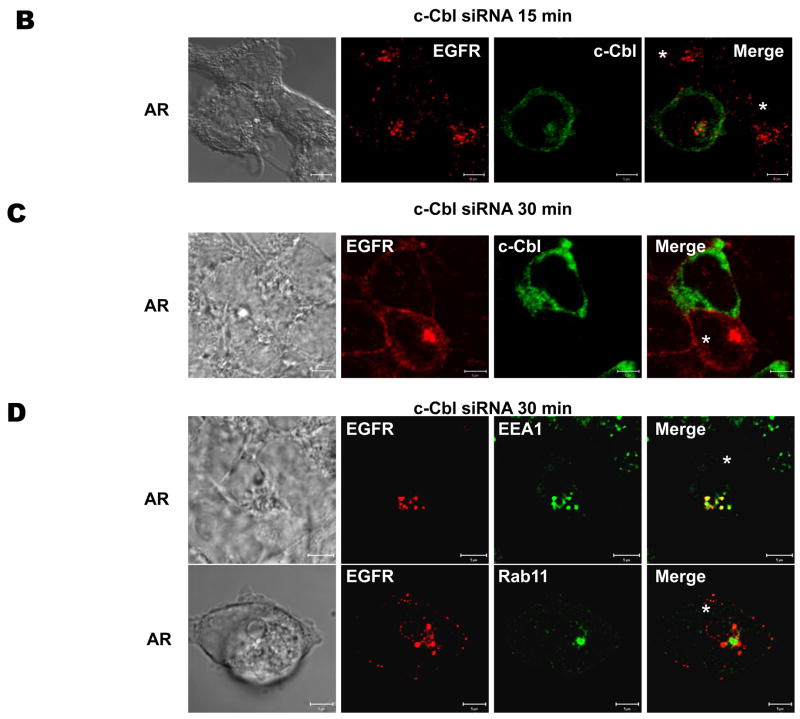
To characterize in greater detail the trafficking of AR-stimulated EGFR in c-Cbl-knockdown cells, we performed side-by-side comparisons of trafficking in HEK293 cells with little silencing of c-Cbl (cells without asterisk) and those with marked silencing (cells with asterisk) (Fig. 6). Having established that under synchronized conditions AR-activated EGFR undergo endocytosis (Fig. 3 and 5C), we analyzed the localizations of AR-induced EGFR in control and c-Cbl-knockdown cells (Fig. 6 B–D). As shown in Fig. 6B, following a 15 minute chase with AR, internalization of the EGFR was apparent (evidenced by localization of EGFR in intracellular vesicles) regardless of whether c-Cbl expression was knocked down or not. To further investigate the roles for c-Cbl in post-endocytotic, AR-induced EGFR trafficking, we examined the localization of EGFR 30 minutes after a synchronized ligand pulse in c-Cbl-depleted cells (Fig. 6C and D). We observed that c-Cbl-knockdown greatly diminished AR-induced EGFR recycling, as evidenced by intracellular accumulations of EGFR in cells in which c-Cbl was knocked down (Fig. 6C, cell with asterisk), as compared with those in which c-Cbl was not knocked down, in which there was localization of EGFR at the cell surface (Fig. 6C, cell without asterisk). It should be noted that similar findings were observed when the control and c-Cbl-knockdown cells were continuously treated with AR for 30 min (data not shown). Figure 6D shows representative photomicrographs of the intracellular accumulation of EGFR in the early endosomes of cells treated with c-Cbl siRNA 30 minutes following a synchronized pulse of AR, as evidenced by clear colocalization of EGFR with the early endosomal marker EEA1, and no overlap with Rab11. This should be contrasted with Figure 3C, in which EGFRs were localized to the plasma membrane 30 minutes after a pulse of AR. These results strongly suggest that c-Cbl is required for efficient exit of recycling EGFR from endosomes.
FIGURE 6. Role of c-Cbl in AR-induced EGFR trafficking.
Serum-deprived HEK293 cells, which had been transiently transfected with scrambled (SCR) or c-Cbl siRNA for 72 h, were incubated on ice with 100 ng/ml AR for 45 min, washed free of unbound ligand, and subsequently incubated in pre-warmed ligand-free medium at 37°C for 0 (A), 15 (B), and 30 (C) min. The cells then were fixed, stained with anti-EGFR (visualized with Alexa Fluor 568-conjugated secondary antibody; red) and anti-c-Cbl (visualized with Alexa Fluor 488-conjugated secondary antibody; green) antibodies, and analyzed by confocal microscopy. Fields were chosen to show simultaneously cells in which c-Cbl was depleted and cells in which c-Cbl was not, in order to facilitate direct comparisons. White asterisks show positions of c-Cbl-depleted cells. (D) These micrographs show the intracellular localization of EGFR (visualized with Alexa Fluor 568-conjugated secondary antibody; red), in serum-deprived HEK293 cells, which had been transiently transfected with scrambled (SCR) or c-Cbl siRNA for 72 h, 30 minutes after a synchronized pulse with AR. Dual labeling was performed with markers of subcellular organelles, i.e. early endosomes and the perinuclear recycling compartment (visualized with anti-EEA1 and -Rab11 antibodies, respectively, and Alexa Fluor 488-conjugated secondary antibody; green). White asterisks show positions of c-Cbl-depleted cells. Yellow indicates co-localization. Bar, 5 μm. Data shown are representative of at least three independent experiments.
Role of c-Cbl in AR-induced Signaling
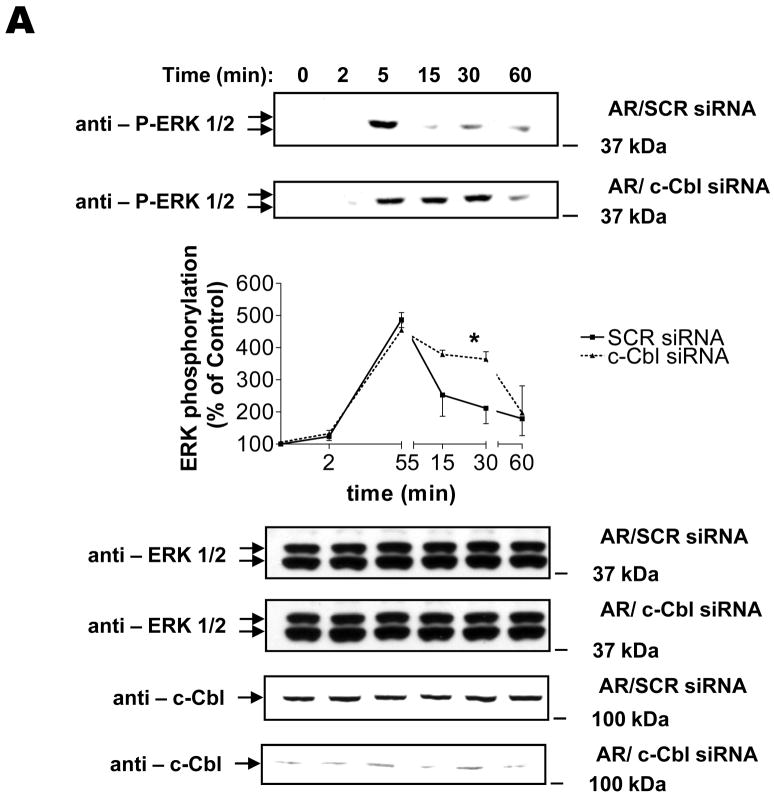
Because one of the main EGFR-mediated signals is activation of the ERK family of MAPKs (28), we focused our attention on potential roles of c-Cbl in ligand-induced ERK1/2 MAPK phosphorylation. Figure 7 compares the ability of AR to induce ERK1/2 MAPK phosphorylation in control and c-Cbl-knockdown cells. In both conditions, phosphorylation of ERK1/2 MAPK peaked at about 500% by 5 min, but the signal was more sustained in c-Cbl-knockdown cells. When similar experiments were performed using EGF, ERK phosphorylation was much more pronounced than for AR (approximately 1,500%) in cells treated with scrambled siRNA. ERK phosphorylation was of similar magnitude in c-Cbl-knockdown cells, and phosphorylation was slightly prolonged (Supplemental Fig. S3A). It should be noted that, as predicted, the EGF-induced degradation of EGFR in our system was significantly blocked in c-Cbl-depleted cells (Supplemental Fig. S3B). Taken together, these results strongly suggest that c-Cbl attenuates later phases of EGFR-induced ERK1/2 MAPK activation.
FIGURE 7. Role of c-Cbl in AR-induced MAPK activation.
Serum-deprived HEK293 cells, which had been transiently transfected with scrambled (SCR) or c-Cbl siRNA for 72 h, were incubated at 37°C with 100 ng/ml AR for 0, 2, 5, 15, 30, and 60 min. After washing with ice-cold PBS, cells were extracted with RIPA buffer, following which cell lysates were immunoblotted with anti-phospho ERK1/2. Blots then were stripped and reprobed for total ERK1/2 or c-Cbl. Results are mean ± S.E. (n=3), *, p < 0.05 versus scrambled siRNA. Insets shown are representative of three independent experiments.
DISCUSSION
The EGFR has been shown to play key roles in the regulation of many normal cellular processes. Despite significant advances, our knowledge about the cellular trafficking and signaling induced by various cognate EGFR ligands is incomplete. In the present study we investigated roles of the ubiquitin ligase c-Cbl in EGFR trafficking and signaling after stimulation with AR, a cognate EGFR ligand that induces receptor recycling. What is new about this work is that our findings implicate c-Cbl as a critical mediator of active sorting of EGFR to the recycling endosomes. To our knowledge, this is the first demonstration that c-Cbl mediates the efficient exit of the EGFR from early endosomes into recycling pathways. Our results also illustrate a potential role for c-Cbl in controlling the temporal dynamics (transient vs. sustained) of ERK1/2 MAPK phosphorylation by the EGFR.
The family of EGFR ligands has expanded throughout the years and now includes seven members, i.e. EGF, HB-EGF, BTC, TGF-α, AR, EPR and EPG. These ligands variably induce degradation or recycling of the EGFR. Although the mechanisms underlying EGF-induced downregulation of EGFR continue to be carefully studied, much less is known regarding the trafficking of EGFR following stimulation with other members of this family. A recent study by Stern et al. (4) investigated the effects of AR and BTC on EGFR. The results suggested that AR did not lead to EGFR degradation, but the effects on recycling were not examined. Those studies were performed on cells overexpressing EGF receptors exposed to supraphysiological concentrations of the ligands. Although experiments with ectopic expression of EGFR present several technical advantages, their interpretation is problematic due to the fact that endocytosis and lysosomal sorting are saturable processes (1, 29, 30), and that receptor overexpression can disrupt normal trafficking (unpublished observation). Therefore, we investigated the effects of AR and EGF on endogenous EGFR expressed in HEK293 cells. We demonstrated herein differential intracellular trafficking of EGFR following stimulation with those ligands. Following endocytosis, the receptors enter early endosomes where they undergo sorting to either recycling or degradative fates (31). In that regard, our results suggest that when EGF and AR are applied in concentrations that result in equivalent levels of short-term EGFR phosphorylation, EGF targets EGFR to degradation, whereas AR targets EGFR to a recycling pathway.
With regard to recycling, it is known that internalized EGFR can recycle back to the plasma membrane directly from early endosomes through Rab4-positive endosomes, or from perinuclear Rab11-positive recycling endosomes (32, 33). Although it was not our intention to directly address this matter, our results indicated that AR-activated EGFR returned to the plasma membrane predominantly from the perinuclear area. It should be noted, however, that according to emerging models, endosomes can be viewed as a mosaic of distinct but interconnected domains containing different combinations of the Rab family of small GTPases [reviewed in (34)].
There are at least three possible mechanisms that could explain why AR stimulation results in recycling, rather than EGFR degradation and downregulation. It is possible that AR could induce hetero-dimerization with other members of ErbB family of receptors, in a fashion distinct from that of EGF (35). In that regard, HEK293 cells express endogenous levels of all four ErbB receptors (data not shown). Another possibility is that the AR-EGFR ligand-receptor interaction is weaker than EGF-EGFR within the acidic environment of endosomes (2). A third possibility is that AR is ineffective for inducing c-Cbl-mediated EGFR ubiquitination (12). Because Johnson et al. (36) provided strong evidence that AR functionally coupled primarily to EGFR, we felt it unlikely that AR triggered EGFR-ErbB2 or -ErbB3 hetero-dimerization. Although AR has been shown to have a much lower affinity for EGFR than does EGF (20), pH-sensitive binding of AR to EGFR has not yet been investigated. Inasmuch as we normalized the concentrations of AR and EGF to similar levels of phosphorylation of EGFR, we did not further investigate the stability of AR-EGFR and EGF-EGFR binding in intracellular vesicles. Instead, we focused our attention on the possibility that AR and EGF induce a differential engagement with c-Cbl. Indeed, AR clearly induced only a transient EGFR association with c-Cbl, and subsequently less sustained receptor ubiquitination, supporting a possible role for the sustained EGFR ubiquitination in the efficient receptor sorting to lysosomal degradation. It should be noted that our results are consistent with those with ectopic expression of EGFR and supraphysiological concentrations of AR (up to 136 nM), in which decreased receptor degradation, ubiquitination and association with c-Cbl also were observed (4). Together, our data and others (3, 4) suggest that AR and TGF-α use common mechanisms to modulate receptor recycling.
Although our data that show that AR (like TGF-α) induces transient EGFR association with c-Cbl and receptor ubiquitination point to potential roles of c-Cbl in the trafficking and signaling of recycling EGF receptors, these possibilities heretofore have received little attention. Despite considerable debate in the literature regarding the role of c-Cbl in endocytosis (9, 13–15, 23, 37–41), one still might speculate that c-Cbl is required for AR-induced EGFR internalization. Recent work by Huang et al. (15), demonstrating a mutational analysis of EGFR lysine residues, suggests that a Cbl-mediated ubiquitination event is involved in EGFR internalization, yet the ubiquitination of the receptor itself is not necessary. Our results document that substantial knockdown of c-Cbl does not interfere with AR-induced EGFR internalization in native HEK293 cells (Fig. 5C). In light of recent findings suggesting that both c-Cbl and Cbl-b must be depleted simultaneously in order to detect the effect of siRNAs on EGFR endocytosis (9), we also investigated whether double knockdown of c-Cbl and Cbl-b could interfere with AR-induced EGFR endocytosis (Supplemental Figure S2). Neither condition appeared to significantly affect receptor endocytosis. It should be noted, however, that out results do not rule out the possibility that a residual pool of c-Cbl/Cbl-b is sufficient to support EGFR endocytosis or that Cbl-c could facilitate EGFR endocytosis (38).
In any case, the most critical observation made was that knockdown of c-Cbl (Fig. 5C and Supplemental Figure S2C) -- as well as overexpression of a c-Cbl dominant negative mutant (Fig. 5F) -- altered receptor recycling to the plasma membrane. Our findings challenge the current dogma that the role of Cbl in EGFR sorting is limited merely to the degradative pathway. We demonstrated herein that c-Cbl-knockdown and an inactive c-Cbl mutant delay sorting of AR-activated EGFR to the recycling endosomes. Because AR-activated EGFR was delayed in the early endosomes in c-Cbl-depleted cells, it appears that c-Cbl is required for EGFR to efficiently exit from these endosomal vesicles. Taken together, we speculate that c-Cbl has the ability to govern the AR-induced active sorting of EGFR from the early endosomes to recycling endosomes.
The results presented here suggest at least two mechanisms by which c-Cbl can regulate active sorting of receptors to recycling endosomes. It is possible that the AR-induced transient EGFR ubiquitination is required at the initial checkpoint to “pre-sort” constitutively trafficked unliganded EGFR from liganded receptors that are further sorted to degradative or recycling pathways. It should be noted, however, that although our studies strongly point to a critical role for the ubiquitin ligase activity of c-Cbl in EGFR recycling, our studies do not warrant that initial AR-induced EGFR ubiquitination is involved in the active sorting of EGFR to recycling endosomes. A systematic mutational analysis of EGFR lysine residues (15) is essential to address this issue. On the other hand, our results demonstrating that AR-induced c-Cbl phosphorylation (a surrogate of its activity) lasted longer than ubiquitination of EGFR (Fig. 4A and B, respectively) suggest an alternative or more complex mechanism.
Alternatively, perhaps, c-Cbl interacts with or regulates key vesicular components that are integral for active recycling. In that regard, Hanyaloglu et al. (42) identified a novel function of hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) in mediating recycling of certain G protein-coupled receptors. They reported that this function was distinct from a previously defined role of Hrs in lysosomal sorting (43), as it did not require receptor ubiquitination. They also hypothesized that Hrs may mediate distinct sorting functions depending upon whether receptors were ubiquitinated or nonubiquitinated. Additionally, Stern et al. (44) have shown that c-Cbl modulates Hrs ubiquitination, phosphorylation and protein levels, thus controlling the composition of sorting machinery. It is tempting to speculate that c-Cbl may conspire with Hrs to determine whether EGFR exit early endosomes into recycling and/or degradative compartments. Taken together, our investigation raises an intriguing possibility that c-Cbl regulates EGFR sorting to recycling pathways. Future studies are necessary to better understand the molecular mechanisms underlying this process.
Although c-Cbl has been implicated in the activation of various MAPKs in response to cell surface receptor activation (16), limited information is available regarding the role of c-Cbl in EGFR-mediated MAPK signaling. Our results presented here (Supplemental Fig. S3A) are consistent with those ffrom other groups, which have reported that compromising ubiquitin ligase activity of c-Cbl leads to enhanced EGF-stimulated ERK1/2 MAPK activation (45–47). However, the potential role of c-Cbl in the activation of ERK1/2 MAPK induced by ligands that do not cause EGFR degradation has not yet been explored. In the present report, we showed that AR induced much more sustained ERK1/2 MAPK phosphorylation in c-Cbl-knockdown cells, as compared with control cells. This finding is significant in that recent studies have indicated that small changes in the strength and/or duration of ERK1/2 MAPK signaling may evoke strikingly different responses, e.g. cytoplasmic versus nuclear localization of ERK1/2 MAPK, differential gene expression, or proliferation versus differentiation (reviewed in (48, 49)). Given the fact that aberrant endosomal trafficking and EGFR signaling have been linked to a number of diseases, including cancer (50), the roles of c-Cbl in trafficking and signaling of the recycling EGF receptors are worthy of closer examination.
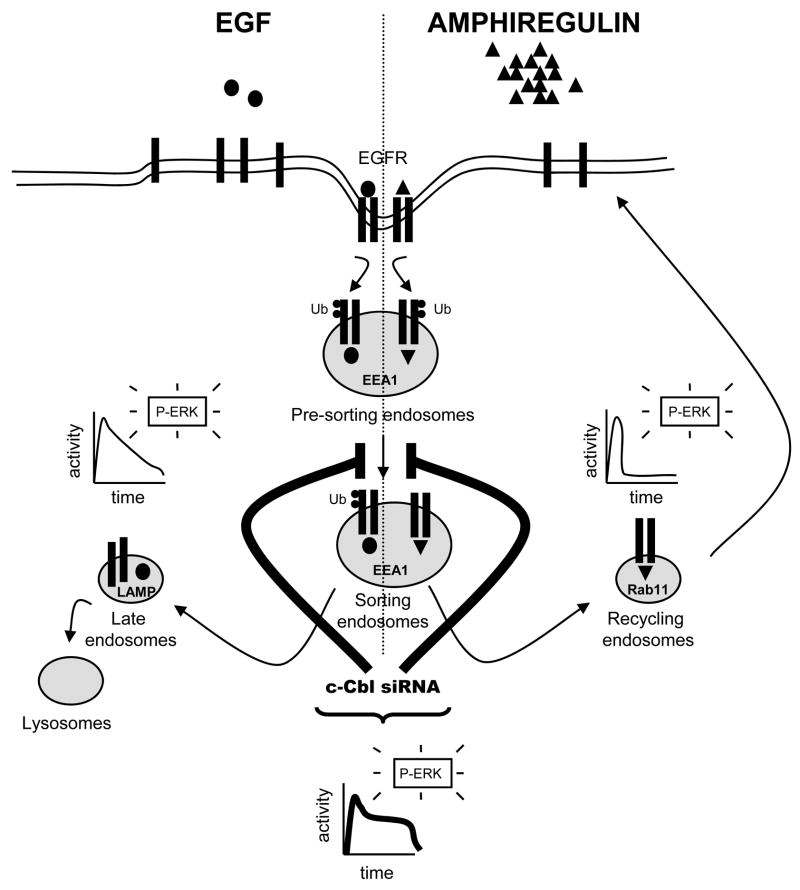
In summary, in the present study we addressed several fundamental issues regarding the processing, sorting and signaling of the EGFR following stimulation with AR (Fig. 8). Our study points to a potential global role for c-Cbl in regulating vesicular sorting of EGFR, regardless of whether the receptors have been destined for recycling or degradation. It appears that the function of c-Cbl is not merely limited to the regulation of receptor degradative pathways. Our work supports additional roles for c-Cbl in (1) mediating differential effects of EGF and AR on EGFR, (2) controlling efficient exit of EGFR from EEA1-positive endosomes into a recycling pathway, and (3) controlling the kinetics of EGFR-stimulated ERK1/2 MAPK. In the future, it will be of great interest to investigate the mechanisms through which c-Cbl regulates trafficking and signaling of recycling EGFR.
FIGURE 8. Roles of c-Cbl in EGFR recycling and signaling.
Binding of AR (triangles; right-side of scheme) or EGF (ovals; left-side of scheme) to the EGFR triggers receptor endocytosis. Following internalization into early pre-sorting endosomes (EEA1-positive), the AR- or EGF-stimulated EGFR is sorted into recycling endosomes (Rab11-positive), or to late endosomes and lysosomes (LAMP-positive), respectively. Differential trafficking of EGFR correlates with differential patterns of ERK activation, i.e. unlike AR, which causes transient phosphorylation of ERK, EGF results in much more persistent activation of ERK. AR induces transient ubiquitination of EGFR (small circles, Ub), whereas EGF induces more sustained ubiquitination. Regardless of EGFR ligand, c-Cbl regulates exit into both recycling and degradative EGFR trafficking pathways. In the absence of c-Cbl, the disrupted sorting of EGFR causes receptor retention within the early endosomes, which consequently is associated with more sustained phosphorylation of ERK.
Supplementary Material
Differential trafficking of EGFR induced by AR and EGF (Supplemental Fig. S1), roles of c-Cbl and Cbl-b in AR-induced EGFR recycling (Supplemental Fig. S2), and role of c-Cbl in EGF-induced EGFR degradation and MAPK activation (Supplemental Fig. S3). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by Department of Veterans Affairs Merit and Research Enhancement Award Program grants (to J.R.R. Sr.), by National Institutes of Health Grants NIH DK052448 and GM063909, by an American Heart Association (Mid-Atlantic) fellowship (to A.B.) and by a laboratory endowment jointly supported by the Medical University of South Carolina, Division of Nephrology and Dialysis Clinics, Inc. (to J.R.R. Sr.).
We thank the MUSC Hollings Cancer Center Molecular Imaging Facility for the use of Zeiss confocal microscope. We also express our gratitude to Dr. Hamid Band, who provided us with the GFP-c-Cbl-WT and GFP-c-Cbl-N constructs.
The abbreviations used are
- AR
amphiregulin
- BTC
betacellulin
- EEA1
early endosome antigen 1
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EPG
epigen
- EPR
epiregulin
- ERK1/2 MAPK
extracellular-signal-regulated kinase 1/2 mitogen-activated protein kinase
- HEK293
human embryonic kidney 293 cells
- HB-EGF
heparin-bound EGF-like growth factor
- Hrs
hepatocyte growth factor-regulated tyrosine kinase substrate
- LAMP
lysosome-associated membrane protein
- TGF-α
transforming growth factor-α
References
- 1.Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 2.Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Fiore PP, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–488. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- 6.Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 8.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 9.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 10.de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. doi: 10.1242/jcs.114.11.2167. [DOI] [PubMed] [Google Scholar]
- 11.Yokouchi M, Kondo T, Houghton A, Bartkiewicz M, Horne WC, Zhang H, Yoshimura A, Baron R. Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J Biol Chem. 1999;274:31707–31712. doi: 10.1074/jbc.274.44.31707. [DOI] [PubMed] [Google Scholar]
- 12.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 14.Ravid T, Heidinger JM, Gee P, Khan EM, Goldkorn T. c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J Biol Chem. 2004;279:37153–37162. doi: 10.1074/jbc.M403210200. [DOI] [PubMed] [Google Scholar]
- 15.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci U S A. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 17.Lill NL, Douillard P, Awwad RA, Ota S, Lupher ML, Jr, Miyake S, Meissner-Lula N, Hsu VW, Band H. The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J Biol Chem. 2000;275:367–377. doi: 10.1074/jbc.275.1.367. [DOI] [PubMed] [Google Scholar]
- 18.Shelly M, Pinkas-Kramarski R, Guarino BC, Waterman H, Wang LM, Lyass L, Alimandi M, Kuo A, Bacus SS, Pierce JH, Andrews GC, Yarden Y. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J Biol Chem. 1998;273:10496–10505. doi: 10.1074/jbc.273.17.10496. [DOI] [PubMed] [Google Scholar]
- 19.Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem. 2003;278:35781–35790. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- 20.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 21.Stein BS, Bensch KG, Sussman HH. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984;259:14762–14772. [PubMed] [Google Scholar]
- 22.Myromslien FD, Grovdal LM, Raiborg C, Stenmark H, Madshus IH, Stang E. Both clathrin-positive and -negative coats are involved in endosomal sorting of the EGF receptor. Exp Cell Res. 2006;312:3036–3048. doi: 10.1016/j.yexcr.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol Biol Cell. 2004;15:3591–3604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willingham MC, Haigler HT, Fitzgerald DJ, Gallo MG, Rutherford AV, Pastan IH. The morphologic pathway of binding and internalization of epidermal growth factor in cultured cells.Studies on A431, KB, and 3T3 cells, using multiple methods of labelling. Exp Cell Res. 1983;146:163–175. doi: 10.1016/0014-4827(83)90334-8. [DOI] [PubMed] [Google Scholar]
- 25.Kharchenko MV, Aksyonov AA, Melikova MM, Kornilova ES. Epidermal growth factor (EGF) receptor endocytosis is accompanied by reorganization of microtubule system in HeLa cells. Cell Biol Int. 2007;31:349–359. doi: 10.1016/j.cellbi.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Shitara Y, Kato Y, Sugiyama Y. Effect of brefeldin A and lysosomotropic reagents on intracellular trafficking of epidermal growth factor and transferrin in Madin-Darby canine kidney epithelial cells. J Control Release. 1998;55:35–43. doi: 10.1016/s0168-3659(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 27.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 29.Wiley HS. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund KA, Opresko LK, Starbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–15723. [PubMed] [Google Scholar]
- 31.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci 113 Pt. 2000;2:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 34.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 35.Lenferink AE, Pinkas-Kramarski R, van de Poll ML, van Vugt MJ, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelen EJ, Yarden Y. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. Embo J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson GR, Kannan B, Shoyab M, Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993;268:2924–2931. [PubMed] [Google Scholar]
- 37.Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, Sorkin A. Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis. Mol Biol Cell. 2005;16:1268–1281. doi: 10.1091/mbc.E04-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 40.Holler D, Dikic I. Receptor endocytosis via ubiquitin-dependent and -independent pathways. Biochem Pharmacol. 2004;67:1013–1017. doi: 10.1016/j.bcp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Szymkiewicz I, Kowanetz K, Soubeyran P, Dinarina A, Lipkowitz S, Dikic I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J Biol Chem. 2002;277:39666–39672. doi: 10.1074/jbc.M205535200. [DOI] [PubMed] [Google Scholar]
- 42.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. Embo J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clague MJ, Urbe S. The interface of receptor trafficking and signalling. J Cell Sci. 2001;114:3075–3081. doi: 10.1242/jcs.114.17.3075. [DOI] [PubMed] [Google Scholar]
- 44.Stern KA, Visser Smit GD, Place TL, Winistorfer S, Piper RC, Lill NL. Epidermal growth factor receptor fate is controlled by Hrs tyrosine phosphorylation sites that regulate Hrs degradation. Mol Cell Biol. 2007;27:888–898. doi: 10.1128/MCB.02356-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- 46.Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. Embo J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu WJ, Tu S, Cerione RA. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell. 2003;114:715–725. doi: 10.1016/s0092-8674(03)00688-3. [DOI] [PubMed] [Google Scholar]
- 48.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 49.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential trafficking of EGFR induced by AR and EGF (Supplemental Fig. S1), roles of c-Cbl and Cbl-b in AR-induced EGFR recycling (Supplemental Fig. S2), and role of c-Cbl in EGF-induced EGFR degradation and MAPK activation (Supplemental Fig. S3). This material is available free of charge via the Internet at http://pubs.acs.org.