Abstract
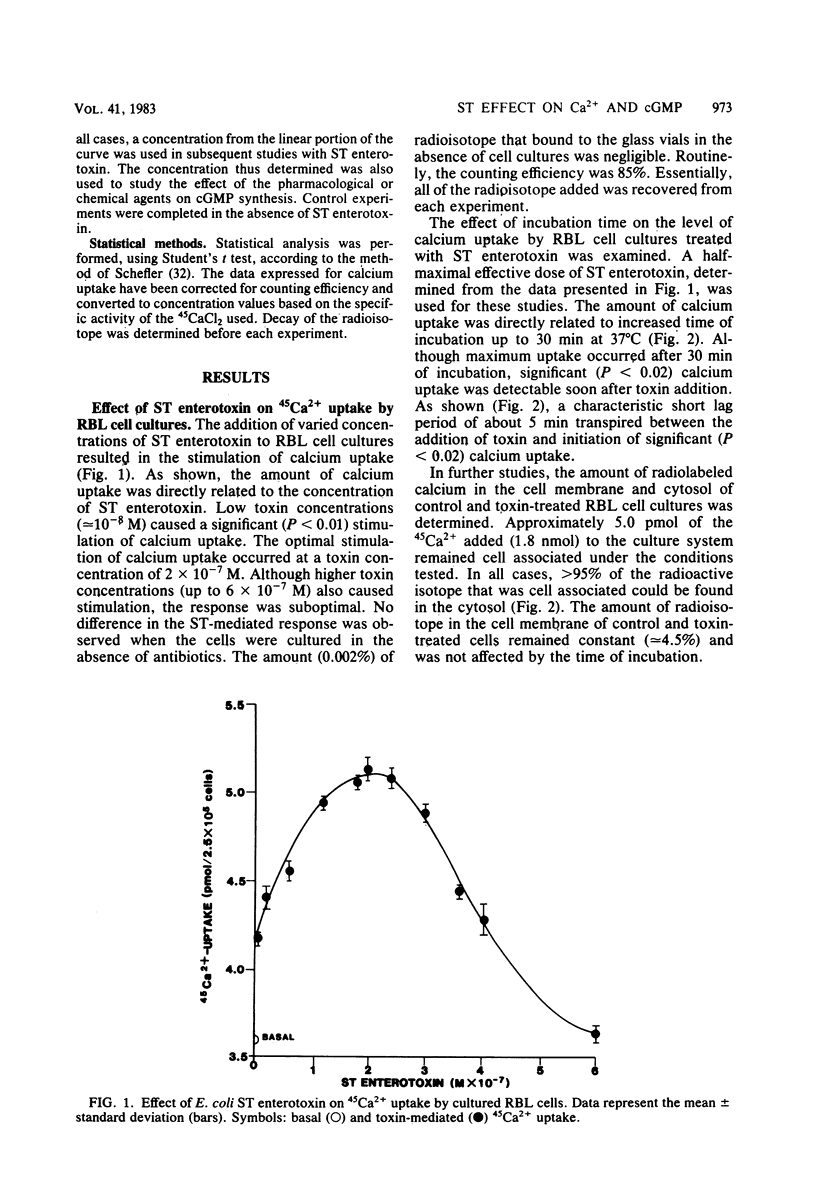
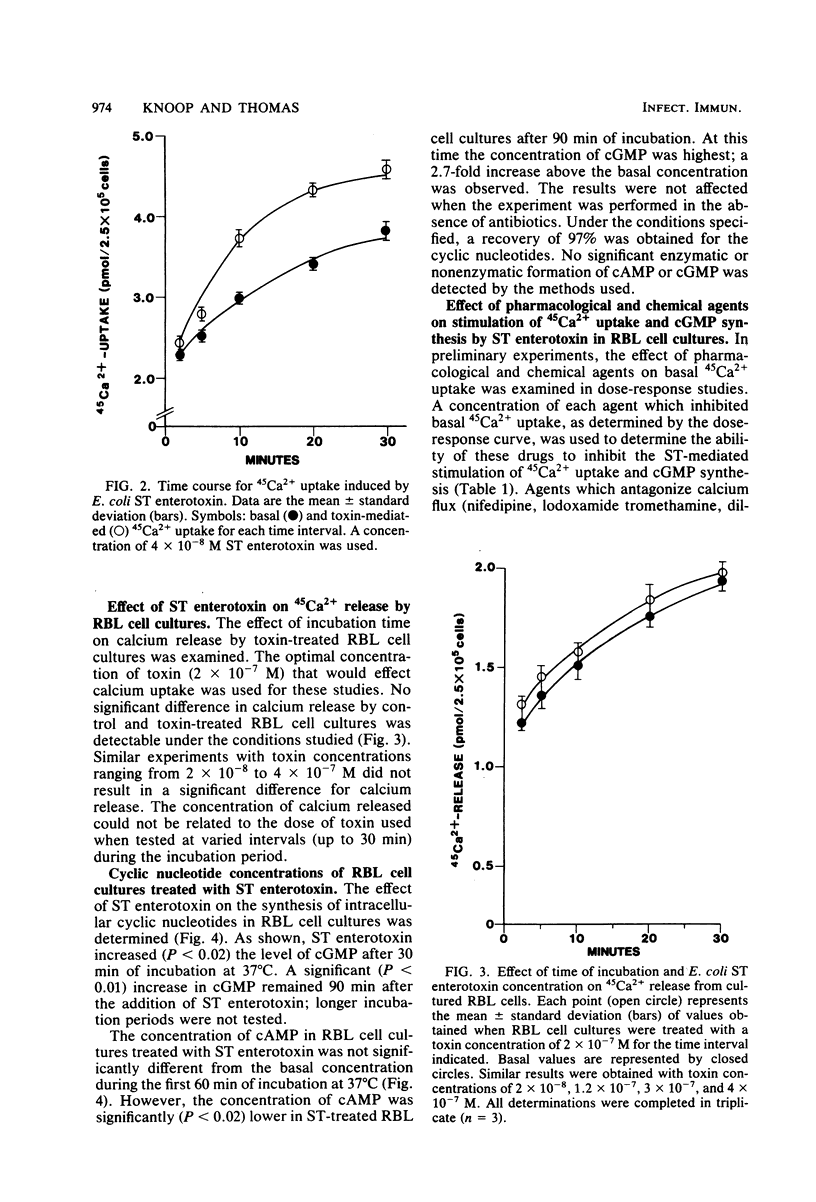
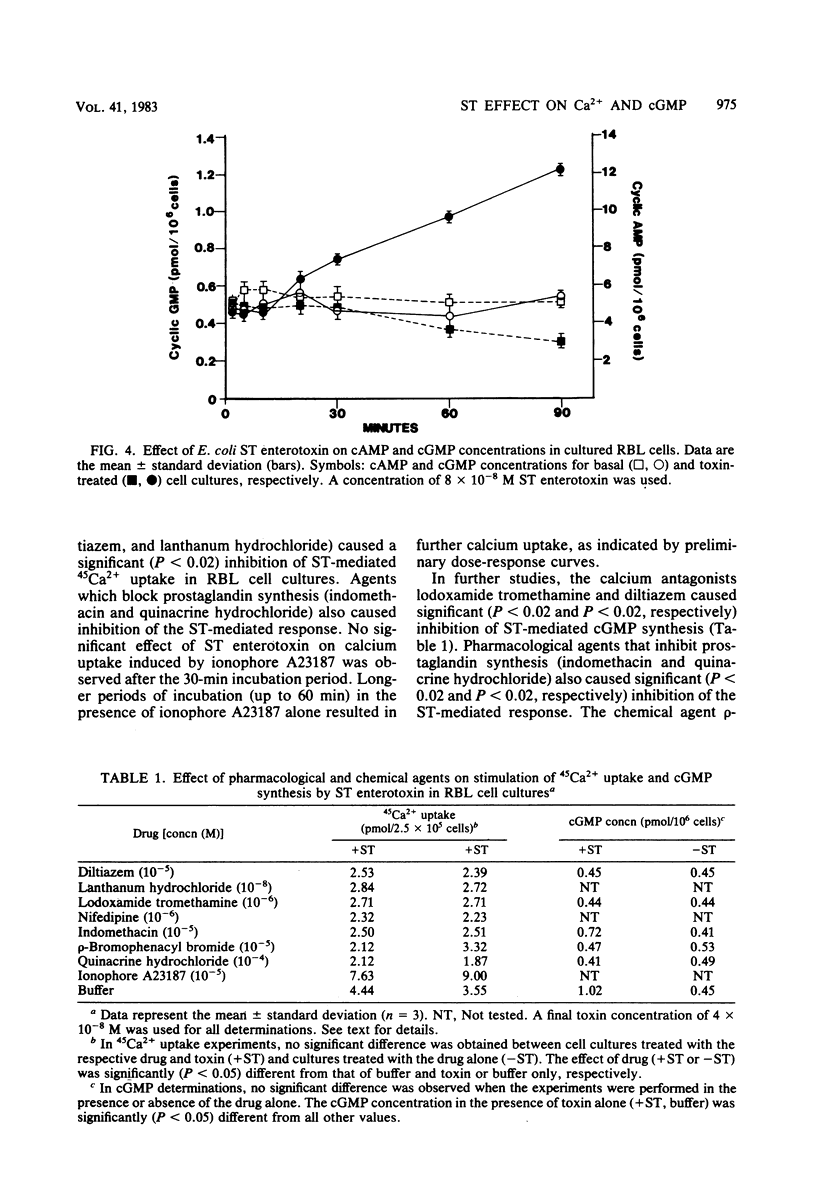
The effect of Escherichia coli heat-stable (ST) enterotoxin on calcium and cyclic nucleotide metabolism in rat basophilic leukemia cell cultures was investigated. Addition of ST enterotoxin to rat basophilic leukemia cell cultures resulted in dose- and time-dependent stimulation of calcium uptake and elevation of the intracellular cyclic GMP (cGMP) concentration. The effect of ST enterotoxin on calcium uptake (P less than 0.02) and cGMP synthesis (P less than 0.02) was demonstrated after 5 and 30 min of incubation at 37 degrees C, respectively. In further studies ST enterotoxin did not enhance calcium release or the intracellular concentration of cyclic AMP. The stimulation of calcium uptake and cGMP synthesis by ST enterotoxin was inhibited by pharmacological and chemical agents which block cellular calcium entry and prostaglandin synthesis. These results demonstrate that ST enterotoxin induces calcium uptake and cGMP synthesis in rat basophilic leukemia cell cultures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbey D. M., Knoop F. C. Effect of chlorpromazine on the secretory activity of Escherichia coli heat-stable enterotoxin. Infect Immun. 1979 Dec;26(3):1000–1003. doi: 10.1128/iai.26.3.1000-1003.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Butcher F. R. Calcium and cyclic nucleotides in the regulation of secretion from the rat parotid by autonomic agonists. Adv Cyclic Nucleotide Res. 1978;9:707–721. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Rohde J. E., Sharp G. W. Intestinal adenyl-cyclase activity in human cholera. Lancet. 1971 May 8;1(7706):939–941. doi: 10.1016/s0140-6736(71)91443-7. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Hadden E. M., Lopez C., Hadden J. W. cGMP and calcium in the initiation of cellular proliferation. Adv Cyclic Nucleotide Res. 1978;9:661–676. [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L., Murad F. Guanylate cyclase assay methods. Adv Cyclic Nucleotide Res. 1979;10:57–67. [PubMed] [Google Scholar]
- Giannella R. A., Drake K. W. Effect of purified Escherichia coli heat-stable enterotoxin on intestinal cyclic nucleotide metabolism and fluid secretion. Infect Immun. 1979 Apr;24(1):19–23. doi: 10.1128/iai.24.1.19-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Evan D. J., Jr, Evans D. G. Mechanism of activation adenylate cyclase in vitro by polymyxin-released, heat-labile enterotoxin of Escherichia coli. J Infect Dis. 1976 Mar;133 (Suppl):103–107. doi: 10.1093/infdis/133.supplement_1.s103. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Cockcroft S., Bennett J. P., Fewtrell C. M. Early events in the activation of Ca2+ dependent secretion: studies with rat peritoneal mast cells. J Physiol (Paris) 1980 Sep;76(5):383–393. [PubMed] [Google Scholar]
- Greenberg R. N., Guerrant R. L., Chang B., Robertson D. C., Murad F. Inhibition of Escherichia coli heat-stable enterotoxin effects on intestinal guanylate cyclase and fluid secretion by quinacrine. Biochem Pharmacol. 1982 Jun 1;31(11):2005–2009. doi: 10.1016/0006-2952(82)90413-0. [DOI] [PubMed] [Google Scholar]
- Greenberg R. N., Murad F., Chang B., Robertson D. C., Guerrant R. L. Inhibition of Escherichia coli heat-stable enterotoxin by indomethacin and chlorpromazine. Infect Immun. 1980 Sep;29(3):908–913. doi: 10.1128/iai.29.3.908-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Murad F., Guerrant R. L. Lanthanum chloride inhibition of the secretory response to Escherichia coli heat-stable enterotoxin. Infect Immun. 1982 Feb;35(2):483–488. doi: 10.1128/iai.35.2.483-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Knoop F. C., Abbey D. M. Effect of chemical and pharmacological agents on the secretory activity induced by Escherichia coli heat-stable enterotoxin. Can J Microbiol. 1981 Aug;27(8):754–758. doi: 10.1139/m81-117. [DOI] [PubMed] [Google Scholar]
- Madsen G. L., Knoop F. C. Inhibition of the secretory activity of Escherichia coli heat-stable enterotoxin by indomethacin. Infect Immun. 1978 Oct;22(1):143–147. doi: 10.1128/iai.22.1.143-147.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen G. L., Knoop F. C. Physiochemical properties of a heat-stable enterotoxin produced by Escherichia coli of human origin. Infect Immun. 1980 Jun;28(3):1051–1053. doi: 10.1128/iai.28.3.1051-1053.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome P. M., Burgess M. N., Mullan N. A. Effect of Escherichia coli heat-stable enterotoxin on cyclic GMP levels in mouse intestine. Infect Immun. 1978 Oct;22(1):290–291. doi: 10.1128/iai.22.1.290-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northover B. J. Indomethacin--a calcium antagonist. Gen Pharmacol. 1977;8(5-6):293–296. doi: 10.1016/0306-3623(77)90001-5. [DOI] [PubMed] [Google Scholar]
- Rao M. C., Guandalini S., Smith P. L., Field M. Mode of action of heat-stable Escherichia coli enterotoxin. Tissue and subcellular specificities and role of cyclic GMP. Biochim Biophys Acta. 1980 Sep 17;632(1):35–46. doi: 10.1016/0304-4165(80)90247-0. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Knoop F. C. Effect of heat-stable enterotoxin of Escherichia coli on cultured mammalian cells. J Infect Dis. 1983 Mar;147(3):450–459. doi: 10.1093/infdis/147.3.450. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Knoop F. C. The effect of calcium and prostaglandin inhibitors on the intestinal fluid response to heat-stable enterotoxin of Escherichia coli. J Infect Dis. 1982 Feb;145(2):141–147. doi: 10.1093/infdis/145.2.141. [DOI] [PubMed] [Google Scholar]
- Volpi M., Sha'afi R. I., Epstein P. M., Andrenyak D. M., Feinstein M. B. Local anesthetics, mepacrine, and propranolol are antagonists of calmodulin. Proc Natl Acad Sci U S A. 1981 Feb;78(2):795–799. doi: 10.1073/pnas.78.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]


