Abstract
We have previously found that aged rats show decreased proximal acidification without changes in NHE3 or V-H+ ATPase expression in brush border membrane vesicles. However, we did not identify any mechanism underlying these observations. The aim of the present work was to evaluate some of the regulatory systems of proximal acidification that could be affected by aging. We measured plasma concentrations of parathyroid hormone (PTH) and the amount of cAMP in the renal cortex of young and old Wistar rats. PTH plasma concentration was increased in old rats, whereas, although it showed a tendency to increase, the cAMP content in the renal cortex of old rats was not significantly different compared with the cortex of young rats. We measured the abundance of NHE8 isoforms of the Na+/H+ exchanger in brush border membrane vesicles from proximal convoluted tubules (PCT) of young and old rats by western blot analysis. We performed RT-PCR experiments in renal cortex homogenates from both experimental groups to evaluate mRNA expression of NHE3, NHE8 and H+ATPase. In senile rats, we detected a decreased abundance (at both gene expression and protein level) of the NHE8 isoform. These results could explain previous observations in which proximal tubule acidification appears affected in aged rats through a decrease in the activity of ethylisopropyl amiloride (EIPA)- and Bafilomycin-sensitive components, without changes in the NHE3 and V-H+ATPase abundance in the apical membrane of the PCT.
Keywords: Aging, cAMP, NHE3, NHE8, Parathyroid hormone, Proximal acidification, V-H+ATPase
Introduction
As part of the normal aging process, the kidney exhibits a progressive deterioration in several structures and functions (Davies and Shock 1950; Baylis 1994). Glomerular filtration rate, renal blood flow and concentrating ability decrease with age (Papper 1973; Corman et al. 1985; Lindeman et al. 1985; Lindeman 1995; Reilly et al. 1995). There is down-regulation of the renin-angiotensin system (RAS) with age, affecting renin mRNA and angiotensin converting enzyme (ACE) levels (Jung et al. 1995). In addition, renal haemodynamics seem to be more dependent on endothelium-derived relaxing factor (NO) in old rats than in young rats (Hill et al. 1997).
Kinsella and Sacktor (1987) found a decrease of Na+/H+ exchanger activity in brush border membrane vesicles (BBMV) from the renal cortex of old animals. In previous studies, we detected a decrease in the capacity of proximal tubular acidification of old rats, without changes in the acid-base balance (MacLaughlin et al. 2001; Fiori et al. 2006). This reduction was due to a decrease in both ethylisopropyl amiloride (EIPA)- and Bafilomycin-sensitive components. EIPA is an inhibitor of apical membrane Na+/H+ exchange, and Bafilomycin inhibits V-H+ATPase. The fall in H-ion secretion was not associated with any change in NHE3 and V-H+ATPase expression as measured by western blot.
Although we described impairment in proximal acidification we were unable to identify the cause/s responsible for the deficiency in the performance of Na+/H+ and V-H+ATPase.
The control of proximal acidification is very complex and depends on many factors including, among others, RAS (Reilly et al. 1995), NO (Amorena and Castro 1997; Manning et al. 1997; Wang 1997), and parathyroid hormone (PTH) (Bank and Aynedjain 1976). The serum concentration of PTH increases with aging in both humans and rats (Fox 1991; Halloran et al. 1996). PTH, through the generation of cAMP, inhibits the transport of phosphate, sodium, bicarbonate and water in the proximal convoluted tubule (PCT; Agus et al. 1971; Dennis 1976; McKinney and Myers 1980). This inhibitory effect of PTH is due to inhibition of the Na+/H+ exchanger in the luminal membrane of PCT cells (Cohn et al. 1983; Dennis 1976; Kahn et al. 1985).
On the other hand, mice with genetic deletion of both NHE2 and NHE3 isoforms have only a 50% reduction in proximal tubule Na+-dependent H+ secretion compared with wild-type mice, suggesting the presence of another apical NHE isoform in the proximal tubule (Choi et al. 2000). NHE8 is a recently identified renal Na+/H+ antiporter isoform (Goyal et al. 2003) that has been localised to the apical membrane of the rat proximal tubule (Goyal et al. 2005) and is highly EIPA sensitive (Zhang et al. 2007). Interestingly, expression of the NHE8 isoform diminishes with age (Becker et al. 2007).
The aim of the present work was to evaluate the occurrence of the NHE8 isoform in BBMV, the concentration of cAMP in kidney cortex homogenates, and the plasma PTH concentration in order to gain some insight into possible factors involved in the decrease of proximal acidification in kidneys of aged rats.
Methods
Animals
All experiments conformed with the principles stated in the American Physiological Society’s Guiding Principles in the Care and Use of Animals. Wistar rats were maintained in 12 h light/12 h dark cycles with ad libitum access to standard laboratory rat chow and tap water. Two groups were studied, young adult (3 months old) and old (18 months old) rats.
Parathyroid hormone analysis
Blood was collected and serum PTH concentration was measured by immunoradiometric assay (Arnaud et al. 1971).
Determination of cAMP levels in renal cortex
Cyclic AMP in renal cortex homogenates was measured by radioimmunoassay (RIA cyclic AMP, Inmunotech, Bio Analytical, Buenos Aires, Argentina). Briefly, kidneys were removed, immersed in cold PBS buffer (in mM: 150 NaCl, 3 KCl, 10 HNa2PO4, 2 H2NaPO4, pH 7.4) and washed with the same buffer, decapsulated, and the renal cortex was separated. The renal cortex was washed with PBS with 1 mM 3-isobutyl-1-methylxanthine (IBMX), an inhibitor of cyclic 3′, 5′-nucleotide phosphodiesterases. Cortex samples were immediately frozen in liquid nitrogen and stored at −80°C until required. Homogenates were obtained by sonication in 2 ml homogenisation buffer (in mM: 20 HEPES, 1 EDTA, 1 IBMX, pH 7.4) per gram of tissue. The concentration of total protein was determined according to Lowry et al. (1951). 125I-cAMP levels were measured in a gamma well counter (LKB Wallac Clinigamma 1272). Results are expressed as femtomoles of cAMP per microgram of protein (fmol/μg) (Castro et al. 1998).
Brush border membrane vesicles
BBMV from renal cortex were isolated from two groups of rats (old and young adults) as described (Fiori et al. 2006). Kidneys were removed, placed in cold HEPES-sucrose-EDTA (HSE) buffer (in mM: 50 sucrose, 10 Tris, 10 HEPES, 0.5 EDTA, pH 7.5) and washed with the same buffer, decapsulated and the renal cortex separated. After differential centrifugation, the vesicle pellet was dissolved in HSE buffer with protease inhibitors (aprotinine 10 µg/ml, leupeptine 10 µg/ml, pepstatin A 10 µg/ml, phenylmethylsulfonyl fluoride 2 mM and dithiothreitol 1 mM). Protein concentration was determined according to Lowry et al. (1951).
Western blot
The abundance in BBMV of the Na+/H+ exchanger, isoform NHE8, was assessed by western blot analysis. BBMV corresponding to 100 μg protein was separated electrophoretically on a 8% sodium dodecylsulfate (SDS) polyacrylamide gel (PAGE), according to Laemmli (1970), transferred to a nitrocellulose membrane and blocked in TBST (in mM: 20 Tris, 150 NaCl and 0.1% Tween 20, pH 7.5) with 5% nonfat milk and 1% BSA (bovine serum albumin). The membrane was incubated with primary antibody (a monoclonal antibody against isoform NHE8, catalogue number MAB3908, Chemicon International; Millipore, Bedford, MA) overnight at 4°C. The membrane was incubated with alkaline phosphatase-labelled secondary antibody (anti-mouse IgG (H+L), AP conjugate, catalogue number S3721; Promega, Madison, WI). Bands were visualised with BCIP/NBT colour development substrate (Promega).
A membrane containing the same samples was incubated with primary antibody against β-actin (antibody against actin Ab5, catalogue number 612656; BD Biosciences, San Diego, CA). The membrane was incubated with a secondary antibody [antibody anti-mouse IgG (H+L) Alkaline Phosphatase Conjugate, catalogue number S372B; Promega].
Band intensities of the NHE8 isoform were determined by densitometric analysis using the ScionImage program (http://www.scioncorp.com) and were normalised to β-actin band intensities on the same blot.
Experiments were repeated four times with protein samples isolated from different groups of young and aged rats.
RNA isolation and RT-PCR
Total RNA was isolated from the renal cortex using TRIzol reagent (Invitrogen, Carslbad, CA). Total RNA (2 μg) was used for first-strand cDNA synthesis using and oligo(dT)12-18 primer (Invitrogen) and Superscript II reverse transcriptase (Invitrogen). Different sets of primers for subunit E from V-H+ATPase, NHE3 and NHE8 isoform from Na+/H+ exchanger were used for PCR analysis. The two primers used for amplifying subunit E from V-H+ATPase were 5′-TGCCTTCAGTTAGAGAGGCCGTGA-3′ (sense), and 5′-TGCCAAGAAGAGTCTGGGACAAGG-3′ (anti-sense), which generated a 221 base pair (bp) H+ATPase product. The two primers for NHE3 were 5′-ACATCCTCTCAGCCATTGAGGACA-3′ (sense) and 5′-TGCCAGATTCTCCATAAGGCAGCTT-3′ (anti-sense), which generated a 662 bp NHE3 product. The two primers for NHE8 were 5′-CCCTCATCCGCCTCGTGGACAT-3′ (sense), and 5′-TGGGAGGCACTGTGGGCTCAGAA-3′ (anti-sense), which yielded a PCR product of 443 bp. Each PCR reaction included 2 μl 10 X PCR buffer minus Mg, 0.4 μl dNTPs (10 mM each), 0.6 μl MgCl2 (50 mM), 1 μl cDNA, 1 μl primers mix (10 μM each), 0.16 μl Taq DNA polymerase (5 U/μl, Invitrogen) and DEPC-treated water. The total reaction volume was 20 µl. Samples were overlaid with mineral oil, denatured at 94°C for 4 min, and followed by 32 cycles consisting of denaturing at 94°C (30 s), annealing at 64°C for NHE3 or 66°C for NHE8, H+ATPase and GADPH (30 s) and extension at 72°C (30 s). PCR was completed by a final extension step of 10 min at 72°C. The amplified products were visualised by electrophoresis on a 1.5% agarose gel, stained with 0.5 µg/ml ethidium bromide, and visualised with UV light. Band intensities were determined by densitometric analysis using the ScionImage program and were normalised to the GADPH product in the same reaction.
Statistics
Results are expressed as means ± SE. Data were analysed using Student’s t test. Differences were considered statistically significant at P < 0.05.
Results
Parathyroid hormone
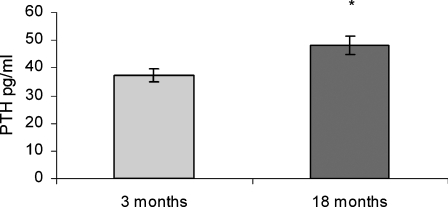
Serum levels of PTH, expressed in pg/ml, were significantly elevated in aged rats (18–22 months) compared with young adults rats (3 months; Fig. 1).
Fig. 1.
Parathyroid hormone (PTH) concentration measured by immunoradiometric assay in the serum of young (3-month- old) and old (18-month-old) rats. Values are means ± SEM. * P < 0.05 vs 3 months old
Determination of cAMP levels in renal cortex
The cAMP concentration was determined in homogenates of renal cortex from young adult and old animals by immunoradiometric assay. The cAMP concentration in the renal cortex of 3-month-old rats was 57.6 ± 8 fmol (µg protein)−1. Aging rats showed no significative increase in cAMP levels [80.6 ± 10.3 fmol (µg protein)−1], relative to young adult animals. The observed values of cAMP are in agreement with results reported in the literature (Steiner et al. 1972).
Western blot experiment
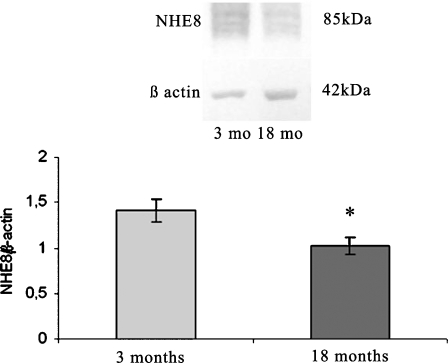
The abundance of protein corresponding to the NHE8 isoform of the Na+/H+ exchanger relative to β-actin was determined in BBMV from PCTs of young and old rats by western blotting using a monoclonal antibody. The purity of the brush border membrane fraction was assessed by measuring the activity of γ-glutamyl transferase (Orlowsky and Meister 1965) and the activity of Na+/K+ ATPase (Pecci et al. 1994) in the homogenate and vesicle pellet. Na+/K+ ATPase activity was not detectable, but the activity of γ-glutamyl transferase increased 10-fold in both groups compared with the original homogenate. The amount of NHE8 isoform was decreased in aging animals compared with control rats (Fig. 2).
Fig. 2.
a Western blot developed using an antibody against the NHE8 isoform of the Na+/H+ exchanger in brush border membrane vesicles (BBMV) of the proximal convoluted tubule (PCT) from young (3-month-old) and old (18-month-old) rats. NHE8 is visualised at 85 kDa while the band at 42 kDa is β-actin. b Densitometric measurements. Data are presented as the ratio of NHE8 to β-actin protein levels. Values are means ± SEM. Four blots were made, each from a pool of tissues taken from three rats
RT-PCR
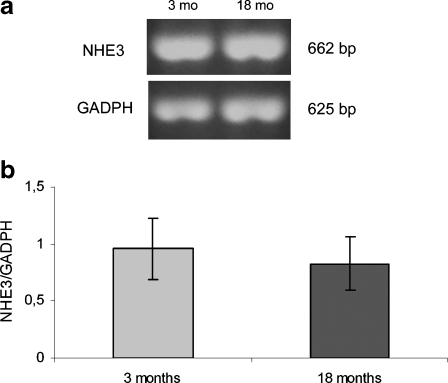
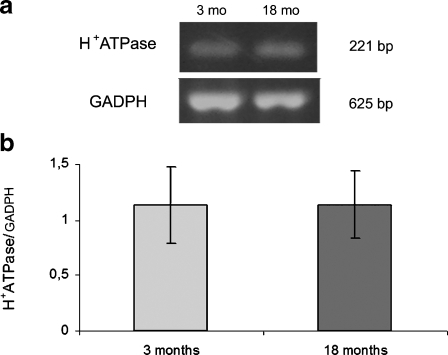
Relative expression of the NHE3 and NHE8 isoforms of the Na+/H+ exchanger and subunit E of V-H+ATPase were assessed by RT-PCR in homogenates of renal cortex from young adult and aged rats. There was no significant difference in mRNA expression of the NHE3 isoform of the Na+/H+ exchanger (Fig. 3) or subunit E of H+ATPase (Fig. 4) in old compared with young adult rats. These results were consistent with the quantification of these proteins by western blot reported previously (Fiori et al. 2006), indicating that mRNA and protein abundance were the same in both experimental groups.
Fig. 3.
Expression of mRNA of the NHE3 isoform of the Na+/H+ exchanger in homogenate of renal cortex in young (3 months) and aging (18 months) rats. a Ethidium bromide-stained 1.5% agarose gel of NHE3 (662 bp) and GADPH (625 bp) PCR products. b Densitometric measurements. The amount of NHE3 mRNA in each sample was divided by the normalisation factor (GADPH) of the sample. Data are presented as means ± SEM
Fig. 4.
Expression of subunit E from V-H+ATPase mRNA in homogenate of renal cortex in young (3 months) and aging (18 months) rats. a Ethidium bromide-stained 2% agarose gel of subunit E from H+ATPase (221 bp) and GADPH (625 bp) PCR products. b Densitometric measurements. The amount of subunit E mRNA in each sample was divided by the normalisation factor (GADPH) of the sample. Data are presented as means ± SEM
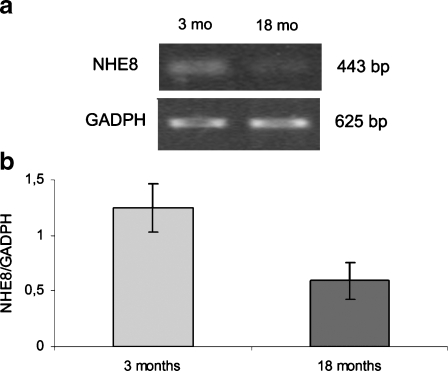
Figure 5 shows the mRNA abundance of the NHE8 isoform in young adult and old rats. Densitometric analysis shows that the mRNA level of NHE8 was decreased in old rats compared with young ones. This result indicates that decreased amounts of NHE8 protein detected by western blot could be a consequence of reduced mRNA abundance in aged animals.
Fig. 5.
Expression of NHE8 isoform mRNA in homogenate of renal cortex in young (3 months) and aging (18 months) rats. a Ethidium bromide-stained 1.5% agarose gel of NHE8 (443 bp) and GADPH (625 bp) PCR products. b Densitometric measurements. The amount of NHE8 mRNA in each sample was divided by the normalisation factor (GADPH) of the sample. Data are presented as means ± SEM. * P< 0.05 vs 3 months old
Discussion
In a previous study, we found that aging is accompanied by a decrease in H+-ion flux in BBMV of PCTs (MacLaughlin et al. 2001). Furthermore, we determined that EIPA- and Bafilomycin-sensitive components were affected, without changes in the amount of the NHE3 isoform or the V-H+ATPase detected by Western blot in BBMV of the PCT of senile rats (Fiori et al. 2006).
In our earlier work, we were unable to detect a fall in NHE3 abundance and, in the present paper, we confirm that there are no also differences in mRNA expression for this isoform or in expression of V-H+ATPase mRNA, supporting our previous observations with western blot analysis (Fiori et al. 2006). There is evidence that the NHE3 isoform is not the sole mediator of proximal tubule apical Na+/H+ exchange. NHE3−/− mice have only a 50% reduction in brush border Na+-dependent H+ secretion compared with NHE3+/+ mice (Schultheis et al. 1998; Wang et al. 1999). It has been pointed out that the NHE2 isoform does not contribute to proximal tubule acidification in mice lacking both NHE2 and NHE3 isoforms (Wang et al. 1999; Choi et al. 2000). Goyal et al. (2003, 2005) described a new member of the family of mammalian NHE exchangers, the NHE8 isoform. This new isoform mediates Na+-dependent acid extrusion across the apical membrane of proximal tubule cells (Zhang et al. 2007). NHE8-mediated transport is retained in NHE3/NHE2 null mice and can be inhibited by EIPA (Choi et al. 2000; Zhang et al. 2007). Thus, if NHE2 does not participate in proximal bicarbonate reabsorption in the proximal convoluted tubule of the rat, it is tempting to speculate that the EIPA-sensitive H+-ion transport described by Choi et al. (2000) and Goyal et al. (2003, 2005) is also affected in old rats.
In the present study, we have demonstrated decreased mRNA and protein abundance of the NHE8 isoform of the Na+/H+ exchanger in senile rats. These results strongly suggest that the proximal tubule acidification diminution observed in old rats could reflect the fall in NHE8 expression in the brush border membrane. A fall in NHE8 expression has been described during growth. Indeed, NHE8 is present in greatest abundance on BBMV during postnatal maturation at a time when NHE3 is virtually undetectable. Interestingly, most NHE8 in adult proximal tubular cells is intracellular and is not on the brush border membrane (Becker et al. 2007). Our findings, along with those of Becker et al., would thus indicate a progressive diminution of NHE8 in the PCT brush border throughout the lifespan of the animal.
The observed decrease in NHE8 abundance in the present work would be consequence of a fall in mRNA abundance and/or redistribution between brush border and cytosol. While details of the control of the intracellular redistribution of NH8 are presently unknown, the decay of NH8 mRNA could be due to a number of well described processes that occur during aging. For instance, in kidney, cellular response to PTH is decreased by an age-related decline in PTH-stimulated adenyl cyclase activity (Armbrecht et al. 1986; Hanai et al. 1989), and this could explain why NH8 mRNA expression decreases with aging despite higher serum PTH.
The Na+/H+ exchanger localised in the apical membrane of the proximal tubule is regulated by many factors including, among others, RAS (Reilly et al. 1995; Saccomani et al. 1990), NO⋅ (Amorena and Castro 1997; Manning et al. 1997; Wang 1997), and PTH (Bank and Aynedjain 1976).
The observed reduction in proximal acidification was due to a reduction in EIPA- and Bafilomycin-sensitive components (Fiori et al. 2006). Nitric oxide modulates Na+/H+ exchange (Amorena and Castro 1997; Díaz-Sylvester et al. 2001; Wang 1997) and aging is associated with a marked decrease in basal as well as stimulated endothelial NO bioactivity (Barton 2005). Thus, we cannot discount the hypothesis that the activity of the Na+/H+ exchange in old rats is affected by changes in the NO pathway.
Parathyroid hormone inhibits the Na+/H+ exchanger (Pollock et al. 1986). This hormone produces this effect through multiple signal pathways, including cAMP-PKA (protein kinase A), phospholipase c-PKC (protein kinase C) and phospholipase A2 (Baum and Hays 1988; Kahn et al. 1985; Pollock et al. 1986). We detected increased levels of PTH in aging rats, and these results coincide with values reported in the literature (Armbrecht et al. 1986; Fox 1991; Kalu and Hardin 1984; Uden et al. 1992). In intact animals, PTH inhibits renal cortical apical membrane Na/H exchange by dual mechanisms: immediate inhibition of the intrinsic transport activity of NHE-3 followed by redistribution of NHE-3 transporter away from the apical membrane to a nonapical pool. Acute regulation of NHE-3 activity by PKA activation has been shown to be associated with NHE-3 phosphorylation (Fan et al. 1999). Although cAMP levels in aged rats did not reach statistical significance, there was a marked tendency to increase. It is possible that the increase in cAMP was marginal because of the well known decrease of sensitivity to PTH in aging (Liang et al. 1990; Hanai et al. 1989). On the other hand, blocking PTH-induced cAMP production does not affect this hormone’s stimulation of bone resorption, which suggests the existence of another intracellular messenger system (Reid et al. 1990).
In conclusion, the diminished capacity of bicarbonate reabsorption in PCT of old rats seems to be consequence of a decreased expression of the NHE8 isoform of the Na+/H+ exchanger, although a decrease in the NHE3 activity due to an increase in PTH cannot be completely discounted.
Acknowledgements
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica, (PICT 05-08305), Universidad Nacional de General San Martín (SB06/045) and Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 0851).
References
- Agus ZS, Puschett JB, Senesky D, Goldberg M (1971) Mode of action of parathyroid hormone and cyclic adenosine 3’-5’-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest 50:617–626. doi:10.1172/JCI106532 [DOI] [PMC free article] [PubMed]
- Amorena C, Castro AF (1997) Control of proximal tubule acidification by the endothelium of the peritubular capillaries. Am J Physiol 272:R691–R694 [DOI] [PubMed]
- Armbrecht HJ, Boltz MA, Forte LR (1986) Effect of age on parathyroid hormone and forskolin stimulated adenylate cyclase and protein kinase activity in the renal cortex. Exp Gerontol 21(6):515–522. doi:10.1016/0531-5565(86)90004-5 [DOI] [PubMed]
- Arnaud CD, Tsao HS, Littledike T (1971) Radioinmmunoassay of human parathyroid hormone in serum. J Clin Invest 50(1):21–34. doi:10.1172/JCI106476 [DOI] [PMC free article] [PubMed]
- Bank N, Aynedjian HS (1976) A micropuntura study of the effect of parathyroid hormone on renal bicarbonate reabsorption. J Clin Invest 58:336–344. doi:10.1172/JCI108477 [DOI] [PMC free article] [PubMed]
- Barton M (2005) Ageing as a determinant of renal and vascular disease: role of endothelial factors. Nephrol Dial Transplant 20:485–490. doi:10.1093/ndt/gfh689 [DOI] [PubMed]
- Baum M, Hays SR (1988) Phorbol myristate acetate and dioctanoylglycerol inhibit transport in rabbit proximal convoluted tubule. Am J Physiol 254:F9–F14 [DOI] [PubMed]
- Baylis C (1994) Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 94(5):1823–1829. doi:10.1172/JCI117531 [DOI] [PMC free article] [PubMed]
- Becker AM, Zhang J, Goyal S, Dwarakanath V, Aronson PS, Moe OW et al (2007) Ontogeny of NHE8 in the rat proximal tubule. Am J Physiol Renal Physiol 293:F255–F261. doi:10.1152/ajprenal.00400.2006 [DOI] [PMC free article] [PubMed]
- Castro AF, Amorena C, Müller A, Ottaviano G, Tellez-Iñon MT, Taquini AC (1998) Extracellular ATP and bradykinin increase cGMP in vascular endothelial cells via activation of PKC. Am J Physiol 275:C113–C119 [DOI] [PubMed]
- Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S et al (2000) Novel amiloridesensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest 105(8):1141–1146. doi:10.1172/JCI9260 [DOI] [PMC free article] [PubMed]
- Cohn DE, Klahr S, Hammerman MR (1983) Metabolic acidosis and parathyroidectomy increase Na+/H+ exchange in brush border vesicles. Am J Physiol 245:F217–F222 [DOI] [PubMed]
- Corman B, Pratz J, Poujeol P (1985) Changes in anatomy, glomerular filtration, and solute excretion in aging rat kidney. Am J Physiol 248:R282–R287 [DOI] [PubMed]
- Davies DF, Shock NW (1950) Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29(5):496–507. doi:10.1172/JCI102286 [DOI] [PMC free article] [PubMed]
- Dennis VW (1976) Influence of bicarbonate on parathyroid hormone-induced changes in fluid absorption by the proximal tubule. Kidney Int 10:373–380. doi:10.1038/ki.1976.123 [DOI] [PubMed]
- Díaz-Sylvester P, Mac Laughlin M, Amorena C (2001) Peritubular fluid viscosity modulates H+ flux in proximal tubules through NO release. Am J Physiol Renal Physiol 280:F239–F243 [DOI] [PubMed]
- Fan L, Wiederkehr MR, Collazo R, Wang H, Crowder LA, Moe OW (1999) Dual mechanisms of regulation of Na/H exchanger NHE-3 by parathyroid hormone in rat kidney. J Biol Chem 274:11289–11295. doi:10.1074/jbc.274.16.11289 [DOI] [PubMed]
- Fiori M, Radrizzani M, Díaz-Sylvester P, Müller A, Corti T, Monserrat A et al (2006) Relative contribution of V-H+ATPase and NA+/H+ exchanger to bicarbonate reabsorption in proximal convoluted tubules of old rats. Aging Cell 5(5):367–372. doi:10.1111/j.1474-9726.2006.00229.x [DOI] [PubMed]
- Fox J (1991) Regulation of parathyroid hormone secretion by plasma calcium in aging rats. Am J Physiol 260:E220–E225 [DOI] [PubMed]
- Goyal S, Vanden Heuvel G, Aronson PS (2003) Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284:F467–F473 [DOI] [PubMed]
- Goyal S, Mentone S, Aronson PS (2005) Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288:F530–F538. doi:10.1152/ajprenal.00229.2004 [DOI] [PubMed]
- Halloran BP, Lonergan ET, Portale AA (1996) Aging and renal responsiveness to parathyroid hormone in healthy men. J Clin Endocrinol Metab 81:2192–2197. doi:10.1210/jc.81.6.2192 [DOI] [PubMed]
- Hanai H, Liang CT, Cheng L, Sacktor B (1989) Desensitization to parathyroid hormone in renal cells from aged rats is associated with alterations in G-protein activity. J Clin Invest 83:268–277. doi:10.1172/JCI113869 [DOI] [PMC free article] [PubMed]
- Hill C, Lateef AM, Engels K, Samsell L, Baylis C (1997) Basal and stimulated nitric oxide in control of kidney function in the aging rat. Am J Physiol 272(6 Pt 2):R1747–R1753 [DOI] [PMC free article] [PubMed]
- Jung FF, Kennefick TM, Ingelfinger JR, Vora JP, Anderson S (1995) Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol 5(8):1573–1580 [DOI] [PubMed]
- Kahn AM, Dolson GM, Hise MK, Bennett SC, Weinman EJ (1985) Parathyroid hormone and dibutyryl cAMP inhibit Na+/H+ exchange in renal brush border. Am J Physiol 248:F212–F218 [DOI] [PubMed]
- Kalu DN, Hardin RR (1984) Age, strain and species differences in circulanting parathyroid hormone. Horm Metab Res 16:654–657 [DOI] [PubMed]
- Kinsella JL, Sacktor B (1987) Renal brush-border Na+/H+ exchange activity in the aging rat. Am J Physiol 252:R681–R686 [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0 [DOI] [PubMed]
- Liang CT, Hanai H, Ishida M, Cheng L, Sacktor B (1990) Regulation of renal sodium calcium exchange by PTH: alteration with age. Environ Health Perspect 84:137–140. doi:10.2307/3430714 [DOI] [PMC free article] [PubMed]
- Lindeman RD (1995) Renal and urinary tract function. In: Masoro EJ (ed) Handbook of physiology, section 11: aging. Oxford University Press, New York, pp 485–503
- Lindeman RD, Tobin J, Shock NW (1985) Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33(4):278–285 [DOI] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagents. J Biol Chem 193:265–275 [PubMed]
- MacLaughlin M, Damasco MC, Igarreta P, Amorena C (2001) In vitro and in vivo evaluation of proximal tubular acidification in aging rats. Am J Physiol 280:R1627–R1631 [DOI] [PubMed]
- Manning RD Jr, Hu L, Reckelhoff JF (1997) Role of nitric oxide in the arterial pressure and renal adaptations to long-term changes in sodium intake. Am J Physiol 272:R1162–R1169 [DOI] [PubMed]
- McKinney TD, Myers P (1980) Bicarbonate transport by proximal tubules: effect of parathyroid hormone and dibutyryl cyclic AMP. Am J Physiol 238:F166–F174 [DOI] [PubMed]
- Orlowsky M, Meister A (1965) Isolation of γ-glutamyl transpeptidase from hog kidney. J Biol Chem 240:338–347 [PubMed]
- Papper S (1973) The effects of age in reducing renal function. Geriatrics 28(5):83–87 [PubMed]
- Pecci A, Cozza EN, Devlin M, Gomez-Sanchez CE, Gomez-Sanchez EP (1994) Endothelin-1 stimulation of aldosterone and zona glomerulosa ouabain-sensitive sodium/potassium-ATPase. J Steroid Biochem Mol Biol 50(1–2):49–53. doi:10.1016/0960-0760(94)90171-6 [DOI] [PubMed]
- Pollock AS, Warnock DG, Strewler GJ (1986) Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol 250:F217–F225 [DOI] [PubMed]
- Reid IR, Lowe C, Cornish J, Gray DH, Skinner SJ (1990) Adenylate cyclase blockers dissociate PTH-stimulated bone resorption from cAMP production. Am J Physiol 258:E708–E714 [DOI] [PubMed]
- Reilly AM, Harris PJ, Williams DA (1995) Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am J Physiol 269:F374–F380 [DOI] [PubMed]
- Saccomani G, Mitchell KD, Navar LG (1990) Angiotensin II stimulation of Na+-H+ exchange in proximal tubule cells. Am J Physiol 258:F1188–F1195 [DOI] [PubMed]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR et al (1998) Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19(3):282–285. doi:10.1038/969 [DOI] [PubMed]
- Steiner AL, Pagliara AS, Chase LR, Kipnis DM (1972) Radioimmunoassay for cyclic nucleotides. II Adenosine 3′-5′-monophosphate and guanosine 3’-5’-monophosphate in mammalian tissues and body fluids. J Biol Chem 247(4):1114–1120 [PubMed]
- Uden P, Halloran B, Daly R, Duh QY, Clark O (1992) Set-Point for Parathyroid Hormone release increases with postmaturational aging in the rat. Endocrinology 131(5):2251–2256. doi:10.1210/en.131.5.2251 [DOI] [PubMed]
- Wang T (1997) Nitric oxide regulates HCO3− and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol 272:F242–F248 [DOI] [PubMed]
- Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G et al (1999) Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol 277:F298–F302 [DOI] [PubMed]
- Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW (2007) Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol 293(3):F761–F766. doi:10.1152/ajprenal.00117.2007 [DOI] [PMC free article] [PubMed]