Abstract
Increased serum insulin levels and reduced peripheral insulin activities seen in insulin resistance syndrome are associated with age-dependent cognitive impairment and Sporadic Alzheimer’s Disease (SAD), suggesting a disturbance in the insulin signalling system in the brain and possibly being one of the causes of dementia. Therefore, the streptozotocin (STZ)-induced animal may be an appropriate model for the investigation of SAD and related dementia. This study was designed to investigate the beneficial effect of Curcumin (CUR), a neuroprotective agent, on intracerebroventricular (ICV) STZ-induced cognitive impairment in rats. For this purpose, adult male Wistar rats were bilaterally ICV injected with STZ (3 mg/kg). An artificial cerebrospinal fluid (aCSF) was given to the control group (SHAM) instead of STZ on days 1 and 3. Learning and memory performance were assessed using the “passive avoidance task” and the “Morris water maze test”. After confirmation of acquisition impairment with these tests, the STZ group was divided into two subgroups: STZ + vehicle (Vh) and STZ + CUR. The rats in the SHAM and STZ + Vh groups were administered intraperitoneally with 0.5 ml Vh and the rats in the STZ + CUR group were treated intraperitoneally with CUR (300 mg kg−1 day−1 in Vh) for 10 days starting from the 25th day after STZ injection. The Morris water maze test was reapplied on the 35th day after STZ injection and all of the rats were sacrificed on day 36 for quantitation of IGF-1 and for histopathological evaluation. Rats in the STZ + CUR group were found to have a higher performance in cognitive tests than rats in the STZ + Vh group (P < 0.01). In parallel with the cognitive tests, IGF-1 levels were decreased in all of the STZ-injected groups (1.78 ± 0.34) compared to the SHAM group (3.46 ± 0.41). In contrast, CUR treatment significantly increased IGF-1 levels (P < 0.001). The degree of neuronal loss decreased after CUR treatment compared to the SHAM group (P < 0.02). These results clearly indicate that CUR treatment is effective in reducing the cognitive impairment caused by STZ in rats, and may be a potential therapeutic agent for altering neurodegeneration in SAD.
Keywords: Alzheimer’s disease, Curcumin, Experimental, IGF-1, Streptozotocin
Introduction
Alzheimer’s disease (AD), the most common form of dementia in the elderly, is characterised by extensive atrophy of the brain caused by neuronal loss (Gasparini et al. 2002). The majority of AD patients are late onset (sporadic) and the disease usually develops after 65 years of age. Sporadic AD (SAD) is associated with peripheral insulin abnormalities, which might influence cerebral glucose metabolism in the brain (Lester-Coll et al. 2006). De Santi et al. demonstrated the hypometabolism of hippocampal glucose in patients with AD compared to control individuals (De Santi et al. 2001), and hypothesised that reduced glucose utilisation and energy metabolism may be two of the main causes of the impaired cognition observed in AD (Rivera et al. 2005).
All these findings imply that neuropathies, peripheral and central insulin signalling abnormalities, cognitive impairments, metabolic syndrome and cholesterol abnormalities link AD and diabetes mellitus (Hoyer et al. 2004). Like insulin, the activities of other growth factors, such as insulin-like growth factor-1 (IGF-1), transforming growth factor (TGF) and some other neurotrophic factors are altered in both AD and type 2 diabetes mellitus. It has been reported that IGF-1 promotes neuronal cytoskeleton alterations via phosphorylation of some of its target proteins. Tau protein is one such protein and phosphorylation is required for its normal physiological activity. Intraneuronal neurofibrillary tangles (NFT), a hallmark of AD, are composed mainly of microtubule-associated tau protein present as paired helical filaments containing an abnormally hyperphosphorylated form of tau protein. Tau protein plays a role in the stabilisation of microtubules, which serve as tracks for the movement of mitochondria and various other organelles (Li and Hölscher 2007).
In vitro experiments demonstrated that tau phosphorylation is normally regulated by insulin and IGF-1 (Pei et al. 1998; Hong and Lee 1997). In addition, impaired insulin or IGF-1 signalling can result in the hyper-phosphorylation of tau, which can cause cell death mediated by apoptosis, mitochondrial dysfunction or necrosis (Schubert et al. 2003, 2004), and promote oxidative stress, which contributes to the neurodegeneration cascade and leads to dementia-associated behavioural and cognitive deficits (de la Monte and Wands 2005). Experimental studies have shown that deterioration of cerebral insulin-IGF action by intracerebroventricular (ICV) streptozotocin (STZ), a diabetogenic drug, leads to a deficit in energy metabolism and progressive cognitive impairment in rats (Lannert and Hoyer 1998). In addition, it was demonstrated that both the ICV injection of insulin in animals and the administration of insulin to healthy volunteers improves memory performance, indicating a positive effect on cognition (Park et al. 2000; Kern et al. 2001).
Studies in animal models have suggested that curcumin (CUR) may be beneficial in neurodegenerative conditions such as AD (Calabrese et al. 2003; Lim et al. 2001; Yang et al. 2005) and focal cerebral ischemia (Thiyagarajan and Sharma 2004). CUR can protect hippocampal neurons against excitotoxic and traumatic injury (Sumanont et al. 2006; Wu et al. 2006). Recent findings suggest the possibility that CUR can reduce oxidative damage and cognitive deficits associated with aging (Ruby et al. 1995). CUR (diferuloylmethane), a major component of the spice turmeric, is not toxic to humans (Sharma et al. 2005). In addition to the direct free radical scavenging properties of high concentrations (300 mg/kg) of CUR (Thiyagarajan and Sharma 2004), lower concentrations can activate or inhibit one or more signal transduction pathways in cells (Duvoix et al. 2005; Suh et al. 2007).
CUR modulates the expression of various molecular targets, such as transcription factors, enzymes, cytokines, cell cycle proteins, receptors and adhesion molecules (Shishodia et al. 2005). CUR may antagonise the deficit of glucose energy metabolism or oxidative stress related to cognitive impairment, as seen in SAD.
A useful model of SAD is the ICV STZ animal model. The compound STZ is known to inhibit insulin receptor function, create persistent oxidative stress, mitochondrial dysfunction, impaired energy metabolism and activation of proapoptotic signalling pathways. All these effects of STZ are sufficient to mimic AD-type neurodegeneration (Hong and Lee 1997; Ganguli et al. 2000; Salkovic-Petrisic and Hoyer 2007). Therefore, this study was undertaken to evaluate the effects of CUR on IGF-1, memory deficit and histopathological changes in the STZ-induced memory deficit model of SAD.
Materials and methods
Animals and laboratory conditions
Twenty four adult (12-month old) male Wistar rats (225–275 g) were placed in a quiet, temperature- and humidity-controlled room (22 ± 3°C and 62 ± 7% relative humidity) in which 12-h cycles (12 h light -12 h dark) were maintained (lights on at 7:00 a.m.). The animals were fed ad libitum with a standard dry rat diet and tap water. The experiments performed in this study were carried out according to the rules in the Guide for the Care and Use of Laboratory Animals adopted by the National Institutes of Health (USA) and the Declaration of Helsinki (Ethics Committee of the Gulhane School of Medicine, No. 65/2006).
Intracerebroventricular injection of streptozotocin
Animals were anaesthetised with a mixture of xylazine and ketamine hydrochloride (3 and 75 mg/kg, respectively, IP). The scalp was shaved, cleaned and cut to expose the skull. The head was positioned in a stereotaxic frame and a midline sagittal incision was made in the scalp. Burr holes were drilled in the skull on both sides over the lateral ventricles by using the following coordinates: 0.8 mm posterior to bregma; 1.5 mm lateral to sagittal suture and 3.5 mm beneath the surface of the brain (Sharma and Gupta 2002). Coordinates for placement of cannulae were determined by using the atlas of Paxinos and Watson (1998). The guide cannulae were secured in place using cranioplastic cement and three 2.4 mm stainless steel screws anchored to the skull. Dummy cannulae were inserted to seal the guide cannulae. Animals were allowed 72 h to recover. 33-gauge microinjection cannulae were used to administer bilaterally, right and left. The cannulae were attached to polyethylene tubing (PE-20; Plastics One, Roanoke, VA), connected to a 100 ml Hamilton syringe. The Hamilton syringes were situated on an infusion pump (RAZEL, St. Albans, VT) and the pump was programmed to deliver. The injection cannulae remained in place for an additional minute following infusion to promote diffusion.
The STZ (Sigma, St Louis, MO) was dissolved in artificial cerebrospinal fluid (aCSF) (3 mM KCl, 140 mM NaCl, 1.2 mM CaCl2, 1 mM MgCl2, 0.3 mM NaH2PO4, 1 mM Na2HPO4, 3 mM d-glucose) in a 25 mg/ml solution. This solution was prepared immediately prior to injection.
At beginning of the study, all of the rats were divided into two groups: SHAM (n=8) and STZ (n=15). Artificial CSF was injected bilaterally ICV (a 20 μl injection was given to each rat on each side and this procedure was repeated on the 3rd day) in the SHAM group. The STZ groups were given a bilateral ICV injection of STZ (3 mg/kg) in a total volume of 20 μl. A 20 μl injection was given to each rat on each side and this procedure was repeated on the 3rd day. Following the last ICV STZ injection, the cannulas were left during the anaesthetised period. Subsequently, the burr holes in the scull were closed with bone wax and the skin incision was sutured. Following surgery, the rats operated on were placed in individual Plexiglas cages and were fed as indicated above.
Study design
At the end of 21 days, the animals were tested in a step-through passive avoidance box and Morris water maze to evaluate cognitive decline. After demonstration of acquisition impairment by STZ, the animals were randomly divided into vehicle (n = 7) and CUR (300 mg/kg, IP, n = 8) treated groups. The CUR treatment was applied for 10 days. CUR (Sigma) was formulated in a vehicle (Vh) using a mixture of 1% sodium carboxy methyl cellulose and 1% Tween-80 (Thiyagarajan and Sharma 2004). Cognitive function was evaluated by the water maze retention test to evaluate the effects of CUR on cognitive impairment. After the reevaluation of the memory test described above, at the end of the study all the rats were sacrificed to obtain blood samples and the entire brain.
Learning and memory tests
One trial passive avoidance
STZ-induced memory deficits were evaluated by a step-through passive avoidance box 21 days after the second STZ injection. This apparatus consists of two-grid floor compartments separated by a guillotine door (8 × 4.5 cm) that can be opened. The first compartment (40 × 30 × 26 cm) was illuminated with a 60 W lamp fixed to the lid of the box. The dark compartment (20 × 30 × 26 cm) was made of black Plexiglas. The floor consisted of a stainless-steel grid connected to a shock scrambler. Each rat was placed in the lighted chamber with a 60 s habituation period. When the guillotine door opened, the initial latency to enter the dark chamber was recorded for 60 s immediately after the rat entered the dark chamber from the light chamber. The guillotine door was closed and an electric foot shock of 2 mA, as an unconditioned stimulus, was delivered to the floor grids for 2 s. After 24 h, the rat was placed in the light compartment again and the retention latency time was measured in the same way, but a foot shock was not delivered and the latency time was recorded to a maximum of 300 s (Lester-Coll et al. 2006).
Morris water maze
The water maze is used to monitor spatial learning and memory in rodents (Morris 1984). The water maze was a circular pool (150 cm in diameter, 60 cm high) constructed of stainless steel. The water was maintained at a temperature of 23°C (±1°C) with an automatic heater. Yellow non-toxic watercolour paint was added to make the water opaque. The tank was divided into four quadrants (NE, NW, SE and SW) with two imaginary perpendicular lines crossing in the centre of the tank. During the water maze test, a movable escape platform made of transparent Plexiglas (10 cm in diameter) was located below the water surface in the centre of one of the quadrants and a pilot experiment showed that it was invisible to the rats. The top of the platform was 1 cm below the surface of the water in its raised position, so that the rat could easily climb onto it to escape from the water. The top of the platform was 30 cm below the water surface in its lowered position and thus not accessible for the rats to escape. The apparatus was located in a room with numerous extra-maze cues that remained constant throughout the experiment. The time required for reaching the platform (escape latency), distance swam to the platform and swimming speed as well as the time and distance spent in each quadrant were recorded by a video tracking system (EthoVision Image Analysis 3.1, Noldus Information Technology, Wageningen, The Netherlands).
At 19 days after the last injection, the pre-training period was started for 2 successive days. On the 21st day, the rats were placed for 60 s on the escape platform, which was at the centre of one of the quadrants in the pool without water. On the 22nd day, the rats were again placed on the same platform under the same conditions, except that the pool was filled with water. When the rat climbed off the platform, it was guided back onto the platform. Training was started the following day. During this period, the escape platform was located in the centre of the southwest quadrant and all of the rats were given a daily session of four trials for 4 consecutive days. The rat was placed in the water facing the pool wall at one of four randomly determined starting locations (north, west, east or south poles) for each trial. When the rat climbed onto the platform, it was allowed to stay on it for 30 s. If the rat did not find the platform within 60 s, then it was guided onto it and allowed to remain for 30 s. Next, it was returned to its heated cage for a 240 s inter-trial interval. After the final trial, the rats were dried with a towel and placed in a holding cage under a heating lamp before returning to their home cages. All of the rats were given a daily session of four trials for 4 consecutive days. The acquisition trials were performed on days 21, 22, 23 and 24 after STZ injections.
Ten days after the completion of the acquisition trials, the animals received a single 60 s probe trial for retention memory. The animals had one trial with the platform removed from the pool for testing the memory function on the retention day (day 34 of the STZ injection or day 10 of the CUR treatment). At this time, two parameters were registered: the time spent in the target area, and latency of the first crossing through the place of the platform (Szabados et al. 2004).
Histopathological examination
The entire brain was used for histopathological examination. Rat brains were sectioned coronally and fixed in 10% formaldehyde. After a 24-h fixation and routine tissue processing, they were embedded in paraffin. Sections with a thickness of 4 μm were prepared on a microtome. The hippocampus was evaluated at a coronal level 3.8–4.0 mm caudal to the bregma, and the total pyramidal neuron number in the hippocampus was estimated using the optical fractionator method (West et al. 1991) by two pathologists blinded to the experiment. After the initial examination of Haematoxylon and eosin-stained sections, representative blocks were selected for immunohistochemical staining.
Immunohistochemical staining procedure
Sections of 4 μm from each formalin-fixed paraffin-embedded tissue were stained using a primary polyclonal rabbit antibody against tau (1:100; Dako, Carpinteria, CA) and ubiquitin (1/150; Dako). Immunohistochemical staining was performed with the labelled streptavidin-biotin method using the UltraVision Large Volume Detection System (Cat # TP-060-HL, LabVision, Fremont, CA) kit using autostainer (Neomarkers, Fremont, CA) according to the procedure described briefly below. Sections were deparaffinised and rehydrated in graded ethanol. After rinsing in distilled water, sections were microwaved for 5 min at 600 W in an 0.01 mol/l sodium citrate buffer (pH 6.0). This step was repeated three times. The slides were immersed in 3% H2O2 in distilled water for 5 min and then in blocking solution for 30 min to block endogenous peroxidase activity and unspecific binding sites, respectively. Sections were then rinsed in phosphate-buffered saline (PBS) and incubated at room temperature with the primary antibody for 60 min, followed by a rinse in PBS. Negative controls omitting the primary antibody were performed in parallel. Subsequently, the sections were treated with biotinylated secondary anti-rabbit antibodies in a 1:200 dilution and the antibody-binding sites were finally visualised with an avidinbiotin peroxidase complex solution, using diaminobenzidine as a chromogen.
Evaluation of tau and ubiquitin immunostaining
Immunohistochemically stained slides were reviewed by two pathologists. The number of areas analysed in the brains varied from 10 to 20 high-power areas per sample. The percentage of stained cells and staining intensity were assessed semi-quantitatively. The immunohistochemical staining intensity was graded using a four-stage grading scale: negative (−), mild (1+), moderate (2+) or severe (3+).
IGF-1 content in serum
At the end of the experiment, the rats were anaesthetised with a 3% isoflurane inhalation. Blood was obtained from the inferior vena cava and the serum was isolated and stored at −80°C. The IGF-1 in the serum was determined with an RIA-assay (Diagnostic Systems Laboratories, Webster, TX).
Statistical data analysis
The data are presented as a means ± standard error of the mean. The group differences in the escape latency and distance in the Morris water maze task were analysed using two-way and one-way analysis of variance (ANOVA) with repeated measures. The one-way ANOVA followed by Tukey’s post hoc test multiple group comparison were used to analyse the group differences of the data collected during successive training days, retention trials and biochemical assay. Statistical significance (P < 0.05) was evaluated by using the Student’s t-test or the one-way ANOVA followed by Tukey’s multiple comparison tests where applicable. In the histopathological examination, differences among the groups were tested for significance with the Mann-Whitney U test. Differences and correlations were considered significant at P < 0.05.
Results
Cognitive tasks
The putative STZ-induced cognitive impairments in rats, which resembles sporadic dementia of the Alzheimer’s type, were assessed 21 days after the last STZ injection. Firstly, cognitive performance was tested with the step-through passive avoidance and Morris water maze tasks.
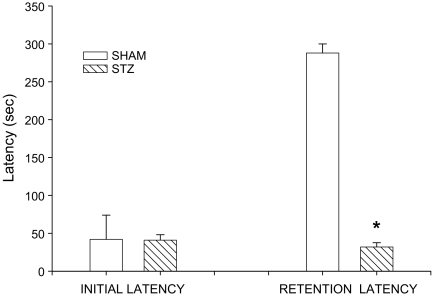
The results for the passive avoidance task are shown in Fig. 1. The mean acquisition or initial latency did not differ significantly between the control and STZ-injected groups. As expected, 24 h after these initial latencies, retention latency was increased only in the SHAM group, not in the STZ group. The STZ treatment significantly impaired retention latency compared to the SHAM group (aCSF-treated controls) (t = 2.747; P < 0.0018). The mean retention latencies were 300 ± 1.01 and 32 ± 5.53 s, respectively.
Fig. 1.
Effect of bilateral intracerebroventricular (ICV) streptozotocin (STZ) injections on the passive avoidance paradigm in rats. The task was performed with the acquisition trial and retention trial. Values are expressed as mean ± SEM. * Significance (P < 0.01) with Student’s t -test
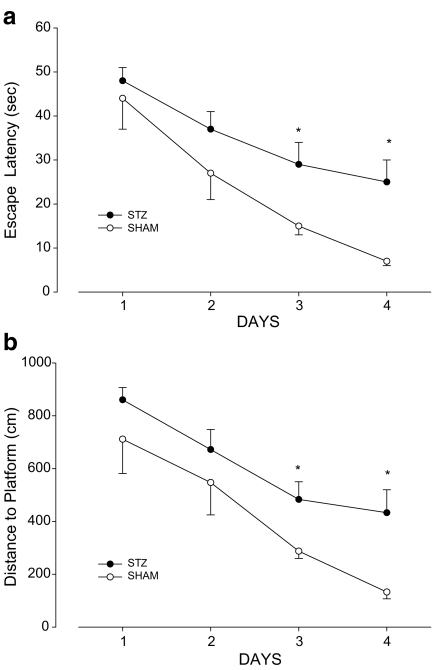
Figure 2 shows the water maze acquisition performance in the control and STZ-injected groups. The acquisition of spatial learning was significantly impaired in the STZ-treated groups compared to the controls (Fig. 2a). Escape latencies decreased during the 4 testing days in the controls [F (3.28)=11.934;P = 0.001] and the STZ group [F (3.56)=5.393, P = 0.048]. The STZ-treated group had significantly longer escape latencies than the control group on days 3 (t = 2.467; P = 0.02) and 4 (t = 2.846; P = 0.010). A two-way repeated ANOVA applied to the escape latencies found a significant group [F (1.21) = 9.373; P = 0.001] and day [F (3.88)=20,364, P = 0.001] effect. The interaction of group and day was insignificant.
Fig. 2.
Comparison of the acquisition performance according to time (a) and distance (b) on the Morris water maze task between the two groups of rats (STZ-induced and SHAM). The task was performed with four trials per day for 4 days for the acquisition test. The sham-operated control group (n = 8) and the STZ group (n = 15) were not given any drugs for 3 weeks after induction of sporadic Alzheimer-type dementia. * Significance (P < 0.01) with Student’s t test. Error bars SEM
As seen in Fig. 2b, similar results were found for the distance to platform [effect of day in control group F (3.28)=17.43; P = 0.001] and in the STZ-treated group [F (3.56)=10.671; P = 0.01]. The STZ group also had a significantly longer distance to the platform than the controls on days 3 (t = 2.640; P = 0.019) and 4 (t = 2.807; P = 0.005). The STZ-treated rats took a more circuitous route to locate the platform.
However, the interaction of group and day was also insignificant in the distance task. The STZ group also had a significantly longer distance to the platform than the controls on days 3 (t = 2.640; P = 0.019) and 4 (t = 2.807; P = 0.005). The STZ-treated rats took a more circuitous route to locate the platform.
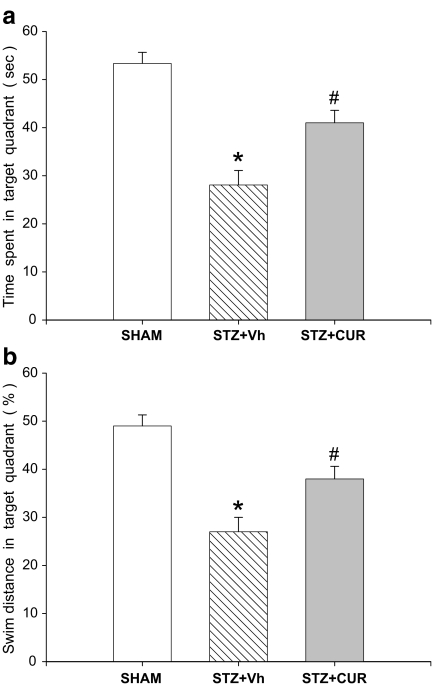
In the probe trial for retention memory, the one-way ANOVA showed a significant difference among the groups [F (2.21) = 12.198; P < 0.01] and Tukey’s post hoc tests showed that the STZ + Vh group spent significantly less time in the target quadrant than the other group. The STZ + CUR group spent significantly more time searching in the target quadrant than the STZ + Vh group (P < 0.05) (Fig. 3a). In addition, like the time spent in the target quadrant, the swimming distance in the target quadrant also showed a significant difference among the groups (Fig. 3b). The results suggest that ICV STZ produces deficits in spatial learning and retention memory in rats. It is possible that the observed deficits in performance could be due to general behavioural or sensory motor impairments and not to deficits in memory. However, the differences among the groups were insignificant when the swimming velocity performances of the groups were compared.
Fig. 3.
Effects on memory retrieval or on retention day in the Morris water maze test of curcumin (CUR) in a 300 mg/kg per day chronic IP injection. Data shows the percentages of time spent (a) and distance (b) in the target area. This test was performed 10 days after the acquisition days. STZ-induced animals showed weaker memory performance in all parameters tested compared to the SHAM and CUR-treated STZ-induced groups (n = 7–8). *P < 0.01 for SHAM vs STZ + vehicle (Vh), # P < 0.05 for STZ + CUR vs STZ + Vh
Histopathological analysis
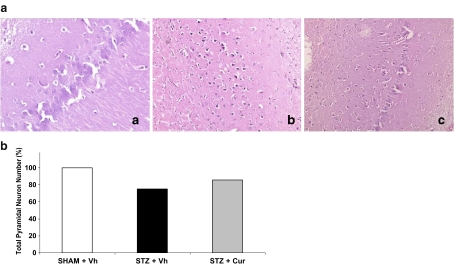
Histopathological examination revealed significant neuronal loss in the STZ-treated group compared to the control group (P < 0.001), and the degree of neuronal loss decreased after CUR treatment compared to the control group (P < 0.02) (Fig. 4). However, staining of tau and ubiquitin in the hippocampus increased slightly after STZ treatment and the decreasing number of tau and ubiquitin staining cells were statistically insignificant after CUR treatment.
Fig. 4.
a Histopathological examination showed a normal hippocampus (a H&E ×200) in the control group, a moderate to prominent neuronal loss in the STZ group (b, H&E ×100) and less neuronal loss in the CUR-treated group (c, H&E ×100) compared to the STZ group. b The percentage of total pyramidal neuron number in the hippocampus in all groups. The degree of neuronal loss decreased after CUR treatment compared to the STZ group in histopathological examination (P < 0.02)
Serum IGF-1 levels
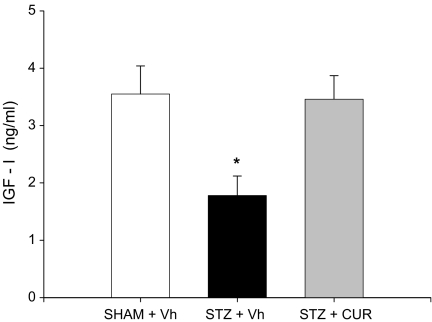
Parallel to the behavioural test performances of all of the rats, IGF-1 levels also decreased (1.78 ± 0.34) compared to the control group after the ICV STZ injection. However, the level of IGF-1 increased (3.46 ± 0.41) following CUR treatment (P < 0.001) as seen in Fig. 5. The difference in IGF-1 levels of rats in the control and CUR-treated groups were insignificant (3.55 ± 0.49 and 3.46 ± 0.41, respectively; P > 0.05).
Fig. 5.
Effects of CUR and bilateral ICV STZ injections on rat insulin-like growth factor-1 (IGF-1) levels. This test was performed 10 days after the acquisition days. The STZ-induced animals showed decreased IGF-1 levels compared to the SHAM and CUR-treated STZ-induced groups (n = 7–8). Data are presented as means ± SEM. * P < 0.01 vs control (SHAM) and STZ-induced group, ANOVA followed by Tukey’s test
Discussion
Several studies have suggested that there is a close link between the pathological and clinical characteristics of AD and type 2 diabetes mellitus (Li and Hölscher 2007; Salkovic-Petrisic and Hoyer 2007). Research has indicated that STZ, a diabetic compound, can disturb insulin signalling and produce oxidative stress. STZ is thought to be responsible for the pathogenesis of the neurodegenerative changes observed in this in vivo model, even in the absence of Aβ aggregation (Grunblatt et al. 2007). The neurodegeneration of AD is associated with oxidative stress, mitochondrial dysfunction, impaired energy metabolism, progressive Aβ agglutination and formation of NFT (Li and Hölscher 2007). Aggregation of misfolded proteins that contain Aβ has been shown to initiate oxidative stress and inflammation and also to be responsible for the neurodegenerative changes and clinical properties of AD (Klein et al. 2001). The ICV STZ model appears to produce cognitive deficits similar to those seen in SAD-type dementia (Hong and Lee 1997; Ganguli et al. 2000; Salkovic-Petrisic and Hoyer 2007). The ICV STZ model mimics AD because of the cognitive impairment created by STZ. However, Aβ aggregation is rarely present in this in vivo SAD model. On the other hand, SAD is a late onset diseases and usually develops after the age of 65. Therefore, adult animals were used intentionally to approach a model that more resembles late onset type AD and that is representative of AD dementia. This method was utilised in the present study, in which middle-aged rodents developed a definitive impairment in learning memory and cognition compared to control rats, as confirmed by the one trial passive- avoidance and Morris water maze tasks. The deficits in behaviour induced by ICV STZ were accompanied by neuronal loss in the hippocampus as well as a significantly decreased IGF-1 concentration in serum.
Lannert and Hoyer (1998) showed that ICV STZ did not cause a systemic diabetes mellitus since STZ was applied at a considerable lower dosage (1%) than that used in systemic application. Consequently, it is thought that the decrease in IGF-1 levels seen here are the result of a central rather than a peripheral effect of STZ. IGF-1 is neurotropic, since it can support neuronal growth, survival and differentiation in the absence of other growth factors. IGF has been shown to promote neurite outgrowth, migration, protein synthesis, neuronal cytoskeletal protein expression and nascent synapse formation (de la Monte and Wands 2005).
Protection of brain cells from degeneration is a useful preventive strategy for AD. Medication suggested for the prevention of AD usually includes compounds that can prevent oxidative damage to neurons. It is known that these antioxidants can increase the resistance of cells to degeneration (Ancelin et al. 2007; Calabrese et al. 2003). Recently, several different in vivo and in vitro studies in animal models revealed that some natural antioxidants, such as CUR, resveratrol and l-acetyl carnitine can be alternatively used as therapeutic agents for AD (Schubert et al. 2003; Kelloff et al. 2000; Olgun et al. 2004). An increasing number of studies have proved that antioxidants, such as acetyl carnitine and CUR, reduce neuronal death occurring in AD. CUR is extensively used in India (Lodha and Bagga 2000) and the number of AD patients between 70 and 79 years of age living in India is approximately four times less than the population of people of the same age living in the United States (Ganguli et al. 2000). In fact, accumulating cell culture and animal model data show that dietary CUR is a strong candidate for use in the prevention or treatment of major disabling age-related neurodegenerative diseases (Cole et al. 2007). These data led us to investigate the neuroprotective activity of CUR, and a demonstration that CUR treatment led to a reduction in Aβ plaque deposition, in both in vivo and in vitro studies (Yang et al. 2005; Lim et al. 2001), encouraged us to test CUR in STZ-induced cognitive impairment and neurodegeneration. Recently, it was also demonstrated that the dienone bridge present in CUR is necessary for reducing plaque deposition and protein oxidation in the Alzheimer’s model (Begum et al. 2008). Frautschy et al. (2001) also demonstrated the beneficial effect of CUR treatment in an ICV Aβ peptide injected experimental AD model. Frautschy used CUR as a protective agent in her study, in contrast to our study, which applied CUR for the treatment of neurodegeneration.
In the present study, CUR treatment led to improved memory and cognitive performance in the presence of STZ-induced neuronal loss in STZ-injected rats as observed in the literature. In contrast to the current literature, the present data show that IGF-1 levels decrease in parallel with impaired cognition, and that CUR treatment improved both IGF-1 levels and impaired cognition in this model. Besides the anti-inflammatory (Lim et al. 2001) and antioxidative (Arafa 2005) properties of CUR, IGF levels in the ICV STZ model were also improved in this study. Improvements in IGF-1 levels with CUR treatment may be a sign of the initiation of neurogenesis. We can speculate that, in addition to neuroprotection, neurogenesis is the main effect of CUR.
Lim et al. (2001) showed that CUR treatment for 6 months significantly lowered the level of oxidized proteins and interleukin-1β (a proinflammatory cytokine), which were elevated in the brains of a transgenic APPSw mouse model (Tg2576). A significant decrease (43–50%) in plaque formation, and in the concentration of insoluble and soluble Aβ, was observed in the same study. In addition, CUR also binds to redox-active metals, iron and copper. This means that the binding of these redox-active metals by CUR may prevent the oxidative damage and inflammation caused by metal-induced NF-κB activation (Arafa 2005).
Thiyagarajan and Sharma (2004) reported that the neuroprotective dose of CUR is 300 mg/kg, and this dose of CUR has more neuroprotective effect and antioxidant activity than doses of 100 mg/kg and less; therefore, we used the high dose of CUR, i.e. 300 mg/kg, in our study. The route of CUR administration is also very important, because of the standardisation of daily dosage. In the present study, CUR was administered intraperitoneally with a constant dose. However, in Frautschy’s study, CUR was given orally in food (Frautschy et al. 2001). Therefore, these results are much more convincing.
In addition, recent preliminary data published by Baum et al. (2008) suggested that CUR treatment can be safely used in patients with AD.
In conclusion, the results obtained from our study demonstrate the effectiveness of CUR in treating cognitive impairment and neurodegeneration caused by ICV STZ injections in rats, and its potential in the treatment of neurodegenerative diseases such as SAD. Primarily, the results also reveal that CUR can be effective in ICV STZ-induced neurodegeneration by increasing IGF-1 levels, which may indicate neurogenesis. Further randomised controlled human studies demonstrating the efficacy of CUR therapy and explaining the potential mechanisms of the insulin signalling pathway interactions should be undertaken.
References
- Ancelin ML, Christen Y, Ritchie K (2007) Is antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidence. Dement Geriatr Cogn Disord 24:1–19 doi:10.1159/000102567 [DOI] [PubMed]
- Arafa HM (2005) Curcumin attenuates diet-induced hypercholesterolemia in rats. Med Sci Monit 11(1):BR228–BR234 [PubMed]
- Baum L, Lam CW, Cheung SK et al (2008) Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28(1):110–113 [DOI] [PubMed]
- Begum AN, Jones MR, Lim GP et al (2008) Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326:196–208 doi:10.1124/jpet.108.137455 [DOI] [PMC free article] [PubMed]
- Calabrese V, Butterfield DA, Stella AM (2003) Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer’s disease. Ital J Biochem 52(4):177–181 [PubMed]
- Cole GM, Teter B, Frautschy SA (2007) Neuroprotective effects of Curcumin. Adv Exp Med Biol 595:197–212 doi:10.1007/978-0-387-46401-5_8 [DOI] [PMC free article] [PubMed]
- de la Monte SM, Wands JR (2005) Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis 7(1):45–61 [DOI] [PubMed]
- De Santi S, de Leon MJ, Rusinek H et al (2001) Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22(4):529–539 doi:10.1016/S0197-4580(01)00230-5 [DOI] [PubMed]
- Duvoix A, Blasius R, Delhalle S et al (2005) Chemopreventive and therapeutic effects of curcumin. Cancer Lett 223(2):181–190 doi:10.1016/j.canlet.2004.09.041 [DOI] [PubMed]
- Frautschy SA, Hu W, Kim P et al (2001) Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging 22(6):993–1005 doi:10.1016/S0197-4580(01)00300-1 [DOI] [PubMed]
- Ganguli M, Chandra V, Kamboh MI et al (2000) Apolipoprotein E polymorphism and Alzheimer disease: the Indo-US Cross-National Dementia Study. Arch Neurol 57(6):824–830 doi:10.1001/archneur.57.6.824 [DOI] [PubMed]
- Gasparini L, Netzer WJ, Greengard P et al (2002) Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci 23(6):288–293 doi:10.1016/S0165-6147(02)02037-0 [DOI] [PubMed]
- Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S (2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101(3):757–770 doi:10.1111/j.1471-4159.2006.04368.x [DOI] [PubMed]
- Hong M, Lee VM (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 272(31):19547–19553 doi:10.1074/jbc.272.31.19547 [DOI] [PubMed]
- Hoyer S (2004) Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol 541:135–152 [DOI] [PubMed]
- Kelloff GJ, Crowell JA, Steele VE et al (2000) Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 130(2S Suppl):467S–471S [DOI] [PubMed]
- Kern W, Peters A, Fruehwald-Schultes B et al (2001) Improving influence of insulin on cognitive functions in humans. Neuroendocrinology 74(4):270–280 doi:10.1159/000054694 [DOI] [PubMed]
- Klein WL, Krafft GA, Finch CE (2001) Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci 24(4):219–224 doi:10.1016/S0166-2236(00)01749-5 [DOI] [PubMed]
- Lannert H, Hoyer S (1998) Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci 112(5):1199–1208 doi:10.1037/0735-7044.112.5.1199 [DOI] [PubMed]
- Lester-Coll N, Rivera EJ, Soscia SJ et al (2006) Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis 9(1):13–33 [DOI] [PubMed]
- Li L, Hölscher C (2007) Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 56(2):384–402 doi:10.1016/j.brainresrev.2007.09.001 [DOI] [PubMed]
- Lim GP, Chu T, Yang F et al (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21(21):8370–8377 [DOI] [PMC free article] [PubMed]
- Lodha R, Bagga A (2000) Traditional Indian systems of medicine. Ann Acad Med Singap 29(1):37–41 [PubMed]
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60 doi:10.1016/0165-0270(84)90007-4 [DOI] [PubMed]
- Olgun A, Kisa O, Yildiran ST, Tezcan S, Akman S, Erbil MK (2004) Antimicrobial efficacy of l-carnitine. Ann Microbiol 54(1):95–101
- Park CR, Seeley RJ, Craft S et al (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68(4):509–514 doi:10.1016/S0031-9384(99)00220-6 [DOI] [PubMed]
- Paxinos G, Watson C (1998) The rat brain in stereotaxiccoordinates. Academic, San Diego
- Pei JJ, Grundke-Iqbal I, Iqbal K et al (1998) Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res 797(2):267–277 doi:10.1016/S0006-8993(98)00296-0 [DOI] [PubMed]
- Rivera EJ, Goldin A, Fulmer N et al (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis 8(3):247–268 [DOI] [PubMed]
- Ruby AJ, Kuttan G, Babu KD et al (1995) Anti-tumor and antioxidant activity of natural curcuminoids. Cancer Lett 94(1):79–83 doi:10.1016/0304-3835(95)03827-J [DOI] [PubMed]
- Salkovic-Petrisic M, Hoyer S (2007) Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm Suppl 72:217–233 doi:10.1007/978-3-211-73574-9_28 [DOI] [PubMed]
- Schubert M, Brazil DP, Burks DJ et al (2003) Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci 23(18):7084–7092 [DOI] [PMC free article] [PubMed]
- Schubert M, Gautam D, Surjo D et al (2004) Role of neuronal insuline resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101:3100–3105 doi:10.1073/pnas.0308724101 [DOI] [PMC free article] [PubMed]
- Sharma M, Gupta YK (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71(21):2489–2498 doi:10.1016/S0024-3205(02)02083-0 [DOI] [PubMed]
- Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41(13):1955–1968 doi:10.1016/j.ejca.2005.05.009 [DOI] [PubMed]
- Shishodia S, Sethi G, Aggarwal BB (2005) Curcumin: getting back to the roots. Ann N Y Acad Sci 1056:206–217 doi:10.1196/annals.1352.010 [DOI] [PubMed]
- Suh HW, Kang S, Kwon KS (2007) Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem 298(1–2):187–194 doi:10.1007/s11010-006-9365-6 [DOI] [PubMed]
- Sumanont Y, Murakami Y, Tohda M et al (2006) Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci 78(16):1884–1891 doi:10.1016/j.lfs.2005.08.028 [DOI] [PubMed]
- Szabados T, Dul C, Majtényi K et al (2004) A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res 154(1):31–40 [DOI] [PubMed]
- Thiyagarajan M, Sharma SS (2004) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74(8):969–985 doi:10.1016/j.lfs.2003.06.042 [DOI] [PubMed]
- West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231(4):482–497 doi:10.1002/ar.1092310411 [DOI] [PubMed]
- Wu A, Ying Z, Gomez-Pinilla F (2006) Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol 197(2):309–317 doi:10.1016/j.expneurol.2005.09.004 [DOI] [PubMed]
- Yang F, Lim GP, Begum AN et al (2005) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280(7):5892–5901 doi:10.1074/jbc.M404751200 [DOI] [PubMed]