Abstract
Background & Aims:
While multiple clinical trials support the efficacy of psychological treatments for reducing IBS symptoms, the mechanisms responsible for symptomatic improvement are unknown. One hypothesis is that psychological treatments work by alleviating comorbid psychological distress implicated in the worsening of bowel symptoms and quality of life. An alternative hypothesis assumes that changes in distress are not strictly a cause but a consequence of IBS that will decrease with symptomatic improvement.
Methods:
We evaluated these two hypothesis by applying structural equation modeling (SEM) to the data set of a large number (N = 147) of Rome II diagnosed participants randomized to cognitive behavior therapy (CBT), psychoeducation, or wait list. Per Rome guidelines, the primary endpoint was global improvement of GI symptoms measured two weeks after a 10-week regimen. Secondary endpoints were distress and quality of life (QOL).
Results:
SEM analyses lend support to a model in which CBT is associated with improvements in IBS symptoms, but that therapeutic gains are not dependent on changes in patients' overall level of psychological distress. Symptom severity, but not clinical status (pain catastrophizing, predominant bowel habits, symptom duration, abuse, diagnosable psychiatric disorder) or relevant sociodemographic variables (e.g., gender, age) moderated treatment outcome.
Conclusion:
CBT has a direct effect on global IBS symptom improvement independent of its effects on distress. Improvement in IBS symptoms is associated with improvements in the QOL, which may lower distress. Symptom improvements are not moderated by variables reflecting the mental well being of IBS patients.
Introduction
Irritable bowel syndrome (IBS) is a prevalent, costly, and potentially disabling gastrointestinal disorder for which there is no universally agreed upon medical option1. Conventional drug and dietary treatments tend to fall short of therapeutic objectives for more severely affected patients 2-4 whose symptoms are often accompanied by psychological dysfunction (e.g., depression, anxiety, somatization) and quality of life impairment 5. The shortcomings of medical therapies, coupled with the influence psychosocial factors exert on the expression and trajectory of IBS 6, have promoted the development of a number of psychological therapies. Their viability has been summarized in several recent systematic reviews that weighed their apparent efficacy profile in light of methodological imperfections of clinical trials. Recent narrative6, 7 and quantitative 8 reviews converge in the conclusion that psychological therapies are, as a group, efficacious in reducing key GI symptoms (pain, bowel dysfunction), comorbid psychological distress, and quality of life impairment9, 10 at least when pitted against passive control comparisons (e.g., waiting list, no-treatment conditions, treatment as usual).
Although the relative superiority of any one psychosocial modality (e.g., interpersonal psychotherapy, hypnosis, muscle relaxation training, etc) is unknown, the majority of trials supporting psychological therapies featured a specific treatment called cognitive behavior therapy (CBT). CBT is a time limited, structured, problem focused, and prescriptive therapy based on two central underlying assumptions: (1) symptoms are acquired (learned) and reflect specific skills deficits in domains of cognitive and behavioral functioning and (2) teaching and rehearsing skills for modifying maladaptive behaviors and thinking patterns can remediate these deficits which, in turn, relieves symptoms. Specific technical components of CBT protocols typically include: (a) the provision of information about stress and its relationship to IBS; (b) self monitoring of antecedent and consequent events associated with IBS flare ups; (c) problem solving strategies around stressors that aggravate symptoms; (d) muscle relaxation exercises for cultivating lower physiological arousal and increased sense of mastery over symptoms; and/or (e) cognitive restructuring for modifying faulty threat appraisals that underlie physiological and emotional reactivity.
Currently, it is not known how or why CBT works, when it works, or how to optimize it so that it renders more robust effect sizes. This is an important research question because a sizable (25-30%11-13) proportion of IBS patients do not respond adequately to CBT. Specifying the processes and mechanisms through which it operates may (1) help elucidate factors central to the maintenance if not etiology of IBS and (2) refine and streamline CBT by isolating which technical components of a treatment package are key to bringing about change. Rather than relying on inert, redundant, or even counterproductive procedures, clinicians and researchers can mobilize their resources toward refining, intensifying, and implementing ”active” components with the highest therapeutic yield. Distilling the active ingredients of CBT is also critical to exporting it beyond the relatively small number of academic facilities that provide behavioral treatments (typically in the context of clinical trials) to routine practice settings where the overwhelming majority of IBS patients are seen. In short, developing more simplified, powerful, accessible, and cost-effective self management therapies depends on specifying theoretical change mechanisms and testing whether they are responsible for therapeutic improvements 14.
This task has been difficult because IBS outcome researchers have focused more on whether specific treatments cause therapeutic changes than the question of what is the basis for the changes. In the absence of formal mediational research, our understanding of why treatments promote change is driven more by conjecture and intuition than the evidentiary process of scientific discovery. Conventional wisdom 15, 16 holds that that psychosocial therapies such as CBT derive their therapeutic value by reducing comorbid psychological distress characteristic of more severe IBS patients. In other words, psychological treatments ”work” by helping patients manage the psychological distress that worsens bowel symptoms and quality of life 17, p. 649. This view casts heightened psychological distress as a driving influence of symptom exacerbations and its reduction should be the primary goal of psychological therapies. In other words, CBT presumably improves GI symptoms by reducing comorbid psychological distress. An alternative hypothesis is that psychological distress is not strictly a cause but a consequence of IBS, which will decrease with improvement of its symptoms. By learning symptom self management skills, CBT treated patients improve GI symptoms which in turn has a salutary impact on comorbid distress and quality of life. Simply put, patients who undergo a successful regimen of CBT may feel less distress because they learn more effective strategies for improving their bowel problems.
We sought to gain perspectives on these two hypotheses by applying structural equation modeling (SEM) to the data set of a relatively large number of Rome II diagnosed participants undergoing treatment as part of an NIH funded clinical trial of two psychosocial treatments. An advantage of SEM is that it can provide perspectives on models that include diverse types of relationships, including moderated, mediational and bidirectional influences. Our second goal was to examine the interrelationships among symptom improvement, quality of life, and distress. Are changes in distress associated with changes in quality of life? Is their relationship consistent with models that assume unidirectional or reciprocal causal dynamics? How are changes in these important secondary endpoints associated with the primary endpoint of global improvement in IBS symptoms? While these variables have been studied in relative isolation of one another, they likely operate synergistically and fuel one another in the day to day lives of patients. Understanding such relationships stands to clarify both patients' experience of IBS as well as the quality of their treatment response to biobehavioral therapies. Our third goal was to assess whether clinical status variables associated with more severe IBS patients moderated treatment response. Of particular interest were patients' catastrophic thoughts about pain (i.e., pain catastrophizing), their abuse history, demographic features (age, gender), predominant bowel habit, psychiatric status (diagnosable Axis I DSM IV disorder), and the severity of their IBS symptom, as determined by the study gastroenterologist during pre treatment assessment.
Method
Experimental Design
This study is a secondary analysis of data 18 collected as part of a three arm randomized clinical trial of group based cognitive behavior therapy and two control groups: (1) an ”active” control group that received a psychoeducation intervention that controlled for nonspecific treatment effects (e.g., therapist attention) and (2) a ”passive” wait list control group that controlled for several threats to internal validity (passage of time, maturation, the effects of repeated assessment, statistical regression). Institutional review board approval for the study was obtained.
Participants
Participants were males and females 18-70 years old (inclusive) who were recruited primarily through referral from local specialty (e.g., gastroenterology) and primary care physicians, media coverage and advertisements placed in local media. A total of 970 subjects contacted the Behavioral Medicine Clinic at the University at Buffalo between February 2000 and May, 2003 and underwent a telephone screening interview to assess basic inclusion-exclusion criteria (bowel symptom frequency of at least twice weekly, no comorbid GI disease). Four hundred seventy seven were scheduled for a full medical and psychological assessment to confirm eligibility for treatment allocation, obtain pretreatment baseline data and secure informed consent. Of individuals who met eligibly criteria, 147 participants completed pre pretreatment assessment and treatment. Inclusion criteria included Rome II IBS diagnosis4 established during a medical examination conducted by a board certified gastroenterologist; IBS symptoms of at least moderate severity; willingness to maintain a stable dosage of any IBS medications during the 4 week pre treatment baseline period prior to randomization; and ability to provide written consent. Exclusion criteria were presence of a comorbid organic GI disease (e.g., IBD, lactose intolerance) or mental retardation; concomitant participation in psychotherapy or lifetime participation in psychotherapy featuring cognitive-behavioral techniques; a history of current or past diagnosis of schizophrenia or other psychotic disorders; or current diagnosis of unipolar depression with suicidal ideation; or current diagnosis of psychoactive substance abuse or dependence. Moderate to severe IBS was operationally defined as a score of 2 or greater on physician administered global ratings of IBS severity scale19-21 (1 = mild, 2 = moderate, 3 = severe, 4 = very severe). The average duration of IBS symptoms was 17 years. Patients were classified on the basis of their predominant bowel habit: 47% were diarrhea-predominant 13% constipation-predominant, and 40% were alternating or mixed. Determination of predominant bowel habit was made after medical examination using Rome II guidelines4 and clinical impression of the study gastroenterologist. After evaluation, the study research coordinator who had no therapeutic relations with any of the participants used a computer generated treatment allocation scheme to randomly assign patients to one of the three groups (cognitive behavior therapy, psychoeducation, or waiting list control) in an approximate 3:1:1 ratio to balance the ethical obligation to deliver treatment in a timely fashion with the need for sufficient statistical power for mediational analyses of CBT. Participants were informed they had an increased likelihood of being assigned to CBT. Because it is not possible to disguise the ”contents” of psychosocial treatment, we adopted the equivalent practice of assessing patients' credibility of the treatments to which they were assigned22. Participants were allocated to therapists, with the goal of balancing the number of patients treated in each condition by each therapist.
Treatment Conditions
Participants meeting study criteria received either 10, weekly 90 minute group (3-6 patients per) sessions of cognitive behavior therapy or psychoeducation/support, or assigned to a wait list condition after a four week pretreatment baseline period. Detailed treatment manuals were used for each session and are available upon request. Participants in the cognitive behavior therapy (CBT, n = 95) learned to conduct functional analyses of situational factors associated with symptoms flare ups; to identify, challenge, and dispute negative skewed or biased thinking patterns (e.g. catastrophizing, overestimation of threat stimuli); and to remediate problem solving deficits for coping with stressors associated with symptom flare ups. The goals of the psychoeducation support (PE, n = 28) condition was to disseminate information about IBS, its clinical features, epidemiology, diagnostic criteria, medical tests, and treatment options; facilitate the sharing of experience among group members; encourage the expression of emotions through empathic listening, unconditional support, and warmth; and foster a sense of cohesion among patients with similar experiences. PE specifically avoided cognitive restructuring, problems solving training, advice giving, or prescription of behavior change advice featured in the CBT condition. Patients assigned to waiting list (WL; n = 24) condition were crossed over to begin cognitive behavior therapy after completing 10 weeks of symptom monitoring and undergoing follow up assessment. None of the patients received any other psychotherapy during the waiting list or treatment phase of the trial.
Therapists and Treatment Integrity
Three doctoral level clinical psychologists (1 male, 2 female) with an average of 10 years of experience delivering psychological treatment of painful medical disorders such as IBS provided treatment in three conditions under the supervision of an experienced Ph.D level psychologist (JML). Prior to delivering treatment, therapists underwent over 40 hours of training under the supervision of the senior author (EBB). Training involved review of both concepts and techniques of both treatments, topic-by-topic review of the manuals, listening to audio taped examples of therapist implementing the treatment, role play and practice exercises, discussion of case examples and rehearsing strategies for difficult or challenging cases; and practice cases. To ensure fidelity of treatment delivery and to minimize therapist drift, therapists followed a structured and manualized treatment protocol which provided detailed guidance on session content and guidelines for implementing specific techniques. A checklist of procedures specific to each session was also used by therapists to assure compliance. Sessions were audio taped and rated by independent evaluators. A sample of audio taped (35%) sessions revealed no violation of treatment procedures for either condition. The senior author provided annual calibration clinical training and regular consultation. No therapist effects were detected in the data.
Assessment
Per Rome recommendations 23, 24, the primary clinical endpoint for this study was patients' estimation of global improvement of IBS symptoms (pain/discomfort and bowel symptoms) which we measured using an psychometrically sound visual analog scale 25 (−100 = substantial worsening to 0 = no change to 100 = substantial improvement). Secondary endpoint measures included overall psychological distress (Global Severity Index of the Brief Symptom Inventory26) and health related quality of life (IBS Quality of Life27). Outcome measures were obtained at intake and two weeks after treatment ended (Week 12) with the exception of global relief which was assessed only at Week 12. Abuse, pain catastrophizing, and participant's expectations for treatment's success and the credibility of the assigned treatment's rationale were assessed before treatment procedures were implemented using standardized self report instruments 28 29, 30. The presence and temporal onset of Axis I psychiatric disorders was assessed during baseline assessment using the Structured Clinical Interview for DSM-IV Axis I Disorders 31. Efforts were made to keep assessors unaware of treatment assignment during post treatment follow up. Adverse events were monitored during treatment and elicited during question of post treatment evaluation. No adverse events were reported.
Results
The results are organized in three sections. The first section reports preliminary analyses that describe means and standard deviations on the key variables and that compares the active and passive control groups. The second section reports the primary structural equation analyses. The third section reports the theoretically interesting interaction analyses that were uncovered during routine checks for specification error.
Preliminary Analyses
Descriptive Statistics
Table 1 presents means and standard deviations for all of the major continuous variables. In terms of demographic variables, age ranged from 22 to 79 with a median age of 50 (and a median absolute deviation of 10.0). The sample was 82% female and the median income was $50,000. The sample was predominately European American (93%). Seventy percent of participants met DSM-IV diagnosis for an Axis I psychiatric disorder based on SCID assessment. Approximately 50% of patients with a diagnosable Axis I disorder indicated that it preceded IBS onset. The most common psychiatric disorder was Generalized Anxiety Disorder, an anxiety disorder characterized by chronic anxiety, tension, hypervigilance, and excessive and uncontrollable worry. Patients' baseline expectations for success and the credibility of the rationale for their assigned therapy were equivalent across the active conditions.
Table 1.
Baseline Demographic Characteristics of the Study Sample
| CBT |
PE |
WL |
||
|---|---|---|---|---|
| Variable | (n=95) | (n=28) | (n=24) | |
| Mean Age | 47.71 (14.11) | 54.37 (12.35) | 47.57 (15.71) | |
| Female, n (%) | 77.9 | 89.3 | 87.5 | |
| Ethnicity, n (%) | ||||
| Caucasian | 91.6 | 96.4 | 91.6 | |
| African American | 6.3 | 0.0 | 4.2 | |
| Other | 2.1 | 4.6 | 4.2 | |
| Mean Symptom Duration (years) | 17.14 (15.79) | 18.56 (13.98) | 14.48 (15.17) | |
| Predominant Bowel Habit | ||||
| Diarrhea | 44.2 | 57.1 | 45.8 | |
| Constipation | 12.6 | 7.2 | 16.7 | |
| Alternating | 43.2 | 35.7 | 37.5 | |
| Marital Status | ||||
| Single | 17.9 | 14.3 | 12.5 | |
| Married | 60.0 | 50.0 | 54.2 | |
| Living with Significant Other | 2.1 | 3.6 | 4.2 | |
| Divorced | 12.6 | 25.0 | 12.5 | |
| Widowed | 4.2 | 7.1 | 16.7 | |
| Education Level | ||||
| Less then High School | 3.2 | 3.6 | 4.2 | |
| Graduated from High School | 18.9 | 25.0 | 20.8 | |
| Some College | 24.2 | 21.4 | 25.0 | |
| Graduated from College | 27.4 | 14.3 | 12.5 | |
| Postgraduate School | 26.3 | 35.7 | 37.5 | |
Note. N = 147. CBT indicates cognitive behavior therapy,; PE, psychoeducation; WL, wait list
Random Assignment
To ensure that random assignment to conditions was successful, group differences on age, education, gender, income, marital status, ethnicity and the pretest scores of the major outcome variables were tested. Consistent with random assignment, no statistically significant effects were observed as a function of condition.
Active Versus Passive Control Groups
Differences between the active and passive control groups (psychoeducational support versus wait list control) were tested on the three primary post-intervention variables, global GI improvement, distress, and quality of life (Table 2). In no case did the mean differences remotely approach statistical significance, yielding p values of 0.41, 0.54 and 0.80. Data for the two homogenous control groups were therefore collapsed to maximize statistical power of contrasts and to simplify statistical modeling. Analyses reported below focus on comparisons of the cognitive behavior therapy condition against this pooled control group. All outcome data exclude post crossover patients.
Table 2.
Pre- and Post Treatment Means and SDs for Patients Receiving CBT, PE, and WL
| CBT |
PE |
WL |
||||
|---|---|---|---|---|---|---|
| Variable Mean (+/− SD) | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 |
| IBS-QOL (Total) | 59.08 (20.67) | 73.98 (16.53) | 57.03 (21.01) | 67.72 (21.81) | 60.47 20.31) | 63.97(22.59) |
| Global Severity Index (GSI) | 57.05 (9.76) | 53.22 (9.85) | 56.17 (9.77) | 55.50 (10.63) | 57.41 (8.05) | 56.20 (9.43) |
| Global Improvement (unit change) | -------------- | 52.61 (33.67) | --------------- | 25.39 (36.37) | -------------- | 16.95 (37.18) |
| IBS Symptom Severity (MD rating) | 3.18 (0.86) | ---------------- | 3.08 (0.90) | ---------------- | 3.38 (0.69) | -------------- |
Note. IBS-QOL indicates IBS Quality of Life measure. GSI measures global severity of psychological distress
Structural Equation Analysis
The General Logic of SEM
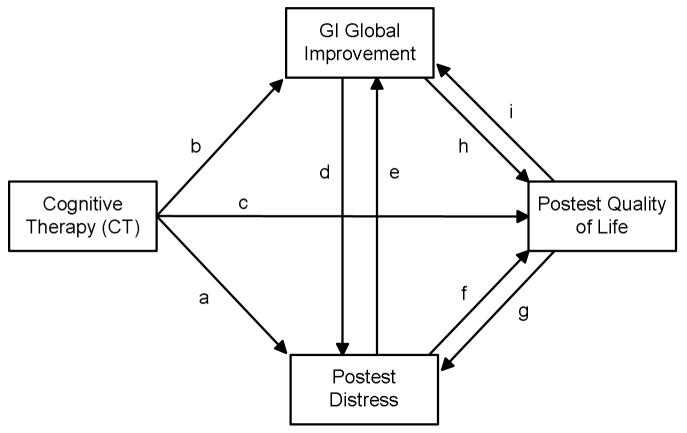
A dummy variable representing the CBT versus the control groups was created (where 1 =CBT and 0 = Control) and incorporated into SEM analyses. Figure 1 illustrates some of the possible causal dynamics that might operate. An arrow represents a presumed causal impact of one variable on another. The variable from which the arrow emanates is the presumed cause and the variable to which the arrow points is the presumed effect. Each arrow is referred to as a path. In cases where two variables are connected by two arrows in opposite directions, a bidirectional causal relationship is posited. For example, psychological distress is assumed to influence the quality of life (path f) and the quality of life is assumed to influence psychological distress (path g). The two hypotheses can be described with reference to Figure 1. Hypothesis 1 states that CBT impacts global improvement (path a) and this, in turn, impacts GI global improvement (path e). CBT impacts global improvement, but it is thought to do so through its impact on distress. This is referred to as an indirect effect, with the effect of CBT on GI global improvement being mediated by distress. Hypothesis 2 states that CBT impacts GI global improvement directly (path b) and this, in turns, reduces distress (path d).
Figure 1.
Conceptual Model for Elaborating Relationships Between Cognitive Therapy, GI Global Improvement, Distress, and Quality of Life
SEM is an extension of familiar regression methods that focus on a single regression equation 32. However, multiple linear equations are evaluated simultaneously. A causal model, often represented as a path diagram per Figure 1, dictates the equations that are estimated. Each path in a causal model has associated with it a path coefficient that is interpreted like a regression coefficient. However, SEM permits one to go beyond traditional regression analysis to gain additional perspectives on the viability of causal models. A causal model makes predictions about how the correlations between the measured variables should pattern themselves. If the data do not pattern themselves in the predicted fashion, then the causal model is rejected. If the data pattern themselves in a way that is consistent with the model, then this does not prove the model is correct (i.e., one cannot make causal inferences). Rather, it increases one's confidence in the model. Sometimes, more than one model can account for the same data, a fact that also must be considered. When observed variables rather than latent variables are the focus of analysis, the approach is often referred to as path analysis.
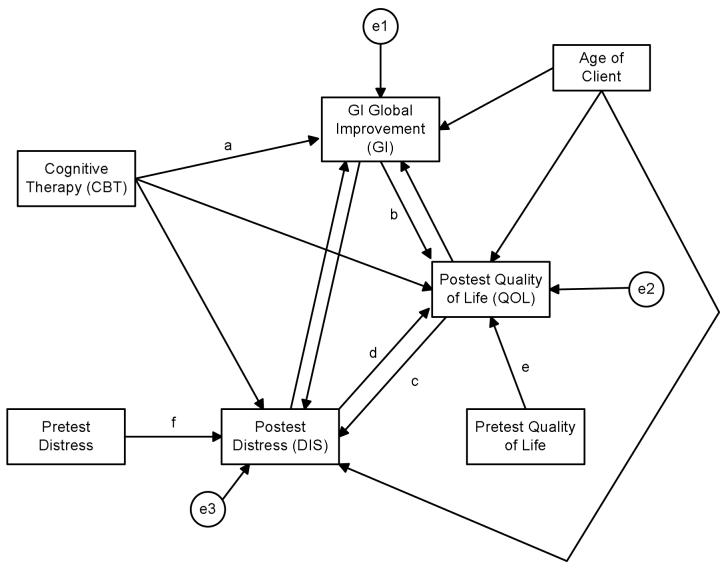
The formal SEM modeling with the present data was complex and is described in the appendix. Preliminary analyses were conducted to test for non-normality, outliers and to deal with missing data. Following standard practice for clinical trials, baseline covariates of each outcome variable, where available, were included in the model so as to focus the analysis on the analysis of change 33. Age of the client also was included as a covariate. The final model that was evaluated is presented in Figure 2 (see appendix for details). The model yielded a good fit to the data, but a number of the path coefficients were not statistically significant. Paths in Figure 2 with letters next to them yielded statistically significant path coefficients. The values of these coefficients and their associated confidence intervals are in Table 3. Table 3 also presents coefficients for selected total effects. We consider the key coefficients in the model here, in turn.
Figure 2.
Final Model from Structural Equation Modeling
Table 3.
Path Coefficients for Model in Figure 2
| Unstandardized Path Coefficient |
Standardized Coefficient |
95% CI | |
|---|---|---|---|
| Selected Direct Effects | |||
| Path a: CBT on GI | 31.62* | 0.40 | 18.40 to 44.90 |
| Path b: GI on QOL | 0.12* | 0.24 | 0.05 to 0.20 |
| Path c: QOL on DIS | −0.12* | −0.24 | −0.21 to −0.05 |
| Path d: DIS on QOL | −0.38* | −0.20 | −0.61 to −0.14 |
| Total Effects | |||
| CBT on QOL | 7.07* | 0.18 | 2.57 to 11.53 |
| CBT on DIS | −2.55* | −0.12 | −4.77 to −0.36 |
| GI on QOL | 0.12* | 0.24 | 0.05 to 0.19 |
| Residual Variances | |||
| e1: GI | - | 0.87 | - |
| e2: QOL | - | 0.34 | - |
| e3: DIS | - | 0.40 | - |
Note. Path letters and residual labels refer to Figure 2; CBT = Cognitive-Behavior Therapy; GI = Gastrointestinal global improvement; QOL = Quality of life; DIS = Psychological distress.
signifies p < 0.05. P value for unstandardized coefficient applies to the standardized coefficient as well. Squared multiple correlations are 1.00 minus the standardized residual variance. Confidence intervals are for the unstandardized path coefficients
The Effect of CBT on Global GI Improvement
The estimated mean difference in global improvement between the CBT and the control groups as reflected by path a of Figure 1 was 31.62 with individuals in the CBT condition showing statistically significantly greater improvement than those in the control conditions (p < 0.05, 95% CI = 18.40 to 44.90). Given random assignment to conditions, a formal causal inference is reasonable for this portion of the model.
The Effect of CBT on Quality of Life
Although the path coefficient linking the intervention dummy variable directly to quality of life was statistically non-significant (see Figure 2), the pattern of statistically significant path coefficients was consistent with the proposition that CBT improves quality of life. This is because CBT was associated with increased improvement in global GI symptoms (path a) and this improvement in GI symptoms, in turn, was associated with improvement in the patients' quality of life (path b), which also was statistically significant (path coefficient = 0.12, p < 0.05, 95% CI = 0.05 to 0.20). This indirect effect, also captured by the product of the path coefficients a and b, was statistically significant (p < 0.05). Stated another way, for every 10 units that global GI improvement ratings increased, the quality of life rating was predicted to increase by 1.2 units. Since the CBT group was approximately 32 units higher on global GI improvement than the control group, this resulted in a mean quality of life score that was just about 3.6 units higher in the CBT condition than in the control condition. This indirect effect was statistically significant (p < 0.05, 95% CI = 1.67 to 6.77). Taking into account all the causal dynamics implied by the model in Figure 2, the estimated total effect of CBT on quality of life was such that those clients in the CBT condition had a predicted mean quality of life score that was 4.6 units higher than those in the control group (p < 0.05, 95% CI = 1.85 to 8.29). Thus, the data are consistent with the proposition that CBT impacts quality of life, primarily through its improvement on GI symptoms.
The modeling strategy allowed for the possibility of reciprocal causation between quality of life and GI global improvement, but only one of the two path coefficients was statistically significant. This was the path from global GI improvement to quality of life (see Appendix for statistical details). This is consistent with the proposition that improving GI symptoms improves the quality of life, but that improving the quality of life does not improve GI symptoms.
The Role of Distress
As noted above, some scientists have argued that the primary impact of CBT is on reducing psychological distress and that this reduced distress, in turn, produces improvement in global GI symptoms. The results from Figure 2 suggest a different scenario. According to the estimated coefficients for the model in Figure 2, CBT is associated with improvement in global GI symptoms which, in turn, are associated with enhanced quality of life of the patient (as discussed above). This improved quality of life, in turn, is associated with decreases in psychological distress in the patient, as reflected by the path coefficient of −0.12 (p < 0.05, 95% CI = −0.20 to −0.05) for path c in Figure 2 (see Table 3). Specifically, for every three units that quality of life changes in a positive direction, psychological distress is predicted to decrease just under one half a scale unit.
Interestingly, the analyses found support for a bi-directional relationship between distress and quality of life. Not only were the data consistent with the proposition that improvements in the quality of life decrease distress, but also that decreases in distress tend to improve quality of life. This latter effect is reflected in the path d in Figure 2, whose coefficient was −.38 (p < 0.05, 95% CI = −0.61 to −0.14). For every one unit that psychological distress increased, the quality of life was predicted to decrease about one third of a unit.
The tested model also allowed for a direct effect of CBT on distress, but we did not find evidence for this once variation in GI improvement and quality of life were statistically held constant. In addition, models were tested that allowed for direct effects of distress on GI global improvement, but evidence for these links were not observed when other key variables were statistically held constant (as per Figure 2).
In sum, somewhat small but statistically significant mean differences in psychological distress were observed for the CBT and control groups (mean difference = −2.55, p < 0.05, 95% CI = −4.77 to −0.36). These mean differences were best accounted for by a model that presumes the differences were the result of induced changes in the patients' quality of life as a result of improvements in GI symptoms, which, in turn, yield lowered levels of distress in the CBT condition.
Interaction Analyses
No statistically significant moderated effects were observed for the model in Figure 2 as a function of abuse history, catastrophizing, predominant bowel syndrome, gender, age and number of DSM IV Axis I psychiatric disorders. An interaction between the number of years that the individual had been experiencing GI problems and the effects of CBT on the patient's quality of life scores was observed (such that the longer individuals had been experiencing GI problems, the more pronounced was the effect of CBT on the patient's quality of life), but this effect proved to be fragile and changed in statistical significance depending on the inclusion or exclusion of different covariates and the method of statistical estimation. This effect is worth noting for future research, but must be viewed as tentative, at best, in the current study.
In the context of explorations of specification error, one interaction effect was noted that could be theoretical interest. It was not predicted a priori and was isolated in the context of exploratory post hoc specification checks. It should be treated as tentative and in need of replication in future research. The interaction was between the severity of the GI symptoms as reported at baseline and the effects of CBT on the patient's quality of life. When an exogenous product term for these variables was included in the model with a path to quality of life, the product term coefficient was 5.67 (p< 0.05, 95% CI = 0.89 to 9.67). This indicates that for every one unit that the severity of GI problems at baseline increased, the subsequent difference between the CBT and control group on quality of life tended to increase by 5.67 units. Thus, the more severe the GI problems were initially, the more pronounced was the effect of CBT on the patient's quality of life.
Discussion
This study addresses the question of how therapeutic gains are achieved in a sample of severely affected, Rome diagnosed IBS patients undergoing symptom self management training (CBT). We were particularly interested in gaining perspectives on whether CBT – arguably the most empirically tested psychosocial treatment 6, 8 – improves GI symptoms by reducing comorbid psychological distress. SEM analyses yielded support for a model in which CBT is associated with statistically significant improvements in IBS symptoms, but that these therapeutic gains are not dependent on or mediated by changes in patients' overall level of psychological distress. Relative to the control group, CBT exerted a direct effect on overall improvement of GI symptoms independent of its effects on distress. To the extent that distress reduction is linked to a treatment condition (CBT versus control), analyses suggested that the effect may be attributable to it being a consequent of improvements in GI symptoms and, in turn, to quality of life. .
In general, our findings dovetail with a recent report 34 concerned with whether changes in comorbid distress variables (depression, anxiety, somatization) explained treatment gains (QOL) in patients who underwent short-term dynamic psychotherapy. While the investigators did not conduct formal mediation analyses, they showed that patients' level of distress accounted for only a limited amount of variance in QOL. A similar pattern of data was reported by Tack et al 35 who found that the improvement23 in abdominal pain in IBS patients treated with the SSRI citalopram was independent of mood change. The data also echo the conclusions of a recent systematic review and meta analysis 8 that psychological treatments in general are more effective in reducing somatic symptoms of IBS (e.g., bowel dysfunction) than comobrdid psychological distress. These data, coupled with the results of the present study, argue, perhaps counter-intuitively, that the greatest therapeutic value of psychological treatments rests in their ability to improve GI symptoms, perhaps through the teaching of such factors as symptom management skills.
Because IBS lacks a treatable biological marker that reliably corresponds with symptoms36, “patient centered” outcomes (e.g., quality of life, distress, GI symptom improvement/relief) are recommended as optimal endpoints for evaluating the viability of treatments for functional GI disorders. Clinical investigators have generally conceptualized and analyzed these endpoints as independent domains. To our knowledge, no attention has been paid to whether treatment response to one endpoint impacts (and is impacted by) other endpoints. Our data suggests that the relationship among endpoints is complex, dynamic, and interdependent. As noted above, the data were consistent with a model in which symptomatic improvements following CBT has a favorable impact on quality of life which, in turn, leads to improvements in overall distress. Improvements in distress, in turn, impact quality of life.
Our efforts at identifying moderators yielded mixed results. The only frequently studies variable that was associated with treatment outcome was intake severity of GI symptoms and it was detected during routine misspecification analysis. The interaction was such that the more severe the GI problems were at intake, the more pronounced was CBT's effect on quality of life. These data lend some empirical support to consensus derived clinical practice guidelines 37, 38 that recommend psychosocial therapies for patients with more severe IBS symptoms. Further research is needed to validate empirically guidelines once they are published to augment their acceptance, adherence and value to practitioners, patients, and institutions responsible for improving health care delivery (e.g., third party payers, government funding agencies).
The limited predictive power of the proposed moderator variables is not altogether surprising given the few reliable prognostic indicators found to influence outcome of IBS therapies. This highlights the value of looking beyond socio-demographic or clinical status (e.g., psychiatric comorbidity, bowel habit type, duration of symptoms) variables for clues about characteristics on which outcome depends. Potentially informative moderators about which little is known include characteristics of the clinician (e.g., clinician expertise, patients' motivation for change); ongoing stressors or “extra-treatment” characteristics such as relationships with family members, work strain, and financial pressures; and features relating to how the therapy was administered (e.g., sequence of procedures, timing of treatment gains, mode of treatment delivery, number of sessions).
In designing this study, our primary intent was to gain insights into the mediational role of psychological distress in reducing global IBS symptoms following CBT. Our modeling efforts argue against the notion that global IBS symptom improvements come about through distress reduction. While our results do not establish the precise processes for how and why CBT works, this does not diminish the scientific value of the study. By challenging the validity of the popular view that CBT's effect is mediated through reduced psychological distress, we hope to stimulate research dedicated to identifying the active ingredients that are theorized to underlie the effects of CBT and other nonpharmacological therapies. We believe that specifying the processes of change is important not merely for theoretical reasons but also so that interventions can be made more effective for the greatest number of patients under diverse conditions. Delineating the critical components of treatment may allow existing interventions to be modified in a manner that enhances their utility as well as modify or eliminate those procedural components that are inert, redundant, or counterproductive.
Previous efforts at addressing the question of what makes CBT work have found no more answers in physiological mechanisms 39, 40 than we find in distress variables. A potentially fruitful approach comes from the broader behavioral medicine literature that shows that significant reduction of painful physical symptoms (e.g., benign headache, musculoskeletal pain, fibromyalgia) following CBT occurs through its impact on cognitive processes (e.g., self efficacy expectancies) 41-46. Whether psychological treatments for IBS alleviate bowel symptoms by modifying patient beliefs is largely uncharted terrain. Two exceptions come from two recent reports 47, 48 that found that negatively skewed patient beliefs of psychotherapy treated (hypnosis; CBT) patients decreased in parallel with IBS symptoms from pre to post-treatment. While these data are consistent with the hypothesis that cognitive changes mediate treatment effects, they are equally compatible with two alternative propositions: (1) changes in IBS symptoms may lead to cognition changes; and (2) changes in cognitions and IBS symptoms may be spuriously correlated because of the causal effect of a third, unspecified variable. Solving this conundrum calls for more sophisticated experimental designs (e.g., analyses of intersession change in process variables and endpoints) and powerful analytic strategies capable of establishing the mediational specificity of mechanisms thought to govern treatment gains. In the end, clinical trials that attend to these methodological issues should advance the field by promoting the development of more effective therapies for a problem whose day to day burden -- in the absence of a more consistently satisfactory medical option -- rests not on the shelf but on the quality of patients' symptom self management skills.
As with any study, the results of the present investigation must be interpreted within the operative methodological constraints. SEM only tests if the patterning of data is consistent with a hypothesized causal model but a good model fit does not prove the model being tested. Other causal structures may account for the data equally well, a phenomenon known as redundant models in the SEM literature. SEM results always must be interpreted knowing that plausible redundant models may exist and the present study is no exception. The measures used in our analyses were subject to measurement error, which can introduce bias in parameter estimates. Although we conducted analyses that suggest its effects are minimal (see Appendix), care also must be taken when working with imperfect indicators of latent constructs.
Several other caveats are worth noting in interpreting the data. Our findings are based on a subset of more severely affected IBS patients with a relatively homogeneous demographic profile drawn from one investigative site and therefore may not necessarily generalize to a more diverse sample or one treated by other research teams. We made no attempt to assess exhaustively the full range of possible mechanisms that may explain what CBT involves or why it works. The mediational value of nonspecific factors (e.g., the therapist – patient relationship) “common” across psychotherapies of equivalent efficacy or unique ones specified by cognitive behavior theory (e.g., catastrophic cognitions) 49-51 is a research question to which clinical investigators should direct their energies. Also unclear is whether our pattern of data would hold up when the primary endpoint (global improvement) is measured using alternative outcome measures such as symptom frequency or severity52 53. Because patients appraisal of improvement and severity of symptoms appear only modestly correlated 25, they are not equivalent endpoints. Research is needed to understand the factors that influence patients' estimation of therapeutic change indices. For example, it is possible that patient appraisal of improvement is a cognitively elaborate construct that may be more sensitive to cognitive interventions than symptom oriented endpoints (e.g., e.g., stool frequency). Because our aim was to study the therapeutic processes that distinguish a successful course of CBT from an unsuccessful one, we relied on treatment completer data. While it is conceivable that data from all participants who initially entered treatment would present a different set of findings, comprehensive analyses of the efficacy of group based CBT for IBS we report elsewhere 18 indicates strong correspondence between treatment completer and intent to treat data.
Despite these limitations, the present study has laid the groundwork for further exploration of the mechanisms by which CBT impacts GI symptoms and quality of life. By showing that most of the effects of CBT on GI symptoms occur independent of distress, attention should turn to the identification of other potential mediators of CBT effects. Future randomized clinical trials can then confirm the impact of these “active ingredients” of the intervention. In addition, the links between global improvement in GI symptoms and overall quality of life should be the subject of further study. The present study suggests an interesting reciprocal dynamic between quality of life and reduction of distress, which requires further empirical attention.
Acknowledgments
Grant support: This research was supported by NIH/NIDDK Grants 54211 and 67878
Abbreviations used
- IBS
irritable bowel syndrome
- CBT
cognitive behavioral therapy
- DSM
Diagnostic and Statistical Manual for Mental Disorders
- SEM
structural equation modeling
- QOL
quality of life
Appendix Technical Details of the Structural Equation Analyses
Preliminary Analyses
Preliminary analyses were conducted to gain perspectives on outliers, missing data, and non-normality. We discuss each, in turn.
Outliers
Both model based and non-model based outlier analyses were pursued. The latter used leverage scores derived from the multivariate profile of the continuous variables in Table 1. An outlier was defined as a person with a leverage score three times the value of the mean leverage. Model based outliers were examined using limited information regression analyses and then examining standardized dfBeta values for each individual. An outlier was defined as individuals who had dfBetas larger than 1.0. No outliers were evident in either analysis.
Missing Data
There were small amounts of missing data amounting to no more than a few cases on any given variable. There was no coherent pattern to the missing data. For those individuals with missing data, values were imputed to conform to covariance estimates consistent with the application of the Expectation-Maximization method with importance re-sampling as described in King, Honaker, Joseph & Scheve54 and implemented in the computer program Amelia 54. Given the small number of instances of missing data, concerns about estimation strategies are moot.
Non-Normality
Maximum likelihood methods were used for the structural equation analyses. These methods assume that the continuous variables in the model are multivariately normally distributed. This was tested using the Mardia test for multivariate kurtosis, which yielded a statistically non-significant result (critical ratio = 0.37, ns). Skewness and kurtosis indices for each continuous variable are presented in Table 1. None of the values appear troublesome.
Primary Analyses
The data were analyzed using the AMOS 7.0 computer program. The chi square fit index for the model was 0.9 (df = 1, p < .35), the CFI was 0.99, the RMSEA was <0.01; the p value for close fit was < 0.43 and the standardized RMR was 0.01. More focused fit tests (examination of modification indices, offending estimates, standardized residuals, and evaluations of overall theoretical coherence) all suggested good fit. For example, no modification indices above 4.0 were observed and none of the discrepancies between pairs of predicted and observed covariances were statistically significant.
Predicted means for variables were derived by estimating means for the exogenous variables and intercepts for the endogenous variables in the models. Significance tests and confidence intervals for the standardized residuals, total effects and indirect effects in Table 3 and in the main text were based on bootstrapping with 2000 bootstrap replicates. Confidence intervals were estimated using the bias corrected bootstrap method as implemented in AMOS 7.0. All of the bootstrap replicate analyses readily converged. In general, the confidence intervals for the bootstrap analyses were close to those for the maximum likelihood estimated confidence intervals.
A variety of alternative models was evaluated to ensure that the separate non-significant path coefficients for reciprocated paths linking two variables were not artifactual. For example, the model in Figure 2 was tested but in place of the reciprocated influence, there was only a single path between distress and global GI improvement with the former influencing the latter. This analysis with the single path was repeated, but where the model was such that global GI improvement impacted distress. In none of the alternative models did the results contradict the basic conclusions made in the main text. Models with reciprocal causation require the presence of instrumental variables to achieve identification. Future research can explore the generalizability of our results across different instruments55.
Parameter estimates for total and indirect effects as reported in the main text and Table 3 include contributions from statistically non-significant paths. There is controversy about whether it is best to report estimates from more saturated models that retain statistically non-significant paths versus trimmed models that explicitly eliminate them 56. The advantage of the former strategy is that it lessens the impact on parameter bias of mis-specification due to left-out-variables error (LOVE) that accumulates across the potentially trimmed variables but is not obvious in any one variable. The disadvantage is slightly reduced power. Our preference is to avoid the LOVE problem given that the study is reasonably powered to begin with. Estimates with and without the non-significant paths included did not vary much from one another.
Extensive tests for specification error were pursued. One set of analyses focused on possible non-linear relationships in the data. Using a limited information estimation strategy, each endogenous variable was regressed onto its core predictors (as dictated by Figure 2) and then quadratic and cubic terms for a given predictor were added to the equations using polynomial regression methods and checked for statistical significance. In no case did we observe statistically reliable non-linearity. Another set of analyses explored specification error due to the omission of interactions between predictors. Using the same limited information estimation strategy, all possible two way interactions were modeled between predictors and tested for statistical significance using strategies discussed in Jaccard & Turrisi 57. None of these effects were even close to being statistically significant. We also tested for possible interactions with variables not included in the model but that theory suggests could be involved in an interaction. It was these analyses that yielded the moderator effects reported in the supplementary section of the main text.
For the interaction analyses reported in the main text, the product term approach described by Marsh, Wen and Hau. 58 for interaction analysis in SEM models was pursued. The interactions were decomposed using methods described in Jaccard and Turrisi 57.
Measurement error can bias parameter estimates. To explore this, we re-estimated the model in Figure 2 as well as the alternative models we pursued but we imposed an a priori determined amount of measurement error onto the observed continuous measures. We used the strategy described by Joreskog and Sorbom 59 of fixing error variances at non-zero values . The amount of unreliability imposed was based on the alpha coefficient for the scale (i.e., the proportion of random error due to measurement error was set to be 1 minus the alpha coefficient for the scale). If no such estimate was available, a reliability of 0.80 was assumed. None of the major conclusions drawn from the original tests were altered in these analyses.
Given that some theoretically interesting links yielded statistically non-significant results, it is useful to provide perspectives on statistical power for the tests of the path coefficients. Power analyses for SEM models are complicated and often rest on assumptions that are impractical or not viable. We followed the practice recommended by Jaccard and Turrisi 60 that provides a rough sense of statistical power by applying power analytic methods for OLS regression as applied to selected linear equations from the set of linear equations implied by the model in question. Given a sample size of 147 and a two tailed alpha level of 0.05, we evaluated the statistical power associated with a path coefficient that represents 5% explained variance over and above a set of three additional covariates. Based on the structural standardized residuals for the key endogenous variables in Figure 2, we evaluated three scenarios where the initial set of covariates accounted for 10% of the variance, 40% of the variance, or 60% of the variance. The approximate statistical power in these three scenarios was always greater than 0.80. For a path coefficient that represents 3% additional explained variance in the same scenarios, the approximate statistical power was 0.59, 0.76, and 0.90. Overall, the approximate power seems adequate for detecting paths that account for at least 5% of the variance of an outcome variable and in some cases, it also is adequate for coefficients that reflect only 3% unique explained variance. In terms of contrasts between the CBT and control groups, the smaller sample size in the control group still yields adequate power greater than 0.80 for medium effects sizes (covariate adjusted mean differences corresponding to a Cohen d of 0.50) and large effect sizes.
The presence of reciprocal causation clouds the interpretation of standardized residuals for the endogenous variables as coefficients of alienation. The relevant squared multiple correlations of the three exogenous variables were calculated using the procedures described in Bentler and Raykov61, 62 and are reported in Table 3.
As with any SEM analysis, we recognize the possible existence of equivalent models and results must be interpreted with caution, accordingly. Good fit indicates the model is consistent with the data but other models might account for the data equally.
Footnotes
We retained the psychosocial therapy dummy variable in the model even though it produced non-significant results because it was part of the broader categorical variable representing type of treatment.
References
- 1.Camilleri M, Mayer EA, Drossman DA, Heath A, Dukes GE, McSorley D, Kong S, Mangel AW, Northcutt AR. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther. 1999;13:1149–59. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradesi S, Tillisch K, Mayer E. Emerging drugs for irritable bowel syndrome. Expert Opin Emerg Drugs. 2006;11:293–313. doi: 10.1517/14728214.11.2.293. [DOI] [PubMed] [Google Scholar]
- 3.Andresen V, Camilleri M. Irritable bowel syndrome: recent and novel therapeutic approaches. Drugs. 2006;66:1073–88. doi: 10.2165/00003495-200666080-00004. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Corazziari E, Talley NJ, Thompson GW, Whitehead WE. Diagnosis, pathophysiology and treatment: A multinational consensus. Degnon Associates; 2000. Rome II. The functional gastrointestinal disorders. [Google Scholar]
- 5.Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45(Suppl II):1125–1130. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–58. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health . Treatment Choice in Psychological Therapies and Counseling: Evidence Based Clinical Guidelines. UK Department of Health; 2001. [Google Scholar]
- 8.Lackner JM, Morley S, Dowzer C, Mesmer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–13. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 9.Creed F, Fernandes L, Guthrie E, Palmer S, Ratcliffe J, Read N, Rigby C, Thompson D, Tomenson B. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology. 2003;124:303–17. doi: 10.1053/gast.2003.50055. [DOI] [PubMed] [Google Scholar]
- 10.Lackner JM, Gudleski GD, Zack MM, Katz LA, Powell C, Krasner S, Holmes E, Dorscheimer K. Measuring health-related quality of life in patients with irritable bowel syndrome: can less be more? Psychosom Med. 2006;68:312–20. doi: 10.1097/01.psy.0000204897.25745.7c. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, Le T, Meyer K, Bradshaw B, Mikula K, Morris CB, Blackman CJ, Hu Y, Jia H, Li JZ, Koch GG, Bangdiwala SI. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard EB. Irritable bowel syndrome: Psychosocial assessment and treatment. APA; 2001. [Google Scholar]
- 13.Blanchard EB, Lackner JM, Gusmano R, Gudleski GD, Sanders K, Keefer L, Krasner S. Prediction of treatment outcome among patients with irritable bowel syndrome treated with group cognitive therapy. Behav Res Ther. 2006;44:317–37. doi: 10.1016/j.brat.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kazdin AE. Progression of Therapy Research and Clinical Application of Treatment Require Better Understanding of the Change Process. Clin. Psychol. 2001;8:143–151. [Google Scholar]
- 15.Boyce P, Gilchrist J, Talley NJ, Rose D. Cognitive-behaviour therapy as a treatment for irritable bowel syndrome: a pilot study. Australian and New Zealand Journal of Psychiatry. 2000;34:300–309. doi: 10.1080/j.1440-1614.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 16.Toner BB. Cognitive-behavioral treatment of irritable bowel syndrome. CNS Spectr. 2005;10:883–90. doi: 10.1017/s1092852900019854. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA, Chang L. Psychosocial factors in the care of patients with GI disorders. In: Yamada T, editor. Textbook of Gastroenterology. Lippincott-Raven; Philadelphia: 2002. pp. 636–54. [Google Scholar]
- 18.Blanchard EB, Lackner JM, Sanders K, Krasner SS, Keefer L, Payne A, Gudleski GD, Rowall D, Sykes M, Kuhn E, Gusmano R, Carosella AM, Firth RS, Dulgar-Tulloch L. A controlled evaluation of group cognitive therapy in the treatment of irritable bowel syndrome. Behaviour Research & Therapy. doi: 10.1016/j.brat.2006.07.003. in press. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA. Do psychological factors define symptom severity and patient status in irritable bowel syndrome? American Journal of Medicine. 1999;107:41S–50S. doi: 10.1016/s0002-9343(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 20.Lackner JM, Quigley BM, Blanchard EB. Depression and abdominal pain in IBS patients: the mediating role of catastrophizing. Psychosomatic Medicine. 2004;66:435–441. doi: 10.1097/01.psy.0000126195.82317.46. [DOI] [PubMed] [Google Scholar]
- 21.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: A circumplex analysis of the communal coping model. Pain. 2004;110:597–604. doi: 10.1016/j.pain.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Lackner JM, Morley S, Mesmer C, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–13. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 23.Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of Treatment Trials for Functional Gastrointestinal Disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead WE, Corazziari E, Prizont R, Senior JR, Thompson WG, van Zanten SJOV. Definition of a responder in clinical trials for functional gastrointestinal disorders: report on a symposium. Gut. 1999;45:78ii–79. doi: 10.1136/gut.45.2008.ii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meissner JS, Blanchard EB, Malamood HS. Comparison of treatment outcome measures for irritable bowel syndrome. Applied Psychophysiology and Biofeedback. 1997;22:55–62. doi: 10.1023/a:1026289709751. [DOI] [PubMed] [Google Scholar]
- 26.Derogatis LR. Brief Symptom Inventory. National Computer Systems; 1993. [Google Scholar]
- 27.Patrick DL, Drossman DA, Frederick IO. A Quality of Life Measure for Persons with Irritable Bowel Syndrome (IBS-QOL): User's Manual and Scoring Diskette for United States Version. University of Washington; Seatle, Washington: 1997. [Google Scholar]
- 28.Leserman J, Li Z, Drossman DA, Hu YJB. Selected symptoms associated with sexual and physical abuse history among female patients with gastrointestinal disorders: The impact on subsequent health care visits. Psychological Medicine. 1998;28(2):417–425. doi: 10.1017/s0033291797006508. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 30.Borkovec TD, Nau Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- 32.Kerlinger FN, Pedhazur EJ. Multiple regression in behavioral research. Holt, Rinehart, and Winston; 1973. [Google Scholar]
- 33.Rausch J, Maxwell S, Kelley K. Analytic methods for questions pertaining to a randomized pretest, posttest, follow-Up design. Journal of Clinical Child and Adolescent Psychology. 2003;32:467–486. doi: 10.1207/S15374424JCCP3203_15. [DOI] [PubMed] [Google Scholar]
- 34.Creed F, Guthrie E, Ratcliffe J, Fernandes L, Rigby C, Tomenson B, Read N, Thompson DG. Does psychological treatment help only those patients with severe irritable bowel syndrome who also have a concurrent psychiatric disorder? Aust N Z J Psychiatry. 2005;39:807–15. doi: 10.1080/j.1440-1614.2005.01686.x. [DOI] [PubMed] [Google Scholar]
- 35.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers A, Janssens J. A controlled cross-over study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. doi: 10.1136/gut.2005.077503. 2006:gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–93. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 37.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 38.Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: A technical review for practice guideline development. Gastroenterology. 1997;112:2120–2137. doi: 10.1053/gast.1997.v112.agast972120. [DOI] [PubMed] [Google Scholar]
- 39.Heitkemper MM, Jarrett ME, Levy RL, Cain KC, Burr RL, Feld AD, Barney P, Weisman P. Self-management for women with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2004;2:585–596. doi: 10.1016/s1542-3565(04)00242-3. [DOI] [PubMed] [Google Scholar]
- 40.Heymann-Monnikes I, Arnold R, Florin I, Herda C, Melfsen S, Monnikes H. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. The American Journal of Gastroenterology. 2000;95:981–993. doi: 10.1111/j.1572-0241.2000.01937.x. [DOI] [PubMed] [Google Scholar]
- 41.Nielson WR, Jensen MP. Relationship between changes in coping and treatment outcome in patients with Fibromyalgia Syndrome. Pain. 2004;109:233–41. doi: 10.1016/j.pain.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Milling LS, Reardon JM, Carosella GM. Mediation and moderation of psychological pain treatments: response expectancies and hypnotic suggestibility. J Consult Clin Psychol. 2006;74:253–62. doi: 10.1037/0022-006X.74.2.253. [DOI] [PubMed] [Google Scholar]
- 43.Rokicki LA, Holroyd KA, France CR, Lipchik GL, France JL, Kvaal SA. Change mechanisms associated with combined relaxation/EMG biofeedback training for chronic tension headache. Appl Psychophysiol Biofeedback. 1997;22:21–41. doi: 10.1023/a:1026285608842. [DOI] [PubMed] [Google Scholar]
- 44.Holroyd KA, Penzien DB, Hursey KG, Tobin DL, Rogers L, Holm JE, Marcille PJ, Hall JR, Chila AG. Change mechanisms in EMG biofeedback training: cognitive changes underlying improvements in tension headache. J Consult Clin Psychol. 1984;52:1039–53. doi: 10.1037//0022-006x.52.6.1039. [DOI] [PubMed] [Google Scholar]
- 45.Andrasik F, Holroyd KA. Specific and nonspecific effects in the biofeedback treatment of tension headache: 3-year follow-up. J Consult Clin Psychol. 1983;51:634–6. doi: 10.1037//0022-006x.51.4.634. [DOI] [PubMed] [Google Scholar]
- 46.Burns JW, Glenn B, Bruehl S, Harden RN, Lofland K. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: a replication and extension of a cross-lagged panel analysis. Behav Res Ther. 2003;41:1163–82. doi: 10.1016/s0005-7967(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 47.Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res. 2004;56:271–8. doi: 10.1016/S0022-3999(03)00076-X. [DOI] [PubMed] [Google Scholar]
- 48.Lackner JM, Lou Coad M, Mertz HR, Wack DS, Katz LA, Krasner SS, Firth R, Mahl TC, Lockwood AH. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther. 2005 doi: 10.1016/j.brat.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; 1986. [Google Scholar]
- 50.Beck AT. Cognitive therapy and the emotional disorders. 1976 [Google Scholar]
- 51.Beck AT, Emery G. Anxiety disorders and phobias. Basic Books; 1985. [Google Scholar]
- 52.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 53.Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. Journal of Consulting and Clinical Psychology. 1995;63:779–786. doi: 10.1037//0022-006x.63.5.779. [DOI] [PubMed] [Google Scholar]
- 54.Honaker J, Joseph A, King G, Scheve K, Singh N, Amelia A. A program for missing data. Department of Government, Harvard University; 2003. [Google Scholar]
- 55.Bollen KA. Structural equations with latent variables. John Wiley & Sons; 1989. [Google Scholar]
- 56.Jaccard J. Multiple regression analysis in clinical child and adolescent psychology. Journal of Clinical Child and Adolescent Psychology. doi: 10.1207/s15374424jccp3503_11. in press. [DOI] [PubMed] [Google Scholar]
- 57.Jaccard J, Turrisi R. Interaction effects in multiple regression. Sage; 2003. [DOI] [PubMed] [Google Scholar]
- 58.Marsh HW, Wen Z, Hau KT. Structural equation models of latent interactions: evaluation of alternative estimation strategies and indicator construction. Psychol Methods. 2004;9:275–300. doi: 10.1037/1082-989X.9.3.275. [DOI] [PubMed] [Google Scholar]
- 59.Joreskog K, Sorbom D. LISREL 8 user's reference guide. Scientific Software; 1997. [Google Scholar]
- 60.Jaccard J, Turrisi R. LISREL analyses of interaction effects in multiple regression. Sage; 1996. [Google Scholar]
- 61.Bentler PM, Raykov T. On measures of explained variance in nonrecursive structural equation models. JAppl Psychol. 2000;85:125–31. doi: 10.1037/0021-9010.85.1.125. [DOI] [PubMed] [Google Scholar]
- 62.Bentler PM, Raykov T. Correction. Journal of Applied Psychology. 2000;85:330. doi: 10.1037/0021-9010.85.1.125. [DOI] [PubMed] [Google Scholar]