Abstract
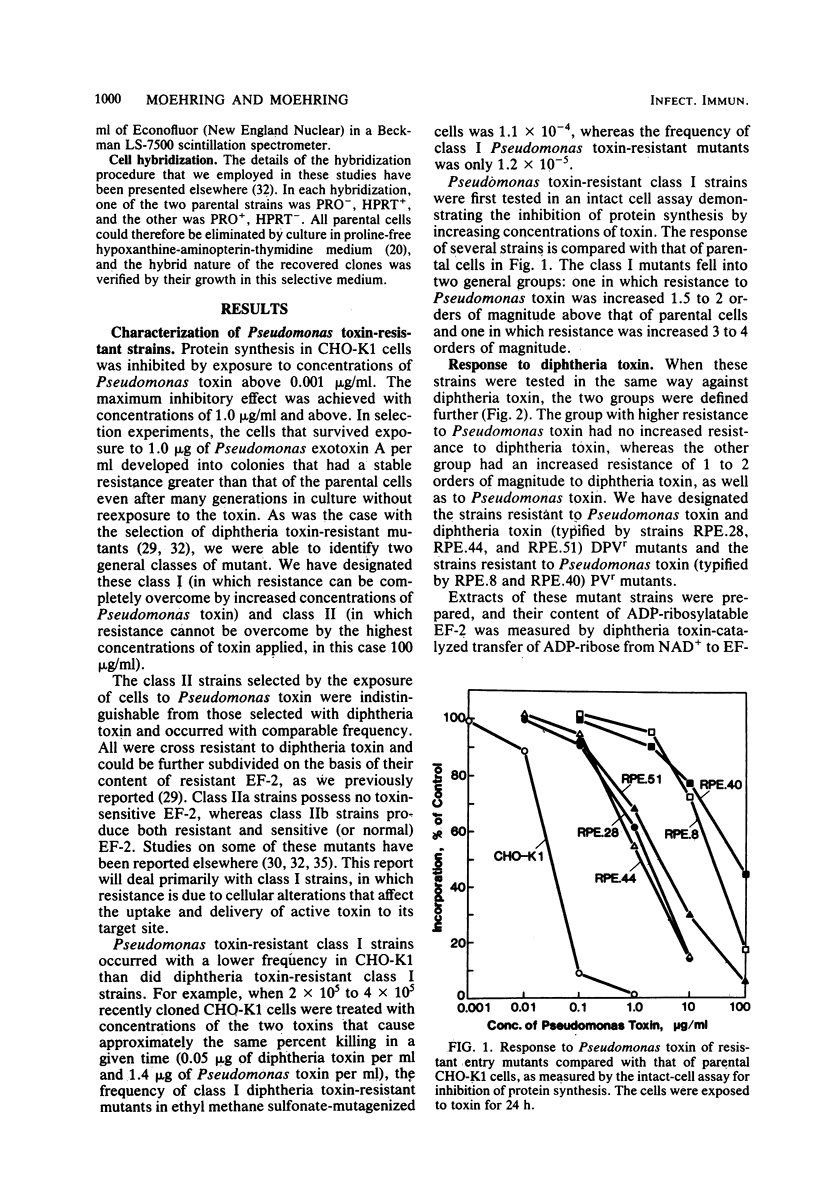
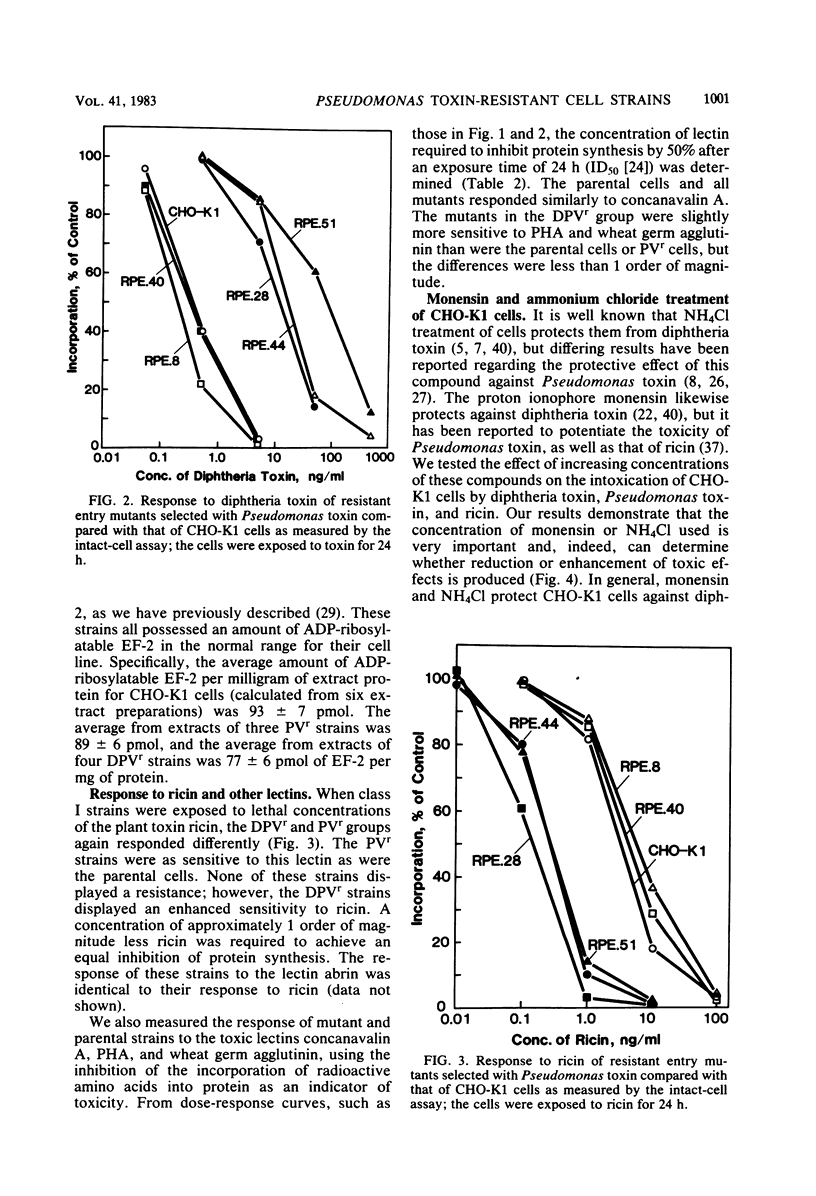
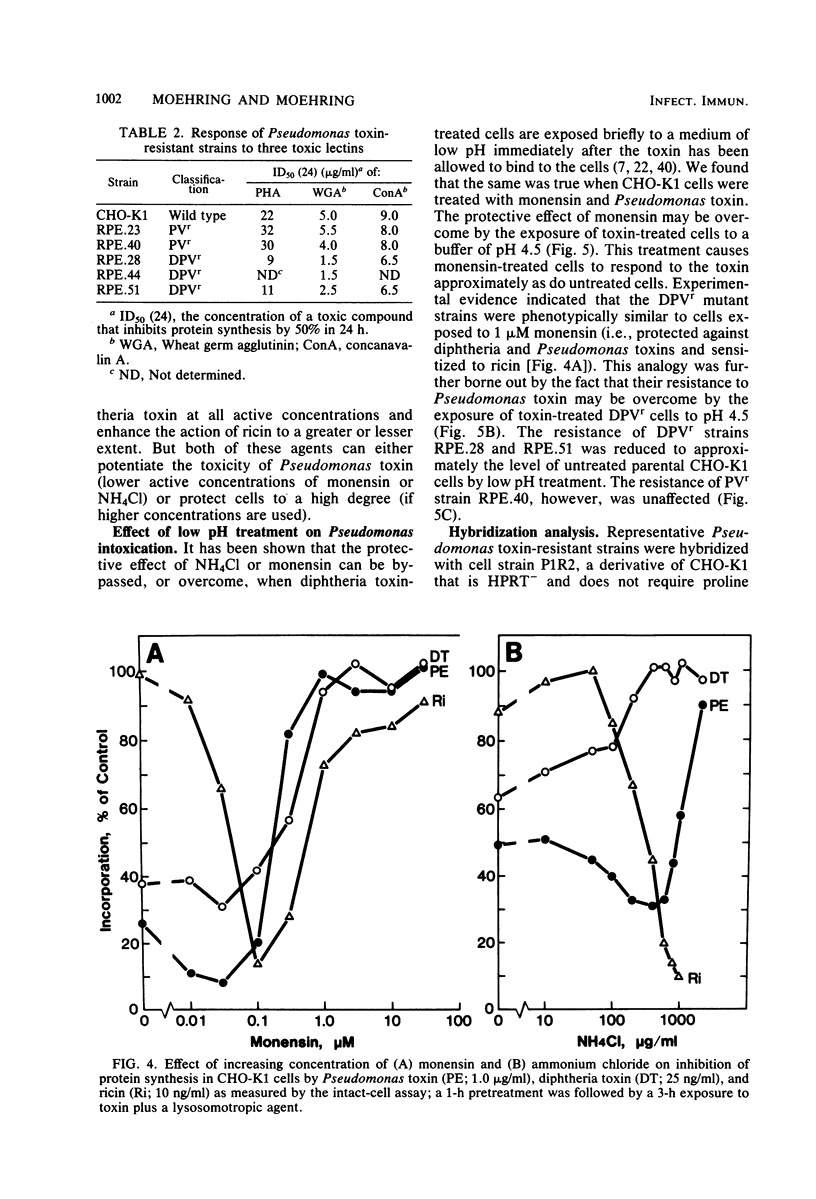
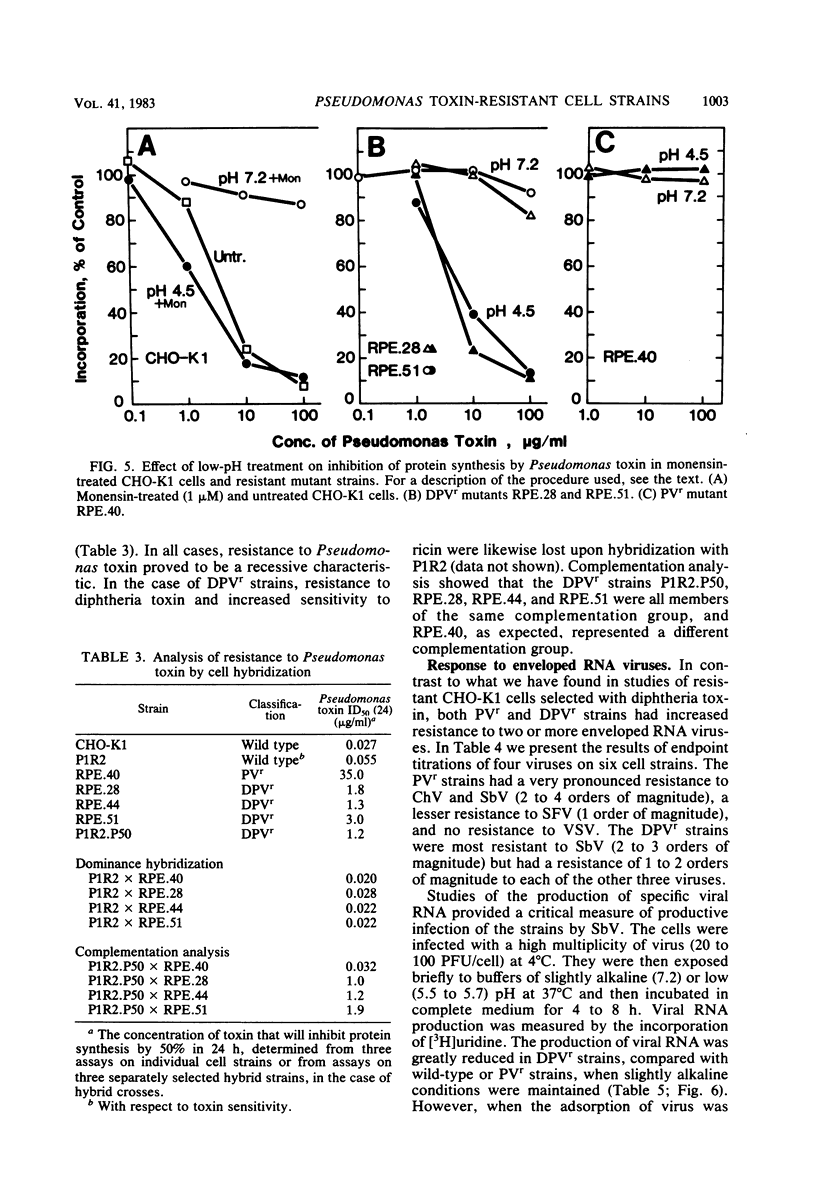
We have investigated two phenotypically distinct types of mutants of CHO-K1 cells that are resistant to Pseudomonas exotoxin A due to a defect in the delivery of active toxin to the target site in the cell, elongation factor 2. Both types contain normal levels of toxin-sensitive elongation factor-2. Hybridization studies have shown that these cells fall into two distinct complementation groups. One group, designated DPVr, is resistant to Pseudomonas toxin, diphtheria toxin, and four enveloped RNA viruses. This group is also hypersensitive to ricin. The resistance of this group is apparently related to a defect in a mechanism for the acidification of endocytic vesicles. The other group, designated PVr, is resistant to Pseudomonas toxin and to three enveloped RNA viruses. The resistance of this group appears to be related to a defect in a cellular mechanism required for the maturation of Sindbis virus that is likewise required for the entry of active Pseudomonas toxin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. H., Brown D. T. Inhibition of Sindbis virus maturation after treatment of infected cells with trypsin. J Virol. 1982 Feb;41(2):692–702. doi: 10.1128/jvi.41.2.692-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J. R., Moehring J. M., Moehring T. J. Binding and uptake of diphtheria toxin by toxin-resistant Chinese hamster ovary and mouse cells. Mol Cell Biol. 1983 Jul;3(7):1283–1294. doi: 10.1128/mcb.3.7.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Effect of ammonium chloride on receptor-mediated uptake of diphtheria toxin by Vero cells. Exp Cell Res. 1981 Aug;134(2):319–327. doi: 10.1016/0014-4827(81)90432-8. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Chin D., Eurey-Owens D., Scheffler I. E., Simon M. I. Biochemical and genetic characterization of three hamster cell mutants resistant to diphtheria toxin. J Cell Biol. 1979 Oct;83(1):116–125. doi: 10.1083/jcb.83.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D., Morris R. E., Saelinger C. B. Essential role of calcium in cellular internalization of Pseudomonas toxin. Infect Immun. 1982 Feb;35(2):715–720. doi: 10.1128/iai.35.2.715-720.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Diphtheria-toxin-resistant mutants of CHO cells affected in protein synthesis: a novel phenotype. Somatic Cell Genet. 1978 Sep;4(5):553–571. doi: 10.1007/BF01542926. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marsh M., White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982 Jan;58(Pt 1):47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H., Martin O. C., Muehl L. A. The exotoxin P. aeruginosa: a proenzyme having an unusual mode of activation. Biochem Biophys Res Commun. 1978 Mar 30;81(2):532–538. doi: 10.1016/0006-291x(78)91567-x. [DOI] [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Stookey M., Draper R. K. Monensin blocks the transport of diphtheria toxin to the cell cytoplasm. J Cell Biol. 1982 Apr;93(1):57–62. doi: 10.1083/jcb.93.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada E., Uchida T., Okada Y. Methylamine stimulates the action of ricin toxin but inhibits that of diphtheria toxin. J Biol Chem. 1981 Feb 10;256(3):1225–1228. [PubMed] [Google Scholar]
- Mento S. J., Siminovitch L. Isolation and preliminary characterization of Sindbis virus-resistant Chinese hamster ovary cells. Virology. 1981 Jun;111(2):320–330. doi: 10.1016/0042-6822(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Characterization of the diphtheria toxin-resistance system in Chinese hamster ovary cells. Somatic Cell Genet. 1979 Jul;5(4):453–468. doi: 10.1007/BF01538880. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J., Danley D. E. Posttranslational modification of elongation factor 2 in diphtheria-toxin-resistant mutants of CHO-K1 cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1010–1014. doi: 10.1073/pnas.77.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The spectrum of virus resistance of a KB cell strain resistant to diphtheria toxin. Virology. 1976 Feb;69(2):786–788. doi: 10.1016/0042-6822(76)90507-9. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Stinebring W. R., Merchant D. J. Survey of interferon production and sensitivity human cell lines. Appl Microbiol. 1971 Jul;22(1):102–105. doi: 10.1128/am.22.1.102-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Danley D. E., Moehring J. M. Codominant translational mutants of Chinese hamster ovary cells selected with diphtheria toxin. Somatic Cell Genet. 1979 Jul;5(4):469–480. doi: 10.1007/BF01538881. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. IV. Isolation of KB cells resistant to diphtheria toxin. Infect Immun. 1972 Oct;6(4):487–492. doi: 10.1128/iai.6.4.487-492.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. V. Concurrent resistance to ribonucleic acid viruses in diphtheria toxin-resistant KB cell strains. Infect Immun. 1972 Oct;6(4):493–500. doi: 10.1128/iai.6.4.493-500.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Selection and characterization of cells resistant to diphtheria toxin and pseudomonas exotoxin A: presumptive translational mutants. Cell. 1977 Jun;11(2):447–454. doi: 10.1016/0092-8674(77)90063-0. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Chinese hamster ovary cell mutants defective in the internalization of ricin. Mol Cell Biol. 1982 May;2(5):535–544. doi: 10.1128/mcb.2.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Enhancement of cytotoxicities of ricin and Pseudomonas toxin in Chinese hamster ovary cells by nigericin. Mol Cell Biol. 1981 Jun;1(6):552–559. doi: 10.1128/mcb.1.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Melsen L. R., Compans R. W. Effects of monensin on morphogenesis and infectivity of Friend murine leukemia virus. J Virol. 1982 Jun;42(3):1067–1075. doi: 10.1128/jvi.42.3.1067-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Iglewski B. H. Comparative toxicities of diphtherial toxin and Pseudomonas aeruginosa exotoxin A: evidence for different cell receptors. J Gen Microbiol. 1978 Oct;108(2):333–337. doi: 10.1099/00221287-108-2-333. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


