Abstract
Lectins have been increasingly important in the study of glycoproteins. Here we report a glycoprofiling method based on the covalent attachment of metal-chelating polymers to lectins for use in an ICP-MS-based assays. The labeled lectins are able to distinguish between glycoproteins covalently attached to a microtiter plate and their binding can be directly quantified by ICP-MS. Since each conjugate contains a different lanthanide, the assays can be conducted in a single or multiplex fashion, and may be readily elaborated to many different assay formats.
Keywords: lectin, glycoprotein, multiplexed assay, lanthanide, ICP-MS
BRIEFS
Employing a lanthanide-chelating polymer tag has enabled the use of lectins in a multiplexed glycoprofiling assay of common glycoproteins.
Introduction
Polysaccharides are crucial components in biological systems. For example, a variety of bacteria use cell-surface polysaccharides for protection from the environment and to mediate interactions with the host immune system.1, 2 In multicellular organisms, protein glycosylation states have been shown by chemical labeling to undergo alterations during embryonic development,3 and changes in cell morphology during apoptosis have been correlated with alterations in cell-surface glycan presentation.4–6 Furthermore, many recent studies have noted differences in degree and type of glycosylation in diseases such as diabetes7 and cancer.8–12 One of the ongoing challenges in these studies is the lack of assays which can rapidly and quantitatively evaluate the changing glycosylation patterns present at the glycoprotein or cellular level.
Lectins have provided a key tool in the analysis of the glycan repertoire in biological samples. There are over 70 lectins available from commercial sources and most lectins recognize a single sugar or a narrow range of similar sugars.13 Some are highly specific for a particular type of both sugar and linkage, as is observed with snowdrop lectin’s (GNA) preference for mannose, especially for terminal α-1,3-mannose residues.14 Others have varying affinities for several different sugars, such as wheat germ lectin (WGA), which has primary affinity for β-GlcNAc, but also recognizes GalNAc and sialic acid.
Recently, the use of lectins in glycoprofiling has focused on microarray analysis with either the lectin or the glycoprotein as the arrayed component. Arrayed lectins have been successfully printed and probed with fluorescently-labeled glycoproteins15–18 or even with whole cells19–21. Alternatively, glycan arrays have been probed with labeled lectins, allowing clinically relevant assays such as the differential detection of pancreatitis and pancreatic cancer.9, 22–25 Yamamoto et al. have described an interesting variant of this approach in a bead-based assay, where various glycoproteins were covalently attached to separate beads, mixed, probed with single biotinylated lectins, and analyzed by flow cytometry.26
The use of lectins in microarrays has proven the potential of rapid glycoprofiling as an important analytical tool. However new techniques which do not rely upon spatial arrays will facilitate expanding the scope of glycoprofiling to more complex assays such as the analysis of single cells from mixed populations by flow cytometric methods. The use of single lectins with flow cytometry has already shown the potential of this approach in identifying new biomarkers on apoptotic cells.4 The ability to use multiple lectins simultaneously in a similar approach would allow rapid glycoprofiling and allow the relative glycosylation patterns on a panel of glycoproteins or between cells to be directly compared.
We have recently introduced a set of new reagents for antibody labeling with metal-chelating polymers for use in immunoaffinity assays with inductively coupled plasma mass spectrometry (ICP-MS)-based detection methods.27 This approach has the potential for rapid massively multiplexed assays.28 The combination of the high sensitivity of ICP-MS (comparable to enzyme linked assays), over seven orders of magnitude in dynamic range, and near absence of signal overlap between collection channels makes ICP-MS an ideal detection method for multiplexed assays.29, 30 These assays have the advantage of speed, and, in comparison with single probe assays, have the added benefit of allowing a direct comparison of the abundances of simultaneously employed probes.
Recently, an interesting example of the potential of multiplexed lectin assays was presented by Pilobello et al.31 They describe the labeling of two different cell extracts with different fluorophores which were then mixed and simultaneously probed on the same lectin microarray. This duplex assay has demonstrated the useful quantitative information which can be obtained by directly comparing glycosylation profiles. Here we report a general method for labeling lectins to be used in multiplexed lectin assays with ICP-MS detection. In this report, we demonstrate the first five-plex assay with lectins on commercial glycoproteins. The tagged lectins are shown to reproducibly and simultaneously probe commercially-available glycoproteins, demonstrating the potential of using lectins in a multiplexed fashion with ICP-MS based detection.
Materials and Methods
Buffers and Salts
Hepes, sodium phosphate, guanidinium hydrochloride, and Tris base were purchased from BioShop (University of Toronto). Molecular biology tested bovine serum albumin (BSA), ovalbumin grade VII, porcine stomach mucin type III (PSM), bovine submaxillary mucin type I-S (BSM), fetal calf fetuin, yeast invertase grade VII, fetal calf asialofetuin type I, concanavalin A type V (ConA), Triticum vulgaris lectin (WGA), Arachis hypogaea lectin (PNA), Galanthus nivalis lectin (GNA), WGA-gold conjugate, MgCl2, MnSO4, CaCl2, natural abundance LnCl3 (TbCl3, EuCl3, GdCl3, YbCl3, DyCl3), β-mercaptoethanol, 5,5’-dithiobis(2-nitrobenzoic acid) (DTNB; Ellman’s reagent), Tween-20, d-(+)-mannose, α-lactose monohydrate, α-cyano-4-hydroxycinnamic acid (αCHCA), and sodium citrate were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). d-(+)-Galactose was purchased from Acros. N-acetylglucosamine was purchased from BioShop (Burlington, Ontario Canada). Trifluoroacetic acid (TFA) was purchased from Caledon. Acetonitrile was purchased from EMD. Sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC), 500 mM tris(2-carboxyethyl)phosphine (TCEP) Bond-Breaker solution neutral pH, bicinchoninic acid (BCA) protein assay kit, and ReactiBind maleic anhydride-activated strip plates were purchased from Pierce. Ultrapure concentrated 35% HCl was purchased from Seastar Chemicals Inc. The Multi Element Solution Standard 1 and 1000 mg/L Ir/10% HCl standard were purchased from Spex CertiPrep and diluted with 10% ultrapure HCl to working concentrations of 2 ppb. All buffers were made using MilliQ water (Millipore).
Polymer Synthesis
Polymer containing diethylenetriaminepentaacetic acid (DTPA) was synthesized similarly to Lou et al.,27 with the exception that the free thiol end group was not elaborated and that a DTPA ligand was used rather than a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) lanthanide ligand.
Protein-Polymer Conjugation
Solid DTPA-containing polymer was dissolved (5.6 mg/mL, 255 µM) in 100 mM Hepes pH 7.5 to give a stock solution. To the stock solution, TCEP and the desired LnCl3 salt were added to give a solution of polymer (39 µM), TCEP (50 mM), and LnCl3 (4 mM) which was incubated overnight at 37°C. Solid BSA, ConA, WGA, PNA, and GNA were each diluted to 1.1 mg/mL in lectin buffer (100 mM Hepes pH 7.5, 1 mM CaCl2, 1 mM MgCl2, 5 mM MnSO4) and stored at 4°C. Freshly prepared Sulfo-SMCC solution (75 µL, stock 0.96 mg/mL, 2.2 mM in water; for ~100-fold excess in reaction) was added to 750 µL of protein solution and incubated at room temperature for six hours to allow the NHS-ester to react with protein lysine residues. Excess unreacted Sulfo-SMCC was removed from the protein solution by washing three times with lectin buffer in a 30 kDa Biomax Ultrafree molecular-weight cutoff (MWCO) spin filter (Millipore). Excess TCEP and Ln3+ were removed from the polymer solution by washing three times with 25 mM sodium citrate pH 7.0 in a 5 kDa Ultrafree MWCO spin filter (Millipore). After resuspension to their original volumes, the activated protein (700 µL) conjugate was mixed with Ln3+-loaded polymer solution (77 µL) (~1.5-fold polymer excess) and incubated overnight at 37°C to allow the polymer thiol to react with the maleimide. Excess polymer was removed by washing three times with lectin buffer in a 30 kDa MWCO spin filter. After resuspension to the original volume in lectin buffer, the protein-polymer conjugate stock solutions were stored at 4°C. Full activity of the conjugates was retained for a minimum of one month.
A sample of each polymer and protein-polymer conjugate stock solution was diluted 10,000-fold in water, mixed with an equal volume of Ir internal standard (final 1 ppb Ir concentration), and the Ln3+ content determined without further dilution by ELAN DRCPlus ICP-MS (Perkin-Elmer SCIEX) as previously described.32 An additional external Multi-Element standard sample was mixed with an equal volume of Ir internal standard (final 1 ppb each Ir and Ln3+ concentration). To determine the protein concentration of the protein-polymer stock, an aliquot was first washed three times in a 30 kDa MWCO spin filter with 100 mM Hepes pH 7.5 to remove manganese, followed by BCA assay using BSA as the standard.
MALDI Analysis
A sample of each native protein and protein-maleimide conjugate prior to mixing with the polymer solution was mixed 1:1 with α-CHCA matrix (50:50 acetonitrile:water, 0.1% TFA), spotted onto a stainless steel target plate, air-dried, and subjected to MALDI-MS (MALDI microMX (Waters); positive linear mode calibrated with BSA). The difference in mass was used to quantify the number of Sulfo-SMCC groups attached (219.1 amu added mass per Sulfo-SMCC).
DTNB Assay
The polymer concentration was determined from thiol end-group analysis using the DTNB assay.33 Briefly, 100 µL of 8.4 mg/mL polymer in 100 mM Hepes pH 7.5 buffer were incubated with 10 µL 0.5 M TCEP overnight at room temperature. Unreacted TCEP was removed by washing three times in a 5 kDa MWCO spin filter with 500 µL 25 mM sodium citrate pH 5.0. Aliquots were analyzed by measuring the absorbance at 412 nm in guanidium hydrochloride/EDTA solution and quantified using β-mercaptoethanol as a standard.
Microtiter Plate Assay
BSA, ovalbumin, PSM, BSM, fetuin, invertase, and asialofetuin were each diluted to 1 mg/mL in water. One hundred microliters of each protein were added to separate wells of the maleic-anhydride activated strip plate and incubated overnight at room temperature while shaking. The protein sample was removed and 1 M Tris pH 7.5 (300 µL) was added to each well and incubated for one hour while shaking at room temperature. This served to both wash away any unbound protein and to quench any unreacted activated groups on the plate surface. The Tris solution was removed and a protein-polymer conjugate (100 µL, 1:100 dilution in lectin buffer) was added, followed by shaking at room temperature for one hour. The conjugate solution was removed and the wells rinsed three times with PBS-Tween (300 µL, 100 mM sodium phosphate pH 7.2 containing 150 mM NaCl and 0.05% Tween-20). An HCl-Ir standard (100 µl, final 1 ppb Ir concentration) was added to each well and analyzed by ICP-MS as above. An additional well contained the external Multi-Element and Ir standard sample (final 1 ppb Ir and Ln3+ concentration) as above.
The saccharide inhibition studies were performed in the same format as the single conjugate assays above to confirm the selectivity of the lectins were not compromised by the polymer labeling. The lectin-conjugates were diluted in a solution of the indicated concentration (50–500 mM) of mono- or disaccharide and incubated at room temperature for 10 minutes before addition to the microtiter plate wells containing the immobilized glycoprotein. The inhibition resulting from the soluble saccharides were then calculated as a percentage of the control signal in the absence of inhibiting saccharide.
Data Analysis
The ICP-MS output data was transferred to Excel for analysis. For each metal analyzed, the raw ICP-MS data in counts were converted to ppb by dividing by the counts for the respective external 1 ppb standard. The 1 ppb Ir internal sample standard was used to compensate for potential long term instrumental drift. The Ln3+ ppb were then normalized by the 1 ppb Ir internal sample standard. For the plate assays, the Ir-normalized data were then reported as the mean ± standard deviation of at least six independent experiments. To further characterize the polymer and conjugate stocks, the ppb values were converted to micromolar values by dividing by the isotopic mass of the element. Ratios of Ln3+/protein multimer or Ln3+/polymer were then calculated by dividing by the molar concentration of monomeric protein or polymer. This Ln3+/protein multimer ratio was also used to normalize the respective ConA and WGA signals to allow comparison with the results obtained by Pilobello et al.18
Results
Lectin Labeling with Lanthanide-Chelating Polymers
In previous work from our laboratory, we conjugated DOTA-containing, maleimide-terminated polymers to free cysteines of partially-reduced antibodies.27 Unlike antibodies, however, lectins vary greatly in their structure and amino acid content, and in addition, some lectins such as WGA require specific disulfide bonds for proper folding. Therefore, an alternative conjugation procedure was developed which covalently attaches the metal-chelating polymer to lysine side chains of the lectins.
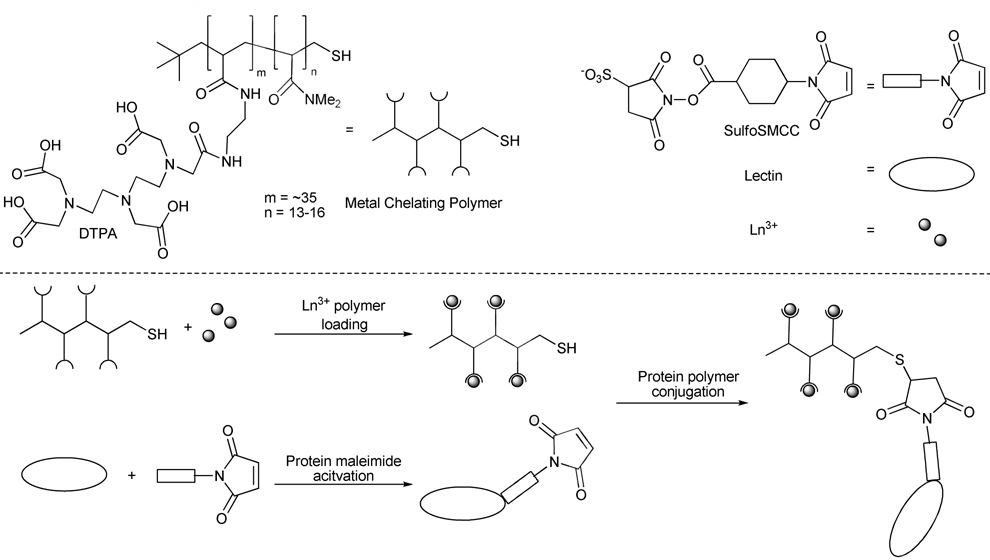
The steps involved in the lectin labeling with the lanthanide-containing polymers are shown in Figure 1. The polymer was obtained with a synthesis similar to that which we have previously used but the terminal thiol was not elaborated further.28 Sulfo-SMCC was chosen for conjugation of the lectin to the metal-chelating polymer, due to its water solubility and its ability to crosslink lysine amino groups of the proteins with the polymer’s terminal thiol. Due to differences in amino acid content and multimeric status between the various lectins, the reaction conditions were chosen to be as general as possible, and were not optimized specifically for any of the proteins (Table 1).
Figure 1.
Synthesis of lectin-polymer conjugates. The lectin (shown as a monomer for clarity) is modified on surface lysine residues with Sulfo-SMCC to allow the conjugation of a thiol-containing polymer pre-loaded with lanthanide ions.
Table 1.
Proteins Used in Conjugation Reactions and Their Specificity for Glycan Structures.
| Protein | Glycan specificity |
|---|---|
| BSA | Non-lectin |
| ConA | α-Man (branched > linear) > β-GlcNAc, α-Glc |
| WGA | β-GlcNAc (trimer > dimer > monomer) > Sia > GalNAc |
| PNA | Gal-β-1,3-GalNAc > terminal Gal |
| GNA | α-1,3-Man and α-1,6-Man |
The lectins were first activated with Sulfo-SMCC and the number of maleimide groups introduced on each lectin was determined by MALDI-MS analysis (Table 2 and Figure S1). Different numbers of maleimide groups were added to each protein tested; however, this number was highly reproducible for each individual protein. Under these reaction conditions, GNA was the only lectin in which less than 100% of protein monomers received at least one maleimide group.
Table 2.
Characterization of Protein-Sulfo-SMCC-Polymer Conjugation Reactions.
| Protein | Ln3+ | Monomer molecular weighta | Average # maleimide / monomerb | Protein multimeric state | Average Ln3+/multimer | Average polymer/multimer | |
|---|---|---|---|---|---|---|---|
| Native | Post activation | ||||||
| BSA | Tb | 66441 | 67889 | 6.6 | 1 | 16 | 0.65 |
| ConA | Eu | 25535 | 26004 | 2.1 | 4 | 38 | 1.5 |
| WGA | Gd | 17069 | 17279 | 0.96 | 2 | 8.4 | 0.43 |
| PNA | Yb | 25168 | 25589 | 1.9 | 4 | 16 | 0.74 |
| GNA | Dy | 12024 | 12269c | <1c | 4 | 21 | 0.74 |
As measured by MALDI.
Each SSMCC = 219.1 amu added.
GNA was the only protein where a significant amount of unlabeled protein remained after incubation with Sulfo-SMCC.
The number of maleimide derivatizations per protein needed to be controlled and this was accomplished by removing excess Sulfo-SMCC reagent prior to polymer conjugation. Wängler et al. observed with derivatized antibodies that it was the number, not size, of modifications that had the greatest effect on affinity and specificity.34 Similar limitations on the number of modified sites have been noted for labeled lectins in the Vector Laboratories’ catalog. Indeed, failure to remove excess Sulfo-SMCC prior to polymer conjugation led to an increase in the number of maleimide additions and an increase in the Ln3+/lectin ratio, but a decrease in binding to glycoproteins (data not shown).
Since hydrophilic polymers usually contain a significant amount of water by weight, a DTNB assay was used to accurately determine the concentration of polymer from the free thiols present at the polymers termini. An average of 70% of the expected value based on mass was found. Additionally, control experiments with Ln3+-loaded polymer demonstrated a 70% recovery of polymer was typical after washing in the spin filter (5 kDa MWCO). These corrected numbers for polymer concentration were used in all further calculations (vide infra).
Initial attempts to synthesize the protein-polymer conjugate prior to loading the Ln3+ into the polymer led to unacceptably high background levels of Ln3+ in the subsequent lectin assays. This presumably arises from nonspecific Ln3+ binding to the proteins that could not be easily removed even after repeated washing with buffer. Reduced background levels of Ln3+ were obtained by incubating the polymer with the desired Ln3+ followed by extensive washing of the metal-loaded polymer with a weakly chelating citrate buffer prior to protein conjugation.
The reaction conditions for polymer conjugation to the maleimide-derivatized protein were also deliberately generalized to allow for future work with new proteins and lectins. The polymer stock was incubated overnight at 37°C with an excess of TCEP (1300-fold) to ensure complete disulfide reduction, and with a 100-fold excess of Ln3+ to ensure maximal metal loading. After extensive washing of the reduced and metal-loaded polymer, it was incubated with the maleimide-activated proteins at a final concentration of 25 µM, representing a 1–3 fold molar excess of polymer : protein multimer.
After removing the unconjugated polymer in a 30 kDa MWCO spin filter, the final concentration of protein-polymer conjugate was determined by a BCA protein assay, and typically approximately 60% of protein was recovered after the conjugation chemistry. While the colorimetric BCA assay involves metal ions, control experiments did not show any interference from the metal-binding polymer. The number of polymers conjugated per lectin was calculated based on the average number of lanthanides per polymer (determined by DTNB and ICP-MS assays) and the number of lanthanides per protein conjugate (determined by BCA and ICP-MS assays; Table 2).
Microtiter Multiplexed Lectin Plate Assay
Stock solutions of the proteins listed in Table 3 were allowed to react overnight with the maleic anhydride-activated microtiter plate (Pierce) (Figure 2), followed by a blocking step with 1 M Tris pH 7.5. Standard protein assays were not sensitive enough to measure the small amount of protein bound to the surface of the plates, either directly or indirectly by assaying a reduction of protein concentration from the incubation solution.
Table 3.
Proteins and Associated Glycans Attached to Maleic-Anhydride Activated Strip Plate.
| Protein | Sugars in glycan† | Reference |
|---|---|---|
| BSA | Non-glycoprotein | |
| ovalbumin | Man, GlcNAc, Gal | 56–58 |
| PSM | GlcNAc, GalNAc, Fuc, Gal | 59 |
| BSM | GlcNAc, GalNAc, Fuc, Gal, Sia | 60 |
| fetuin | Man, GlcNAc, Gal, Sia | 61, 62 |
| invertase | Man, GlcNAc | 39, 63, 64 |
| asialofetuin | Man, GlcNAc, Gal (<0.5% Sia) | 61, 62 |
The glycan structures of ovalbumin, fetuin, invertase, and asialofetuin have been determined. However, BSM and PSM glycans are only fully known by composition due to large size and heterogeneity.
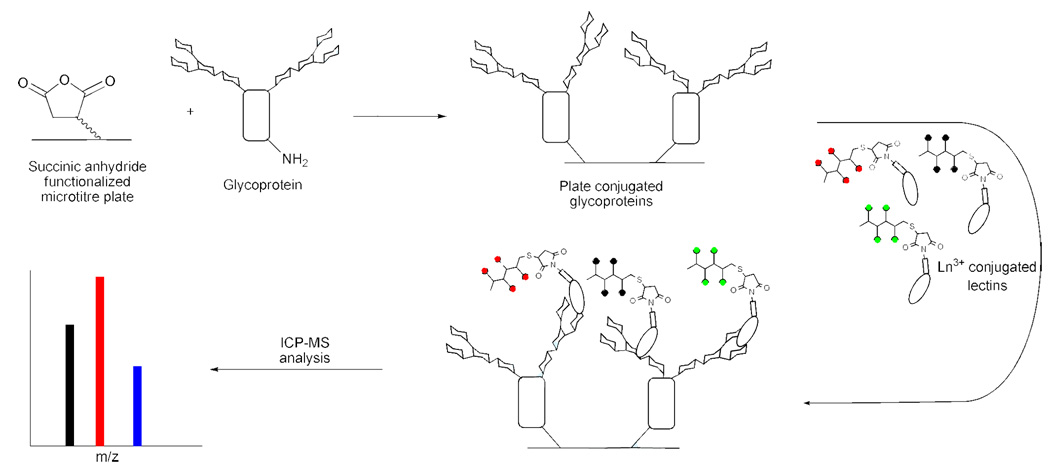
Figure 2.
Microtiter-plate assay to probe glycoproteins with polymer-conjugated lectins. The glycoproteins were covalently attached to the wells of a microtiter plate by the reaction of protein surface lysines with the succinic anhydride groups formed on the plate surface. After washing, single or multiple Ln3+-polymer-lectin conjugates were added to each well to allow binding to the glycoproteins. The wells were washed with PBS-Tween, then concentrated HCl was added to release the Ln ions and the resulting solutions were analyzed by ICP-MS.
To find a working concentration for the plate assay, the protein-polymer conjugate stocks (~0.6 mg/mL total protein) were assayed at a 100-fold and 1000-fold dilutions. Using the lectins at a 6 µg/mL working concentration gave excellent reproducibility, an acceptable signal to noise, and was therefore used as the standard dilution for the plate assays. For the single probe assay, this gave a final conjugate amount of ~0.6 µg, or 60–175 nM due to differences in molecular weight and multimeric composition of the lectins. For the five-conjugate multiplex reaction, the conjugates were each kept at the same concentration as used with the single assay; therefore, the final total conjugate amount was ~3 µg/well. After incubation with the protein-polymer conjugate(s), each well was washed with PBS-Tween 20, then concentrated HCl was added to release the Ln3+ and the samples were analyzed by ICP-MS (Figure 3).
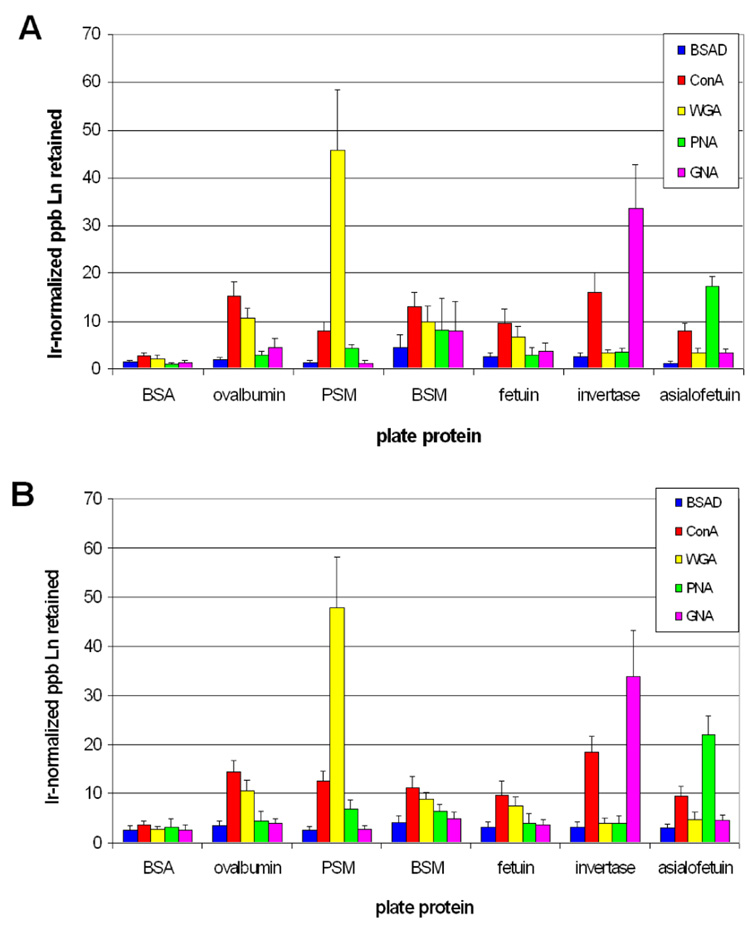
Figure 3.
Differential lectin-polymer conjugate binding to immobilized glycoproteins in single conjugate (A) and multiplex (B) assays. The values represent the average ± standard deviation of the Irnormalized retained ppb Ln3+ for at least six replicate experiments.
Microtiter Multiplexed Lectin Plate Assay
Several observations are immediately evident from Figure 3. First, the BSA-conjugate (non-lectin protein-conjugate control) and the BSA plate protein sample (non-glycoprotein control) each showed no differential affinity towards any of the plate proteins or lectin-polymer conjugates. Second, each lectin-conjugate exhibited differential binding to each glycoprotein. This demonstrates that attaching the ~22 kDa metal-chelating polymer to the lectins did not notably affect their sugar specificities. For example, the observed glycoprotein specificity for ConA is consistent with literature results which have examined the affinity of ConA for glycopeptides derived from ovalbumin and fetuin.35 The of binding of ConA to invertase is also expected due to the large mannose-rich glycan present on this glycoprotein.36, 37 The observed binding pattern for PNA with fetuin and asialofetuin is also consistent with the literature.38 GNA bound strongly to invertase but not to ovalbumin or fetuin, consistent with previous precipitation assays.14 The observed lower binding of ConA to invertase relative to GNA is likely due to the fact that glycan structure of invertase contains a greater portion of linear (rather than highly branched) α-1,6-Mannosides.39 However, since invertase is 50% glycan by weight, ConA binding is still observed even with the reduced preference for this mannose-containing structure. As an additional control, commercially-available WGA-gold nanoparticle conjugate was tested in the same fashion as the lectin-polymer conjugates and analyzed by ICP-MS. The same plate protein specificity trends were observed with this conjugate as with the WGA-polymer conjugate (Figure S2). When normalized for Ln/multimer signal (Figure S3), the ConA and WGA results are also consistent with lectin microarray work by Pilobello et al.18 Saccharide inhibition studies also gave the expected pattern of specificity (Figure S4).40–45 It should be noted that the concentrations of mono or disaccharide required to achieve inhibition were higher than expected; this is probably due to the multivalent interactions associated with the density of the glycan presentation on the microtitre plate. This effect has been noted in previous studies‥46, 47 Third, each conjugate exhibited the same behavior in the multiplex mixture as in single-conjugate assays. This demonstrates that there is no interference between the conjugates at the concentrations used in the assays and importantly there is no exchange of lanthanides between the polymers.
Since each lectin has a range of affinities for different sugars or glycans, it is possible that in a multiplexed assay a competition for glycan binding sites may occur, leading to a differential signal compared with the single lectin assays. Although this was not the case using a ~100 nM solution of each lectin (above), at higher lectin concentrations, a competition between lectins may allow for a greater differentiation between high-affinity and lower-affinity glycan recognition for a given lectin. Preliminary experiments to explore this facet of the multiplexed assay were done with a ten-fold higher concentration of the protein-polymer conjugates (Figure S5). As would be expected, these assays showed an overall increase in the lanthanide signal. However, a decrease in the binding of each conjugate in the multiplexed assay when compared to the single lectin assays was seen, suggesting that the lectins are competing for the glycan binding sites. Comparison of the lectin binding profiles at the higher (~1 µM) and the lower lectin concentrations (~100 nM) did not show a significantly different binding recognition pattern with the chosen glycoproteins. Further studies with synthetic glycans will allow the exploration of potential of competitive lectin binding experiments.
Discussion
Lectins provide a readily accessible set of tools for determining the glycosylation states present in a given biological sample. They are less specific than antibodies but this allows a broader range of glycans to be interrogated, and when multiple different lectins are employed with varying specificities, a fingerprint of lectin activity can be used to identify glycosylation patterns. Although these fingerprints are by no means as high resolution as mass spectrometric techniques, they are more amenable to high-throughput analysis, as little sample preparation is necessary.
Lectin microarrays have been very successful at establishing the glycan profiles of homogenous samples such as a solution of glycoproteins, a suspension of liposomes or even a homogenous cell population. However, these studies rely on one interaction at a time, be it on a single lectin microarray with one homogeneous glycoprotein solution, or a single lectin on array of glycoproteins. The ability to use multiple lectins in a multiplexed solution assay has not previously been investigated. Our results show that lectins can be used in a multiplexed assay and the results obtained are consistent with those of single lectin assays from previous literature examples.18 Furthermore, the protocol for derivatizing BSA and the lectins with Sulfo-SMCC and lanthanide-chelating polymer was deliberately designed to be a general one and should therefore be useful for other proteins. While the need for full lectin activity imposes a limit on the degree of derivatization, the multiple metal-binding sites within each polymer ensures that the attachment of a single polymer to a lectin multimer allows sufficient signal to noise at the conjugate concentrations used in the plate assay. Since there are 13 stable lanthanides, even the natural abundance lanthanide salts would allow for highly multiplexed lectin assays. However, because of the power of ICP-MS to resolve isotopic masses, extending the series to isotopically-enriched lanthanides would easily allow at least 21 probes, even in the presence of interfering adducts of equal mass.28
As with microarrays, there are some potential limitations to this technique. First, care must be taken that conjugation would not disrupt lectin activity. This may be of particular concern for some sialic acid-binding lectins which employ lysines in their active site for charge interactions.48 However, incubation with substrate prior to reaction with Sulfo-SMCC could potentially block such important lysines and prevent their derivatization. Second, while the prep-to-prep trends for each protein-conjugate are consistent, absolute numbers have some variation due to conjugation efficiency, Ln3+ loading of the polymer, and overall activity after purification. As suggested by Carlsson et al.,7 normalizing the ICP-MS signal to a known glycoprotein standard led to improved prep-to-prep quantitation (data not shown). Third, many commercially-available plant and animal lectins are themselves glycoproteins and could potentially cross-react with each other. In fact, ConA has been used to precipitate LBA.49 Therefore, any lectins used in a multiplex assay must be non-glycosylated. While few bacterial lectins have been described and characterized, none have yet been found to be glycosylated; thus, the identification of new bacterial lectins may provide useful non-glycosylated probes.17 Alternatively, chemical modification or enzymatic removal of any lectin glycan could potentially avoid any probe-probe interactions. Chen et al. have recently used periodate oxidation and dipeptide ligation to block glycans on antibodies while the enzymatic digestion of the glycans did not prevent cross-reaction with lectins.50 There is not yet enough information to speculate on which would be a more generally useful technique. Alternatively, synthetic “lectins” composed of rigid aromatic backbones51 or boronic acids could be used.52–55
Recently our group has developed a flow cytometer interface for an ICP-MS mass spectrometer.65 Using this instrument and a multiplexed lectin-based assay, it will be possible to type individual bacterial cells as well as to investigate differences in cellular glycosylation between diseased and non-diseased cells. The sensitivity of ICP-MS for the lanthanides and the low natural abundance of lanthanides should allow these analysis using the multiplexed lectin approach we have described.32
Supplementary Material
Supporting figures S1–S5 are available.
ACKNOWLEDGMENT
We gratefully acknowledge financial support from NIH grant #GM076127-01A1 for the support of this work. We also thank Professor M. Winnik for helpful discussions.
REFERENCES
- 1.Comstock LE, Kasper DL. Cell. 2006;126:847–850. doi: 10.1016/j.cell.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Whitfield C. Annu. Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz S, Frey B, Sheriff A, Gaipl US, Beer A, Voll RE, Kalden JR, Herrmann M. Cytometry A. 2006;69:230–239. doi: 10.1002/cyto.a.20206. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport EM, Kurmyshkina OV, Bovin NV. Biochemistry (Mosc) 2008;73:393–405. doi: 10.1134/s0006297908040032. [DOI] [PubMed] [Google Scholar]
- 6.Sarter K, Mierke C, Beer A, Frey B, Fuhrnrohr BG, Schulze C, Franz S. Autoimmunity. 2007;40:345–348. doi: 10.1080/08916930701356804. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson J, Gullstrand C, Ludvigsson J, Lundstrom I, Winquist F. Talanta. 2008;76:333–337. doi: 10.1016/j.talanta.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Patwa TH, Zhao J, Anderson MA, Simeone DM, Lubman DM. Anal. Chem. 2006;78:6411–6421. doi: 10.1021/ac060726z. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Patwa TH, Qiu WL, Shedden K, Hinderer R, Misek DE, Anderson MA, Simeone DM, Lubman DM. J. Proteome Res. 2007;6:1864–1874. doi: 10.1021/pr070062p. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Patwa TH, Xu L, Shedden K, Misek DE, Tuck M, Jin G, Ruffin MT, Turgeon DK, Synal S, Bresalier R, Marcon N, Brenner DE, Lubman DM. J. Proteome Res. 2008;7:1693–1703. doi: 10.1021/pr700706s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott K, Aoki K, Lim J, Porterfield M, Johnson R, O'Regan R, Wells L, Tiemeyer M, Pierce M. J. Proteome Res. 2008;7:1470–1480. doi: 10.1021/pr700792g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda K, Katagiri T, Shimada T, Irie S, Sato TA, Nakamura Y, Daigo Y. J. Proteome Res. 2007;6:3475–3483. doi: 10.1021/pr070103h. [DOI] [PubMed] [Google Scholar]
- 13.Sharon N, Lis H. Lectins. 2nd ed. Boston: Kluwer Academic Publishers; 2003. [Google Scholar]
- 14.Shibuya N, Goldstein IJ, Van Damme EJ, Peumans WJ. J. Biol. Chem. 1988;263:728–734. [PubMed] [Google Scholar]
- 15.Chen P, Liu YK, Kang XN, Sun L, Yang PY, Tang ZY. J. Cancer. Res. Clin. Oncol. 2008;134:851–860. doi: 10.1007/s00432-008-0357-7. [DOI] [PubMed] [Google Scholar]
- 16.Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, Hirabayashi J. Nat. Methods. 2005;2:851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- 17.Hsu K-L, Gildersleeve JC, Mahal LK. Mol. Biosyst. 2008;4:654–662. doi: 10.1039/b800725j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. ChemBioChem. 2005;6:985–989. doi: 10.1002/cbic.200400403. [DOI] [PubMed] [Google Scholar]
- 19.Tateno H, Uchiyama N, Kuno A, Togayachi A, Sato TA, Narimatsu H, Hirabayashi J. Glycobiology. 2007;17:1138–1146. doi: 10.1093/glycob/cwm084. [DOI] [PubMed] [Google Scholar]
- 20.Hsu K-L, Mahal LK. Nat. Protoc. 2006;1:543–549. doi: 10.1038/nprot.2006.76. [DOI] [PubMed] [Google Scholar]
- 21.Hsu K-L, Pilobello KT, Mahal LK. Nat. Chem. Biol. 2006;2:153–157. doi: 10.1038/nchembio767. [DOI] [PubMed] [Google Scholar]
- 22.Manimala JC, Roach TA, Li Z, Gildersleeve JC. Angew. Chem. Int. Ed. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 23.Song X, Xia B, Lasanajak Y, Smith DF, Cummings RD. Glycoconj. J. 2008;25:15–25. doi: 10.1007/s10719-007-9066-8. [DOI] [PubMed] [Google Scholar]
- 24.de Boer AR, Hokke CH, Deelder AM, Wuhrer M. Anal. Chem. 2007;79:8107–8113. doi: 10.1021/ac071187g. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Lee MR, Shin I. Nat. Protoc. 2007;2:2747–2758. doi: 10.1038/nprot.2007.373. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K, Ito S, Yasukawa F, Konami Y, Matsumoto N. Anal. Biochem. 2005;336:28–38. doi: 10.1016/j.ab.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Lou X, Zhang G, Herrera I, Kinach R, Ornatsky O, Baranov V, Nitz M, Winnik MA. Angew. Chem. Int. Ed. 2007;46:6111–6114. doi: 10.1002/anie.200700796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornatsky OI, Kinach R, Bandura DR, Lou X, Tanner SD, Baranov VI, Nitz M, Winnik MA. J. Anal. At. Spectrom. 2008;23:463–469. doi: 10.1039/b710510j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner SD, Baranov VI, Bandura DR. Spectrochim. Acta Part B At. Spectrosc. 2002;57:1361–1452. [Google Scholar]
- 30.Baranov VI, Quinn Z, Bandura DR, Tanner SD. Anal. Chem. 2002;74:1629–1636. doi: 10.1021/ac0110350. [DOI] [PubMed] [Google Scholar]
- 31.Pilobello KT, Mahal LK. Curr. Opin. Chem. Biol. 2007;11:300–305. doi: 10.1016/j.cbpa.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Ornatsky OI, Lou X, Nitz M, Schafer S, Sheldrick WS, Baranov VI, Bandura DR, Tanner SD. Anal. Chem. 2008;80:2539–2547. doi: 10.1021/ac702128m. [DOI] [PubMed] [Google Scholar]
- 33.Ellman GL. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Wangler C, Moldenhauer G, Eisenhut M, Haberkorn U, Mier W. Bioconjug. Chem. 2008;19:813–820. doi: 10.1021/bc700308q. [DOI] [PubMed] [Google Scholar]
- 35.Baenziger JU, Fiete D. J. Biol. Chem. 1979;254:2400–2407. [PubMed] [Google Scholar]
- 36.Mislovicova D, Chudinova M, Vikartovska A, Gemeiner P. J. Chromatogr. A. 1996;722:143–149. doi: 10.1016/0021-9673(95)00530-7. [DOI] [PubMed] [Google Scholar]
- 37.Mislovicova D, Masarova J, Svitel J, Gemeiner P. Int. J. Biol. Macromol. 2002;30:251–258. doi: 10.1016/s0141-8130(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 38.La Belle JT, Gerlach JQ, Svarovsky S, Joshi L. Anal. Chem. 2007;79:6959–6964. doi: 10.1021/ac070651e. [DOI] [PubMed] [Google Scholar]
- 39.Lehle L, Cohen RE, Ballou CE. J. Biol. Chem. 1979;254:2209–2218. [PubMed] [Google Scholar]
- 40.Lotan R, Skutelsky E, Danon D, Sharon N. J. Biol. Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- 41.Goldstein IJ, Hollerman CE, Merrick JM. Biochim. Biophys. Acta. 1965;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- 42.Van Damme EJM, Allen AK, Peumans WJ. FEBS Lett. 1987;215:140–144. [Google Scholar]
- 43.Nagata Y, Burger MM. J. Biol. Chem. 1974;249:3116–3122. [PubMed] [Google Scholar]
- 44.Burger MM, Goldberg AR. Proc. Natl. Acad. Sci. U.S.A. 1967;57:359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen AK, Neuberger A, Sharon N. Biochem. J. 1973;131:155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith EA, Thomas WD, Kiessling LL, Corn RM. J. Am. Chem. Soc. 2003;125:6140–6148. doi: 10.1021/ja034165u. [DOI] [PubMed] [Google Scholar]
- 47.Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. ChemBioChem. 2008;9:1836–1844. doi: 10.1002/cbic.200800195. [DOI] [PubMed] [Google Scholar]
- 48.Imberty A, Gautier C, Lescar J, Perez S, Wyns L, Loris R. J. Biol. Chem. 2000;275:17541–17548. doi: 10.1074/jbc.M000560200. [DOI] [PubMed] [Google Scholar]
- 49.Galbraith W, Goldstein IJ. FEBS Lett. 1970;9:197–201. doi: 10.1016/0014-5793(70)80354-4. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, Haab BB. Nat. Methods. 2007;4:437–444. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- 51.Ferrand Y, Crump MP, Davis AP. Science. 2007;318:619–622. doi: 10.1126/science.1148735. [DOI] [PubMed] [Google Scholar]
- 52.Monzo A, Bonn GK, Guttman A. Anal. Bioanal. Chem. 2007;389:2097–2102. doi: 10.1007/s00216-007-1627-y. [DOI] [PubMed] [Google Scholar]
- 53.Edwards NY, Sager TW, McDevitt JT, Anslyn EV. J. Am. Chem. Soc. 2007;129:13575–13583. doi: 10.1021/ja073939u. [DOI] [PubMed] [Google Scholar]
- 54.Zou YJ, Broughton DL, Bicker KL, Thompson PR, Lavigne JJ. ChemBioChem. 2007;8:2048–2051. doi: 10.1002/cbic.200700221. [DOI] [PubMed] [Google Scholar]
- 55.Battistini E, Mortillaro A, Aime S, Peters JA. Contrast Media Mol. Imaging. 2007;2:163–171. doi: 10.1002/cmmi.141. [DOI] [PubMed] [Google Scholar]
- 56.Tai T, Yamashita K, Ogata-Arakawa M, Koide N, Muramatsu T, Iwashita S, Inoue Y, Kobata A. J. Biol. Chem. 1975;250:8569–8575. [PubMed] [Google Scholar]
- 57.Tai T, Yamashita K, Ito S, Kobata A. J. Biol. Chem. 1977;252:6687–6694. [PubMed] [Google Scholar]
- 58.Ohyama Y, Kasai KI, Nomoto H, Inoue Y. J. Biol. Chem. 1985;260:6882–6887. [PubMed] [Google Scholar]
- 59.Starkey BJ, Snary D, Allen A. Biochem. J. 1974;141:633–639. doi: 10.1042/bj1410633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuiki S, Hashimoto Y, Pigman W. J. Biol. Chem. 1961;236:2172–2178. [PubMed] [Google Scholar]
- 61.Spiro RG, Bhoyroo VD. J. Biol. Chem. 1974;249:5704–5717. [PubMed] [Google Scholar]
- 62.Baenziger JU, Fiete D. J. Biol. Chem. 1979;254:789–795. [PubMed] [Google Scholar]
- 63.Neumann NP, Lampen JO. Biochemistry. 1967;6:468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- 64.Gascon S, Neumann NP, Lampen JO. J. Biol. Chem. 1968;243:1573–1577. [PubMed] [Google Scholar]
- 65.Tanner SD, Bandura DR, Ornatsky O, Baranov VI, Nitz M, Winnik MA. Pure App. Chem. 2008 doi: 10.1039/b710510j. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting figures S1–S5 are available.