Abstract
Parkinson Disease (PD) is a late onset disorder with age-dependent penetrance that may confound genetic studies since affected individuals may not demonstrate clinical manifestations at the time of evaluation. The use of endophenotypes, biologic surrogates for clinical disease diagnoses, may permit more accurate classification of at-risk subjects. Positron emission tomography (PET) measurements of 6-[18F]fluorodopa ([18F]FDOPA) uptake indicate nigrostriatal neuronal integrity and may provide a useful endophenotype for PD linkage studies. We performed [18F]FDOPA PET in 11 members of a large, multi-incident Amish family with PD, 24 normals and 48 people with clinically definite idiopathic PD (PD controls). Clinical diagnoses in the Amish were clinically definite PD in four, clinically probable in one, clinically possible in five, and normal in one. Abnormal [18F]FDOPA posterior putamen uptake was defined as less than three standard deviations below the normal mean. The criteria were applied to the Amish sample to determine a PET endophenotype for each. We performed genetic simulations using SLINK to model the effect phenoconversion with the PET endophenotype had on logarithm of odds (LOD) scores. PET endophenotype confirmed the status of two clinically definite subjects. Two clinically definite Amish PD subjects had normal PETs. Two possible PD were converted to “PET definite PD”. The remainder had normal PETs. The average maximum LOD score with the pre-PET was 6.14±0.84. Simulating phenoconversion of subjects with unknown phenotypes increased the LOD score to 7.36±1.23. The [18F]FDOPA PET endophenotype permits phenoconversion in multi-incident PD families and may increase LOD score accuracy and power of an informative pedigree.
Keywords: Parkinson’s disease, Positron Emission Tomography, Linkage, Endophenotype, Amish
Introduction
The discovery of α-synuclein,(Polymeropoulos et al. 1997) parkin,(Kitada et al. 1998) ubiquitin carboxyterminal hydrolase L1,(Leroy et al. 1998) DJ-1,(Bonifati et al. 2003) genes has provided new insights into the pathophysiology of PD. However, it is not entirely clear what role these genes play in sporadic PD. The search for genetic factors that contribute to late onset neurodegenerative disorders is confounded substantially by age dependent penetrance. Misclassification can falsely increase or decrease LOD scores and can occur due to phenocopies (sporadic cases thought to be genetic) or young age at time of clinical assessment. Age of onset has substantial variability in identical twins with PD.(Dickson et al. 2001) Phenocopies and age dependent penetrance likely confound the search for risk factor genes for PD. Theoretically, identification of a narrow phenotype may reduce the rate of phenocopies, although young onset PD due to parkin can be indistinguishable from sporadic young onset PD.
Neuroimaging biomarkers such as [18F]FDOPA PET may be more sensitive than clinical examination for identification of defects in nigrostriatal pathways associated with PD(Heiss and Hilker 2004) and help identify affected individuals, especially in this age-dependent disease. Previous studies demonstrate that [18F]DOPA PET may detect a nigrostriatal defect several years prior to the onset of clinical manifestations in PD(Brooks 1991) or subclinical lesions in monkeys.(Guttman et al. 1988) We hypothesize that [18F]DOPA PET may provide an endophenotype that will increase the power and accuracy of genetic linkage studies. Patients with PD have reduced [18F]FDOPA uptake in the striatum, especially in posterior putamen, that correlates with loss of neurons in the substantia nigra pars compacta.(Morrish et al. 1996;Morrish et al. 1998) We now use PET-based criteria to segregate research subjects by abnormal, normal or uncertain nigrostriatal neuronal integrity and define this as an endophenotype for PD.
Methods
This study was approved by the Washington University School of Medicine Human Studies Committee and the Radioactive Drug Research Committee.
Subjects
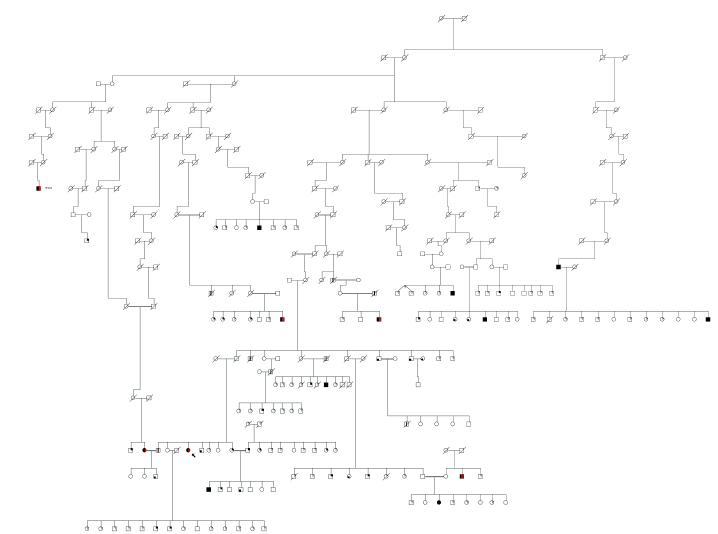
We studied three groups of subjects: people of Amish decent, non-Amish normal controls, and non-Amish people with clinically definite idiopathic PD. Amish subjects were participants in a genetic study of PD.(Racette et al. 2002) Amish subjects’ ages ranged from 43-81; seven were men and four were women. They were ascertained by initially contacting (with their permission) relatives of the proband or by identifying subjects with known PD through community volunteers Recruiting from all communities was by word of mouth, local print media, regional Amish weekly newspapers, and through the national Amish newspaper The Budget. The pedigree was constructed with the program Cyrillic 2.1(Cherwell Scientific) using family books obtained from subjects and Amish libraries. All known PD genes or loci have been excluded in this pedigree by either direct sequencing of the gene or linkage analysis of markers flanking the known loci.
We obtained relevant PD history with a validated and weighted questionnaire(Duarte et al. 1995) and performed a neurologic examination including a Unified Parkinson’s Disease Rating Scale(Fahn et al. 1987) (UPDRS) motor subsection 3 and Mini-Mental State examination (in English)(Folstein et al. 1975) on each subject. Based upon neurological examination, we classify individuals as clinically possible, probable, or definite PD using previously reported criteria.(Racette et al. 1999)
A movement disorders specialist blinded to the subjects’ clinical history performed the repeat UPDRS3 examination at the time of the [18F]FDOPA PET studies. Inter-rater reliability between the initial examiner and the repeat examiner was determined by rating a video tape on a group of ten PD subjects followed in the Movement Disorders Center. The intra-class correlation for the total UPDRS3 (without rigidity) was 0.70.
Controls Subjects
We studied 10 male and 14 female non-Amish normal controls. Sixteen of them were age-matched within two years to the Amish (mean age: 58 years old (range: 40-65)). Controls were unrelated, PD patient spouses/caregivers and had normal neurologic examinations. We also studied 48 non-Amish people (mean age: 60 years old (range: 38-80); 29 males and 19 females) with clinically definite PD (as defined above). These people had no neurological abnormality beyond PD.
[18F]FDOPA PET Studies
All PD medications were held for twelve hours prior to the study (when relevant). Subject preparation and PET scans were collected, reconstructed to about 6 mm FWHM, and aligned as previously described (Racette et al. 2001) using a Siemens/CTI 953B scanner in 3D-mode.(Spinks et al. 1993)
A reviewer blinded to the clinical status of the subject outlined an occipital region as well as the entire caudate, anterior and posterior putamen on each side of a magnetic resonance image (3D MPRAGE) for each subject. This MR image was co-registered to the [18F]FDOPA PET image using a validated method.(Black et al. 2001) and this transformation matrix was used to resample the MR-identified regions of interest.
Decay-corrected regional tissue-activity PET measurements were extracted from the dynamic series of PET images, and an influx constant Ki was calculated with the occipital region as the input using data from 24 to 94 minutes after injection(Patlak et al. 1983;Logan et al. 1987)
An Amish subject or non-Amish PD control subject was considered to have an abnormal [18F]FDOPA PET if the Ki for the one of the posterior putamen was more than three standard deviations below the normal mean.
Genetic simulations
Simulated linkage studies were performed with the program SLINK.(Terwilliger et al. 1993) We assumed a rare autosomal recessive, age-dependent penetrant disease locus with a disease gene frequency of 0.0001 based upon the observed population prevalence in this community. The following penetrances were used based upon subjects’ age at time of clinical evaluation: 0% ages <35; 10% ages 35-40; 25% ages 41-60; 50% ages 61+. We assumed a marker locus with five equally frequent alleles (gene frequency 0.2). Due to program constraints, we cut all inbreeding loops by duplicating loop breakers. This method of loop breaking is similar to the automatic loops function in FASTLINK.(Cottingham, Jr. et al. 1993) For each permutation we ran 1000 simulated linkage studies and obtained a minimum, maximum and average LOD score and standard deviation. To determine the power of the pedigree with currently available information, markers were simulated and the phenotypes fixed with the pre-PET clinical diagnostic information. Subjects with possible or probable PD were classified as phenotype unknown. This same analysis was performed with the PET endophenotype fixed (when applicable) to determine the effect of PET on the LOD scores. For the post-PET phenotypes, subjects meeting the PET definition of “PET definite” were converted to affected, subjects with clinically definite PD who were “PET normal” were converted to unknown, subjects with possible or probable PD who were “PET definite” were converted to affected, and subjects with possible or probable PD who were “PET normal” remained unknown for the simulations. To simulate the change in phenotype by phenoconverting more subjects with the PET endophenotype, we simulated the phenotype of living siblings and children under the same assumptions as above. This simulation run permits the SLINK program to assign diagnoses to unknown subjects and provides a very conservative estimate of the overall power of phenoconversion. We also performed a simulated linkage analysis in which we simulated the PET phenoconversion by individually changing possible or probable PD subjects to PET definite.
Results
The pre-PET diagnosis was normal in one Amish subject, possible PD in five, probable PD in one. The mean interval from initial to repeat exam was 2.7±0.8 years. Table 1.The pedigree from which these subjects were taken is in Figure1. Two of the clinically definite subjects have been followed for over five years for clinical management of their PD and have clear response to levodopa, motor fluctuations, and dyskinesias. The other two clinically definite subjects did not desire treatment and were only seen for research clinical assessments and have not been treated for their parkinsonism. Subject # 8 had asymmetric rest tremor, bradykinesia, and rigidity at the initial visit but the tremor seemed to improve with time. Subject # 10 had asymmetric postural tremor, bradykinesia, and rigidity. On one examination she had asymmetric rest tremor but this was not seen on follow-up examination.
Table 1.
Demographic and Clinical Features of Amish Subjects
| Subject | Age* | Gender | Initial UPDRS3 |
Followup UPDRS3 |
UPDRS3 Interval (years) |
Clinical Diagnosis |
Other Neurological Clinical Diagnoses |
PET Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | 33.50 | 43.00 | 2.85 | Definite | Definite | |
| 2 | 70 | M | 3.00 | 5.50 | 0.68 | Possible | Essential Tremor | Normal |
| 3 | 52 | M | 3.00 | 2.00 | 3.02 | Possible | Definite | |
| 4 | 72 | M | 40.00 | 44.00 | 3.10 | Definite | Definite | |
| 5 | 52 | F | 11.00 | 20.50 | 3.10 | Normal | Post-poliomyelitis | Normal |
| 6 | 81 | F | 2.00 | 3.00 | 2.79 | Possible | Essential Tremor/dystonia |
Normal |
| 7 | 43 | M | 4.00 | 19.50 | 3.02 | Possible | Essential Tremor | Definite |
| 8 | 69 | M | 18.50 | 22.00 | 3.23 | Definite | Normal | |
| 9 | 73 | M | 7.00 | 9.00 | 1.76 | Possible | Normal | |
| 10 | 48 | F | 13.50 | 9.00 | 3.25 | Definite | Normal | |
| 11 | 67 | F | 8.00 | 5.00 | 3.25 | Probable | Normal |
Age at time of PET scan
Figure 1.
Amish pedigree demonstrating multiple affected subjects and inbreeding.
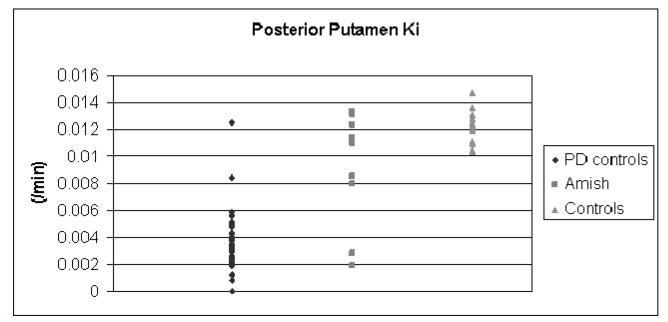
Forty-seven of 48 clinically definite control PD subjects were classified as definite by [18F]FDOPA PET critieria. The one PD control that did not meet these criteria had a 6-month history of unilateral, intermittent resting tremor with slight bradykinesia and rigidity in that limb and a Unified Parkinson Disease Rating Scale motor subscore of 4.5.(Figure 2)
Figure 2.
[18F]FDOPA PET Ki in control subjects and Amish, Forty-seven of 48 PD controls and four Amish met the PET criteria for PD of Ki for one of the posterior putamen more than three standard deviations below the normal mean.
The [18F]FDOPA PET confirmed the status of two clinically definite subjects. Two definite PD subjects had normal PETs. Two clinically possible PD subjects were converted to “PET definite PD”. One probable PD, three possible PD and one normal Amish were classified as PET normal. Table 1
Simulation studies
We modeled the effect of using the PET endophenotype in linkage studies in several ways. Table 2. We first studied the power of the pedigree to detect linkage and obtained an average LOD score of 6.14 reflecting the informativeness of this large, complex pedigree. Phenoconversion of the subjects according to their PET diagnosis resulted in no net change in LOD score due to the conversion of two clinically definite subjects to PET normal and conversion of two clinically possible subjects to PET definite. Next, we simulated phenoconversion in subjects with an unknown phenotype by allowing the SLINK program to simulate phenotypes. This provides a conservative approximation of the additional power gained by performing PET in all available subjects and converting some subjects to normal or definite. The average LOD score for the 1000 simulations increased to 7.36±1.23 with the simulated phenotypes. We also calculated the power of converting individual possible or probable PD subjects to PET definite by performing a simulated linkage analysis after phenoconversion of examined possible or probable subjects. Each additional phenoconverted subject added an average .33 to the LOD score. Table 2.
Table 2.
Phenotype and Genotype Simulations with and without [18F]FDOPA PETEndophenotype
| Pedigree | Minimum LOD score |
Maximum LOD score | Average LODscore | Standard Deviation |
|---|---|---|---|---|
| Current Pedigree | 2.99 | 8.25 | 6.14 | 0.84 |
|
Simulated phenoconversion: 1 possible PD→PET definite |
3.23 | 9.04 | 6.35 | 0.85 |
|
Simulated phenoconversion: 2 possible PD→PET definite |
2.90 | 9.23 | 6.82 | 0.92 |
|
Simulated phenoconversion: 2 possible PD→PET definite |
3.30 | 9.78 | 7.06 | 1.03 |
Discussion
This study demonstrates several important findings. First, “[18F]FDOPA may be used to change an equivocal clinical diagnosis of PD to a PET diagnosis of PD for genetic in linkage studies; however, long term follow-up of these patients will determine the accuracy of this PET endophenotype. Although this pilot, feasibility study of eleven subjects was not powered to produce significant changes in the pedigree’s power, there was a substantial improvement in the power when we simulated phenoconverting other subjects with unknown phenotypes. It is possible to identify subjects who may be at higher risk of PD and convert them to PET definite PD for linkage. Our findings are likely a conservative estimate of the true power of the PET endophenotype in this pedigree since program limitations in SLINK do not permit inclusion of inbreeding loops. Therefore, the genetic complexity modeled is substantially greater than the program would accept.
A second important finding of this study is that phenocopies of late onset neurodegenerative disorders like PD can have a substantial impact on the LOD score in a linkage study. We found that serial examinations and treatment of subjects resulted in the most stable diagnosis over time. There was no change in diagnosis for those who were treated and followed serially. We have followed and treated the majority of the PD subjects available in this community but treatment information was not available for the two subjects converted from “clinically definite” to “unknown”. We suspect that when longitudinal follow-up and response to chronic treatment information is available, the PET endophenotype will be less important. However, in a cross-sectional study, the PET endophenotype may be critical to avoid misclassifying phenocopies and pre-symptomatic subjects.
We do not know the duration of the pre-symptomatic phase during which there is a detectable nigrostriatal defect on [18F]FDOPA PET. Two imaging studies have attempted to measure the pre-symptomatic phase of PD. One study used [18F]FDOPA to estimate the length of time that patients with PD have preclinical abnormalities of the nigrostriatal system prior to the development of clinical symptoms to be 5.2 - 6.5 years.(Morrish et al. 1998) These investigators measured longitudinal [18F]FDOPA uptake and extrapolated backwards from the change in the two PETs in the PD patients to estimate the time that damage to the nigrostriatal system began. Another study used PET and fluorodeoxyglucose (FDG) PET and a completely different method of data analysis to estimate the preclinical period as 4.5 years before symptoms.(Moeller and Eidelberg 1997) However, neither of these studies claim to identify how early one might be able to identify such a preclinical defect.
[18F]FDOPA PET primarily reflects decarboxylase activity(Martin and Perlmutter 1994) and represents just one of several types of PET tracers that can be used to assess integrity of nigrostriatal neurons. Some label presynaptic dopamine transporters, like [11C]CFT(Wullner et al. 1994) or the SPECT tracer beta-CIT, whereas others reflect vesicular monoamine transporter type 2 (VMAT2) sites that occur on presynaptic vesicles that store dopamine for subsequent release.(Marek et al. 2001) VMAT2 sites on presynaptic vesicles are less likely to be regulated than either decarboxylase or DAT(DaSilva et al. 1993;Chan et al. 1999;Lee et al. 2000;Frey et al. 2001). However, it is possible one of these other tracers may be more sensitive for detection of asymptomatic nigrostriatal defects.
Given these caveats, it is certainly possible that we could misclassify subjects who might develop PD in another decade but we believe that the added accuracy of the [18F]FDOPA PET endophenotype may prove to be critical for finding risk factor PD genes in older onset PD pedigrees. Although we anticipate that the [18F]FDOPA PET endophenotype will facilitate detection of pre-symptomatic PD, the [18F]FDOPA uptake is also age dependent and will likely misclassify younger “at-risk” subjects.
Although the two clinically definite subjects were changed to PET normal, we do not necessarily know the correct clinical diagnosis. In our computer modeling we used the PET diagnosis as a definitive diagnostic procedure; however, there is no consensus on how to categorize parkinsonian subjects with normal [18F]FDOPA or other ligand uptake.(The Parkinson’s Study Group 2004) For the purposes of genetic linkage studies, however, selecting a more homogeneous phenotype (clinical PD with reduced [18F]FDOPA uptake) may increase the likelihood of detecting linkage. Phenocopies have been seen in two longitudinal PD dopamine agonist trials.(Rascol et al. 2000;Parkinson Study Group 2000) Although one study excluded subjects from analysis,(Whone et al. 2003) there is no published data on the longitudinal follow-up of these subjects to guide data analysis. We chose a conservative strategy in our simulation studies of converting those subjects’ diagnoses to PET normal. In fact, these two subjects had persistent UPDRS3 scores performed over time by two movement disorders specialists and cannot be considered “normal”. Since the sensitivity of biomarkers like [18F]FDOPA PET, B-CIT SPECT, and other markers is unknown, only longitudinal clinical and imaging studies of subjects undergoing PET including pathologic study can clarify this issue.
Acknowledgments
This work was supported by NIH grants K23NS43351, NS41509, NS39913, NS041771 the Greater St. Louis Chapter of the American Parkinson Disease Association, the Jack Buck Fund, and the Elliot H. Stein Fund, the Ruth Kopolow Fund.
Reference List
- Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. Template images for nonhuman primate neuroimaging. 1. Baboon. Neuroimage. 2001;14:736–743. doi: 10.1006/nimg.2001.0752. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. Detection of preclinical Parkinson’s disease with PET. Geriatrics. 1991;46:25–30. [PubMed] [Google Scholar]
- Chan GL, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T, Morrison KS, Adam M, Schulzer M, Calne DB, Ruth TJ. Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med. 1999;40:283–289. [PubMed] [Google Scholar]
- Cottingham RW, Jr., Idury RM, Schaffer AA. Faster sequential genetic linkage computations. Am J Hum Genet. 1993;53:252–263. [PMC free article] [PubMed] [Google Scholar]
- DaSilva JN, Kilbourn MR, Mangner TJ, Toorongian SA. Synthesis of [11C]methoxy derivative of α-dihydrotetrabenazine for PET imaging of monoaminergic nerve terminals. J Lab Comp Radiopharm. 1993;32:257–259. [Google Scholar]
- Dickson D, Farrer M, Lincoln S, Mason RP, Zimmerman TR, Jr., Golbe LI, Hardy J. Pathology of PD in monozygotic twins with a 20-year discordance interval. Neurology. 2001;56:981–982. doi: 10.1212/wnl.56.7.981. [DOI] [PubMed] [Google Scholar]
- Duarte J, Claveria LE, de Pedro-Cuesta J, Sempere AP, Coria F, Calne DB. Screening Parkinson’s disease: a validated questionnaire of high specificity and sensitivity. Mov Disord. 1995;10:643–649. doi: 10.1002/mds.870100518. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members of the UPDRS Development Committee . Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. Macmillan; New York: 1987. pp. 153–163. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Kilbourn MR. Imaging the vesicular monoamine transporter. Adv Neurol. 2001;86:237–247. [PubMed] [Google Scholar]
- Guttman M, Yong VW, Kim SU, Calne DB, Martin WR, Adam MJ, Ruth TJ. Asymptomatic striatal dopamine depletion: PET scans in unilateral MPTP monkeys. Synapse. 1988;2:469–473. doi: 10.1002/syn.890020502. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Hilker R. The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson’s disease. Eur J Neurol. 2004;11:5–12. doi: 10.1046/j.1351-5101.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, Wudel J, Pal PK, Fuente-Fernandez R, Calne DB, Stoessl AJ. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Logan J, Wolf AP, Shiue C-Y, Fowler JS. Kinetic modeling of receptor-ligand binding applied to positron emission tomographic studies with neuroleptic tracers. J Neurochem. 1987;48:73–83. doi: 10.1111/j.1471-4159.1987.tb13129.x. [DOI] [PubMed] [Google Scholar]
- Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, Oakes D, Seibyl J. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57:2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- Martin WRW, Perlmutter JS. Assessment of fetal tissue transplantation in Parkinson’s disease: does PET play a role? Neurology. 1994;44:1777–1780. doi: 10.1212/wnl.44.10.1777. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Eidelberg D. Divergent expression of regional metabolic topographies in Parkinson’s disease and normal ageing. Brain. 1997;120:2197–2206. doi: 10.1093/brain/120.12.2197. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64:314–319. doi: 10.1136/jnnp.64.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of the progresssion in Parkinson’s disease. Brain. 1996;119:585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group Pramipexole vs levodopa as initial treatment for Parkinson disease: A randomized controlled trial. J Am Med Assoc. 2000;284:1931–1938. doi: 10.1001/jama.284.15.1931. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;27:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- Racette BA, Rundle M, Wang JC, Goate A, Saccone NL, Farrer M, Lincoln S, Hussey J, Smemo S, Lin J, Suarez B, Parsian A, Perlmutter JS. A multi-incident, Old-Order Amish family with PD. Neurology. 2002;58:568–574. doi: 10.1212/wnl.58.4.568. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clark CE, Lang AE. A five-year study of the incidence of dyskiensias in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. New Engl J Med. 2000;18:1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Spinks TJ, Jones T, Bailey DL, Townsend DW, Grootoonk S, Bloomfield PM, Gilardi M-C, Casey ME, Sipe B, Reed J. Physical performance of a positron tomograph for brain imaging with retractable septa. Phys Med Biol. 1993;37:1637–1655. doi: 10.1088/0031-9155/37/8/002. [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Speer M, Ott J. Chromosome-based method for rapid computer simulation in human genetic linkage analysis. Genet Epidemiol. 1993;10:217–224. doi: 10.1002/gepi.1370100402. [DOI] [PubMed] [Google Scholar]
- The Parkinson’s Study Group Levodopa and the Progression of Parkinson’s Disease. New Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH, Hauser RA, Brooks DJ. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- Wullner U, Pakzaban P, Brownell AL, Hantraye P, Burns L, Shoup T, Elmaleh D, Petto AJ, Spealman RD, Brownell GL. Dopamine terminal loss and onset of motor symptoms in MPTP-treated monkeys: a positron emission tomography study with 11CCFT. Exp Neurol. 1994;126:305–309. doi: 10.1006/exnr.1994.1069. [DOI] [PubMed] [Google Scholar]