Abstract
OBJECTIVE—Diagnosis of pre-diabetes and early-stage diabetes occurs primarily by means of an oral glucose tolerance test (OGTT), which requires invasive blood sampling. The aim of this study was to determine whether differences exist in breath 13CO2 excretion during a 13C-labeled OGTT between individuals with normal glucose tolerance (NGT) and individuals with pre-diabetes and early-stage diabetes (PDED) and whether these differences correlated with blood glucose kinetics.
RESEARCH DESIGN AND METHODS—Blood and breath samples were collected at baseline and every 30 min for a 10-h period after ingestion of 75 g glucose isotopically labeled with 150 mg [U-13C6]d-glucose.
RESULTS—Age (56 ± 5 vs. 47 ± 3 years) and BMI (31 ± 2 vs. 31 ± 2 kg/m2) were not different between individuals with NGT (n = 10) and PDED (n = 7), respectively. Blood glucose concentrations were significantly higher in those with PDED compared with those with NGT from baseline to 4.5 h after glucose ingestion (P ≤ 0.05). Glucose-derived breath 13CO2 was significantly lower in individuals with PDED compared with those with NGT from 1 to 3.5 h after glucose (P ≤ 0.05). Peak breath 13CO2 abundance occurred at 4.5 and 3.5 h in individuals with PDED and NGT, respectively (36.87 ± 3.15 vs. 41.36 ± 1.56‰ delta over baseline).
CONCLUSIONS—These results suggest that this novel breath test method may assist in recognition of pre-diabetes or early-stage diabetes in at-risk persons without the need for invasive blood sampling, thus making it an attractive option for large-scale testing of at-risk populations, such as children.
Type 2 diabetes and its cluster of disorders (i.e., metabolic syndrome) have become a major public health concern (1,2) and form a predictive risk profile for cardiovascular disease (3–5). Recently, the risk of development of metabolic syndrome has significantly increased in the pediatric population (6). Traditionally, pre-diabetes and early-stage diabetes are assessed in at-risk adult and pediatric populations using a standard oral glucose tolerance test (OGTT) requiring repeated invasive blood sampling over the 2 to 3 h after a glucose load. Postprandially, the mix of fuels used for cellular energy production is dramatically altered when insulin sensitivity is decreased (7–9). The ability to noninvasively detect the shift in fuel source utilization between individuals with normal glucose tolerance (NGT) and those at risk for pre-diabetes and early-stage diabetes (PDED) has considerable clinical utility.
It has been demonstrated previously that 13CO2 in breath is as effective as the hyperinsulinemic-euglycemic clamp method for assessing insulin resistance (10). However, the clinical utility of detecting breath CO2 kinetics using a standard diagnostic infrared 13CO2 breath analyzer between individuals with NGT and those with PDED has not been clearly established. Therefore, the aim of the present study was to evaluate the efficacy of a diagnostic breath analyzer available in many physician offices to characterize the kinetics of glucose-derived CO2 in breath after administration of a standard OGTT in individuals with NGT and PDED. We compared the breath CO2 kinetics from individuals with NGT with those from individuals with PDED for 10 h after an OGTT (75-g glucose load) isotopically labeled with [U-13C6]glucose. In addition, breath CO2 data were correlated with indexes of insulin resistance (whole-body index of insulin sensitivity [WBISI], homeostasis model assessment of insulin resistance [HOMA-IR], and quantitative insulin sensitivity check index [QUICKI]) calculated from measures of blood glucose and plasma insulin. We hypothesized that the standard breath analyzer would be sufficiently sensitive to detect impaired glucose utilization for cellular respiration during a standard OGTT between those with NGT and PDED.
RESEARCH DESIGN AND METHODS
The protocol was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board and conducted in the UTMB General Clinical Research Center (GCRC). All subjects provided written informed consent before participating in the study. Screening of all subjects included fasting blood glucose, blood cell count, thyroid function test, lipid panel, urinalysis, and a urine pregnancy test for premenopausal women. Individuals with a previous diagnosis of type 2 diabetes or taking any medications known to affect glucose or lipid metabolism were excluded from participation. On the basis of their 2-h OGTT blood glucose levels on the study day, subjects were grouped as either having NGT (2-h OGTT blood glucose <7.8 mmol/l, n = 10) as outlined by the American Diabetes Association (http://www.diabetes.org) or having PDED (2-h OGTT blood glucose 7.8–16 mmol/l, n = 7). All subjects with NGT required fasting blood glucose ≤5.6 mmol/l for inclusion. Subject characteristics are shown in Table 1.
Table 1.
Subject characteristics
| NGT | PDED | P value | |
|---|---|---|---|
| 2-h OGTT blood glucose (mmol/l) | 6.2 ± 0.17 | 11.4 ± 0.94* | <0.0001 |
| Sex (female/male) | 5/5 | 4/3 | 0.79 |
| Age (years) | 47 ± 3 | 56 ± 5 | 0.15 |
| Weight (kg) | 89 ± 6 | 92 ± 6 | 0.74 |
| Height (cm) | 166 ± 2 | 172 ± 3 | 0.09 |
| BMI (kg/m2) | 31 ± 2 | 31 ± 2 | 0.96 |
| Fasting blood glucose (mmol/l) | 5.1 ± 0.11 | 6.8 ± 0.56* | 0.003 |
| Fasting plasma insulin (μIU/ml) | 7.0 ± 1.7 | 10.39 ± 2.77 | 0.29 |
| WBISI | 8.00 ± 1.22 | 5.48 ± 1.47 | 0.10 |
| HOMA-IR | 1.63 ± 0.43 | 3.23 ± 0.94* | 0.05 |
| QUICKI | 0.37 ± 0.01 | 0.34 ± 0.01* | 0.04 |
Data are means ± SE.
Significantly different from NGT (P ≤ 0.05).
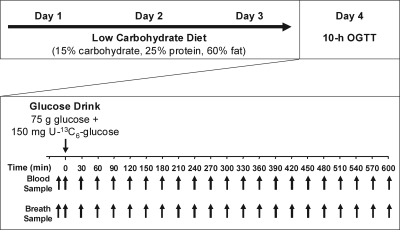
The study protocol consisted of 4 days of study activities (Fig. 1). On days 1–3, subjects consumed a standardized, low-carbohydrate diet containing 15% carbohydrate, 25% protein, and 60% fat. Individual dietary requirements were determined by a registered dietitian and calculated using the Harris Benedict equation (11). During this diet stabilization period, all subjects were provided with breakfast, lunch, and dinner from the UTMB GCRC metabolic kitchen. All meals were picked up on a daily basis for consumption off-site. Subjects were required to completely consume these meals and nothing else except water. Height and weight were recorded daily during the visits to the GCRC for pick- up of the meals. On the morning of the fourth day, a 10-h OGTT was performed in the GCRC.
Figure 1.
Study time line. Subjects followed a 3-day standardized low-carbohydrate diet composed of 15% carbohydrate, 25% protein, and 60% fat. Individual dietary requirements were determined by a registered dietitian and calculated using the Harris Benedict equation. On the fourth day, after an overnight fast, a 10-h stable isotopically labeled OGTT was performed, and blood and breath samples were collected every 30 min.
After an overnight fast (water allowed), subjects were placed in a bed at the GCRC, and an antecubital venous intravenous line was inserted for the collection of blood samples. Baseline blood samples were collected twice over a 15-minute period. During the second baseline blood collection, simultaneous breath samples were collected by having the subjects breathe into breath collection bags fitted with one-way valves. After the baseline sample collection, a drink containing 75 g glucose and 150 mg [U-13C6]glucose was administered and consumed within 1 min. From this point (t = 0 min), blood and single-point breath samples were collected every 30 min for 10 h. For the collection of breath samples, the subjects were instructed to breathe normally, hold their breath for 3 s, and exhale completely into the collection bag provided. Subjects remained at rest throughout the 10-h OGTT and were only allowed to move around the room to use the restroom. Water was provided ad libitum throughout the 10-h OGTT. After collection of the final samples at t = 10 h and removal of the intravenous line, the subjects were given food and discharged.
Blood glucose
Glucose concentrations were determined in whole blood immediately after collection of the samples using a 2300 STAT Plus Glucose analyzer (intra-assay coefficient of variance [CV] = 2.1%).
Plasma insulin
Insulin concentrations were determined in plasma using an Immulite 2000 chemiluminescence immunoassay system (Diagnostic Products Corporation, Los Angeles, CA) (CV = 3.3%).
Breath CO2
The ratios of 13CO2 to 12CO2 in single breath samples were measured using a UBiT-IR300 infrared spectrophotometer (Otsuka Electronics, Hirakata, Osaka, Japan) (CV ≤1.0%). All results are calculated as per mille (‰) change of 13CO2 abundance from the baseline breath sample and expressed as per mille delta over baseline (‰DOB).
Indexes of insulin resistance
WBISI (12), HOMA-IR (13), and QUICKI (14) were calculated from blood glucose (milligrams per deciliter) and plasma insulin (micro-international units per milliliter) measurements using the following equations:
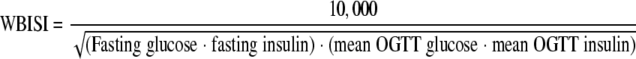 |
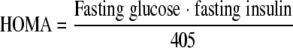 |
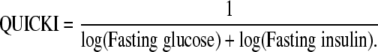 |
Statistics
A repeated-measures analysis using restricted maximum likelihood estimation was used to obtain parameter estimates with the MIXED procedure in SAS (SAS/STAT 9.1 User's Guide; SAS Institute, Cary, NC). Each set of measurements from the same subject was considered a correlated cluster of observations. The AR(1) covariance structures were used. These models allow for inclusion of subjects with small amounts of missing data. The mixed model tested the effects for group, time, and the (group × time) interaction. When the interaction was significant (P < 0.05) we computed two-sample t tests at each time point for descriptive purposes used only in the figures.
RESULTS
There were no differences in age, height, weight, BMI, sex, or fasting plasma insulin between the NGT and PDED groups (Table 1). HOMA-IR was significantly higher in the PDED group, and QUICKI was significantly lower in the PDED group compared with the NGT group (P ≤ 0.05). WBISI was not significantly different between the groups.
Blood glucose.
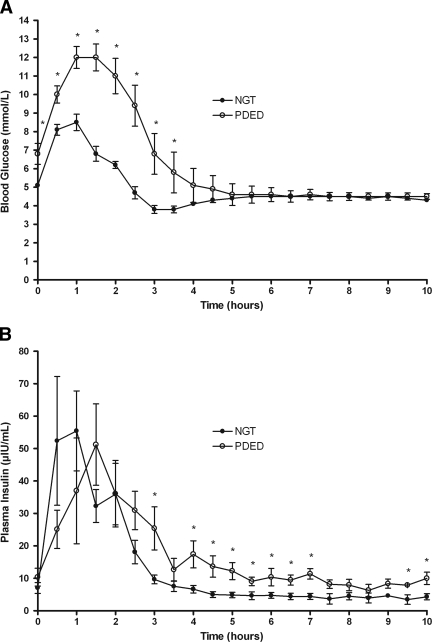
By design, 2-h OGTT blood glucose concentrations were higher in the PDED group (11.4 ± 0.95 mmol/l) compared with the NGT group (6.2 ± 0.19 mmol/l; P < 0.00001). The repeated mixed-model analysis indicated that blood glucose had significant group (P < 0.0001), time (P < 0.0001), and group × time interaction (P < 0.0001). The interaction indicated that blood glucose concentrations were significantly higher in the PDED group compared with the NGT group between 0 and 3 h, as shown in Fig. 2A. Fasting blood glucose concentrations were higher in the PDED group (6.8 ± 0.56 mmol/l) than in the NGT group (5.1 ± 0.13 mmol/l; P < 0.01). Blood glucose levels were significantly elevated after the glucose load in the PDED group compared with the NGT group from t = 0–3.5 h (P < 0.05). There were no differences in blood glucose concentrations from 4 through 10 h after the glucose load between the groups.
Figure 2.
Blood glucose and plasma insulin concentrations in individuals with NGT and PDED during the 10-h OGTT. A: Blood glucose was different between individuals with NGT and PDED over time as determined by mixed-model repeated measures (P < 0.05). *Different between individuals with NGT and PDED (t test, P < 0.05). B: Plasma insulin was different between individuals with NGT and PDED over time as determined by mixed-model repeated measures (P < 0.05). *Different between individuals with NGT and PDED (t test, P < 0.05).
Plasma insulin
The repeated mixed-model analysis indicated that insulin gave effects for group (P = 0.53), time (P < 0.0001), and group × time interaction (P = 0.03). The interaction indicated that the differences between the PDED and NGT groups were stronger at later time points than at early ones, as shown in Fig. 2B.
Breath CO2
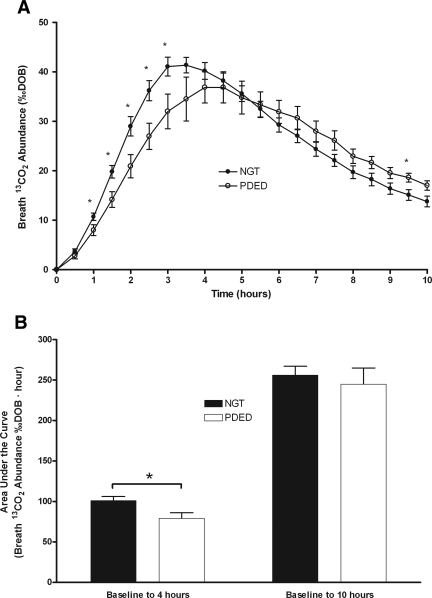
Breath 13CO2 abundance had significant group (P = 0.02), time (P < 0.0001), and group × time interaction (P = 0.0002). The interaction indicated that breath 13CO2 abundance was significantly lower in the PDED group compared with the NGT group between 1 and 3 h after the glucose load as shown in Fig. 3A. Peak breath 13CO2 abundance occurred at 3.5 h in the NGT group (41.36 ± 1.56 ‰DOB) and at 4.5 h in the PDED group (36.87 ± 3.15 ‰DOB). Intersubject CVs at peak 13CO2 appearance were 12 and 22% for the NGT and PDED groups, respectively. Areas under the curve (AUCs) for breath 13CO2 abundance were calculated from 0 to 4 h and from 0 to 10 h (Fig. 3B) as mean ± SEM. Mean breath 13CO2 abundance AUC was lower in the PDED group than in the NGT group from 0 to 4 h after the glucose load (P < 0.05). The difference between the PDED and NGT groups disappeared when AUC was calculated from 0 to 10 h.
Figure 3.
Breath 13CO2 abundance and areas under the curve for individuals with NGT and PDED during the 10-h OGTT. A: Breath 13CO2 abundance was different between individuals with NGT and PDED over time as determined by mixed-model repeated measures (P < 0.05). *Significant difference between individuals with NGT and PDED (t test, P < 0.05). B: AUCs were different between individuals with NGT and PDED when measured from 0 to 4 h but not when measured from 0 to 10 h. *Significant difference between individuals with NGT and PDED (t test, P < 0.05).
Correlation of breath CO2 to indexes of insulin sensitivity
The 2-h breath 13CO2 abundance measurements correlated directly with WBISI (Pearson correlation coefficient r = 0.502, P = 0.04) and QUICKI (r = 0.629, P = 0.007) and inversely with fasting glucose (r = −0.631, P = 0.007), 2-h glucose (r = −0.689, P = 0.002), HOMA-IR (r = −0.555, P = 0.02), and weight (r = −0.546, P = 0.02). Significant correlations were found between all three measured indexes of insulin resistance (P ≤ 0.002). No correlations were found between age and any of the measured parameters.
CONCLUSIONS
Results of the present study demonstrate that a standard infrared breath analyzer can detect differences in the excretion pattern of glucose-derived breath CO2 between individuals with NGT and PDED. Moreover, these differences were detectable in the time frame of a standard OGTT and correlated with the differences in glucose kinetics between the two groups. Thus, this noninvasive breath method may assist in recognition of PDED by providing an alternative approach for large-scale population testing without the need for blood sampling. This type of testing is particularly important to improve acceptance of pre-diabetes screening for certain patient populations, such as pediatric patients, who may be reluctant to undergo screening because of the prospect of repeated blood sampling.
Remarkably, the breath analyzer was capable of detecting marked differences in glucose-derived breath CO2 kinetics between individuals with NGT and PDED within 60 min of a 10-h OGTT. The initial rate of glucose-derived CO2 appearance in breath was significantly lower in individuals with PDED compared with those with NGT. Consistent with the blood glucose measurements, the breath test was able to detect impairments in clearance of exogenous (oral) glucose from circulation in individuals with PDED. Whereas the traditional blood glucose measurements rely on the accumulation of substrate in circulation, the breath test is a more direct measure of intracellular glucose metabolism. Impaired glucose uptake due to diminished pancreatic insulin secretion or impaired insulin action on the target tissue (i.e., skeletal muscle) results in blunted glucose oxidation in individuals with PDED.
A strength in our present study is that breath CO2 kinetics were followed over a longer period (10 h) and included multiple breath CO2 measurements in conjunction with simultaneous measurements of both blood glucose and plasma insulin concentrations. The data expressed as AUC support the assumption that, although slower in individuals with PDED, the eventual fate of the oral glucose was the same as that in individuals with NGT. Although the mean AUC was higher in individuals with NGT than in those with PDED from 1.5 through 4.5 h after glucose ingestion, this difference disappeared within the 10-h period. Thus, our results support the concept that individuals with PDED switch at a slower rate between lipid and glucose as a source of metabolic fuel compared with individuals with NGT. Impaired glucose tolerance has been described as “metabolic inflexibility” of the target tissue to respond to a switch from lipid oxidation to glucose oxidation (7). Under normal physiologic conditions, cellular fuel consists primarily of free fatty acids (FFAs) and glucose, with protein making a minor contribution (7,9,15). In the basal state, >60% of cellular fuel for insulin-dependent peripheral tissues (muscle) is derived from FFAs (15–19). In individuals with NGT, when an oral dose of glucose (75–100 g) is administered, the contribution of FFAs to whole-body fuel oxidation is reduced dramatically, with glucose becoming a prominent cellular fuel (15). In individuals with insulin resistance or pre-diabetes, this response is significantly blunted, resulting in a disproportionate contribution of FFAs to postprandial whole-body energy consumption (20,21). Glucose uptake through GLUT4 is mediated by insulin, whereas non–insulin-dependent glucose uptake occurs through other glucose transporters or mechanisms. In disorders such as type 2 diabetes, the non–insulin-dependent glucose uptake may be upregulated to compensate for the lack of insulin-dependent GLUT4 recruitment, contributing to a higher-than-normal glucose oxidation in the fasting state but lower insulin-mediated glucose oxidation in response to a meal (7,20,22).
Although resting energy expenditure measurements are not available from these experiments, future studies that include indirect calorimetry measures are needed to confirm how resting energy expenditure differences between subjects, as well as metabolic changes in response to glucose ingestion, affect CO2 kinetics in breath. However, it is important to underscore that this breath test technique is intended as a noninvasive alternative to blood draws for the determination of glucose tolerance during a standard OGTT—not as an alternative to indirect calorimetry for measurements of whole-body energy expenditure. This technique is simple to apply and relatively economical because it requires few single-breath collections and no special training, which may allow for the development of at-home kits.
Our results are, in general, consistent with the correlations between glucose-derived CO2 in breath and indexes of insulin resistance reported by Lewanczuk et al. (10). This group recently compared a single measurement of [13C]glucose-derived CO2 in breath taken 90 min after ingestion of a lower dose of glucose to results obtained from a hyperinsulinemic-euglycemic clamp performed on a separate occasion. Interestingly, although the methods used in that study were profoundly different from those used in ours, their correlation coefficient was remarkably similar to our 2-h correlation coefficient between breath CO2 and HOMA-IR (−0.531 [10]; −0.555 [ours]) (10). However, to the best of our knowledge no studies have explored whether glucose-derived CO2 in breath correlated with blood glucose and plasma insulin measurements collected simultaneously during a labeled OGTT. Our study is also the first demonstrating feasibility of the use of a cost-effective diagnostic breath analyzer instead of an isotope ratio mass spectrometer as used previously.
Although low-carbohydrate diets are not typically prescribed before administration of a standard OGTT, the 3-day low-carbohydrate diet stabilization period was intended to remove dietary intake as a variable in the present study. In addition, we intended to decrease hepatic and muscle glycogen stores, thus minimizing these tissues as immediate sources of endogenous glucose. Future research is warranted to characterize the impact of diet and altered gastrointestinal absorption of glucose on the kinetics of breath CO2 appearance.
In summary, the results of the present study demonstrate the clinical applicability of a standard breath analyzer to detect differences in glucose-derived breath CO2 kinetics between individuals with NGT and PDED, which correlate with differences in blood glucose kinetics. After a baseline sample, the optimal time for breath collection was between 1 and 3 h after the oral glucose load, similar to the time frame of a standard OGTT. Both the area under the curve, when multiple measurements are made over time, and the slope, when only before and after measurements are available, were shown to be effective. This noninvasive method may assist in recognition of undiagnosed PDED in at-risk individuals during the pre-diabetes stage of type 2 diabetes in a standard OGTT and, in the future, may also help increase compliance with diabetes screening in specific populations, such as obese children. In addition, the use of a point-of-care diagnostic breath 13CO2 analyzer and storable breath collection bags is suitable for large-scale population testing in research as well as the clinical setting. However, further research is necessary to validate this method as a viable diagnostic tool to be used in addition to or in place of existing methods. For example, it will be important to establish cutoff values between individuals with true NGT and those with PDED, determine within-subject variability and repeatability, elucidate the effects of dietary intake on breath test results, and explore the applicability of this method in pediatric care.
Acknowledgments
This study was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Small Business Innovation Research Grant R43 DK072637-01, awarded to BioChemAnalysis Corporation (M.J.) and subcontracted to UTMB (M.S.-M.). Clinical studies were conducted in the GCRC at UTMB, funded by NIH/National Center for Research Resources, U.S. Public Health Service, GCRC Grant M01 RR00073, and The Claude D. Pepper Older Americans Independence Center, funded by grant P30 AG024832 (J.G.). No other potential conflicts of interest relevant to this article were reported.
We thank William J. Durham, PhD, for his comments during the preparation of this manuscript.
Published ahead of print at http://care.diabetesjournals.org on 15 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW: The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 178:373–383, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM: insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab 88:2399–2403, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Hanley AJ, Festa A, D'Agostino RB Jr, Wagenknecht LE, Savage PJ, Tracy RP, Saad MF, Haffner SM: Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes 53:1773–1781, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR: Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 110:1245–1250, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Frayn KN: The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans 31:1115–1119, 2003 [DOI] [PubMed] [Google Scholar]
- 8.McGarry JD: Banting Lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Randle PJ: Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Lewanczuk RZ, Paty BW, Toth EL: Comparison of the [13C]glucose breath test to the hyperinsulinemic-euglycemic clamp when determining insulin resistance. Diabetes Care 27:441–447, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Harris JA, Benedict FG: A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4:370–373, 1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ: Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Felber JP, Ferrannini E, Golay A, Meyer HU, Theibaud D, Curchod B, Maeder E, Jequier E, DeFronzo RA: Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes 36:1341–1350, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Cahill GF Jr: Starvation in man. Clin Endocrinol Metab 5:397–415, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Fritz IB: Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev 41:52–129, 1961 [DOI] [PubMed] [Google Scholar]
- 18.Henriksson J: Muscle fuel selection: effect of exercise and training. Proc Nutr Soc 54:125–138, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Jequier E: Nutrient effects: post-absorptive interactions. Proc Nutr Soc 54:253–265, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, Goodpaster B, Wing RR, Simoneau JA: Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA: The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 72:96–107, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Wiernsperger NF: Is non-insulin dependent glucose uptake a therapeutic alternative? Part 1: physiology, mechanisms and role of non insulin-dependent glucose uptake in type 2 diabetes. Diabetes Metab 31:415–426, 2005 [DOI] [PubMed] [Google Scholar]