Abstract
OBJECTIVE—The aim of this study was to describe the natural history of insulin secretion and insulin sensitivity in the development of isolated impaired fasting glycemia (i-IFG), isolated impaired glucose tolerance (i-IGT), and combined IFG/IGT.
RESEARCH DESIGN AND METHODS—Baseline and 5-year follow-up data from the Inter99 study were used. Individuals with normal glucose tolerance (NGT) at baseline and i-IFG, i-IGT, combined IFG/IGT, or NGT at the 5-year follow-up were examined with an oral glucose tolerance test (n = 3,145). Insulin sensitivity index (ISI), homeostasis model assessment of insulin sensitivity (HOMA-IS), early-phase insulin release (EPIR), and insulin secretion relative to insulin action (disposition index) were estimated.
RESULTS—Five years before the pre-diabetes diagnoses (i-IFG, i-IGT, and IFG/IGT), ISI, HOMA-IS, EPIR, and disposition index were lower than in individuals who maintained NGT. During the 5-year follow-up, individuals developing i-IFG experienced a significant decline only in HOMA-IS, whereas individuals developing i-IGT experienced significant declines in ISI, EPIR, and disposition index. Individuals with IFG/IGT exhibited pronounced declines in ISI, HOMA-IS, EPIR, and disposition index during the 5-year follow-up.
CONCLUSIONS—A stationary reduced insulin secretion followed by a decline in primarily hepatic insulin sensitivity characterizes the transition from NGT to i-IFG. In contrast, low whole-body insulin sensitivity with a secondary lack of β-cell compensation is associated with the development of i-IGT. Thereby, i-IFG and i-IGT appear to result from different underlying mechanisms, which may have implications for the prevention and treatment of the diabetes that succeeds them.
During the past few years, it has been established that the pre-diabetic conditions of isolated impaired fasting glycemia (i-IFG), isolated impaired glucose tolerance (i-IGT), and combined fasting and postchallenge hyperglycemia (IFG/IGT) represent distinct pathways to diabetes. These pre-diabetic states are characterized by different degrees of insulin sensitivity, insulin secretion, and hepatic glucose output as well as secretion of glucagon and incretin hormones (1–8). Nevertheless, the primary abnormalities inherent in the different pre-diabetic conditions are still unknown.
Randomized trials have shown beneficial effects of lifestyle intervention on diabetes risk in individuals with i-IGT and IFG/IGT (9,10), but whether lifestyle interventions have the same preventive effects in individuals with i-IFG is not known. Indeed, a more profound insight into the pathogenesis of the disease is needed to optimize prevention and treatment of type 2 diabetes. In particular, focus on the initial defects responsible for hyperglycemia in the fasting and postprandial states is essential for interrupting the progression from normal to abnormal glucose metabolism.
Most previous studies have examined the pathophysiology of pre-diabetes in cross-sectional settings without knowing the time of onset of glycemic abnormalities. However, the observed abnormalities in pre-diabetes may be related to traits already apparent in the normoglycemic state. Prospective studies are therefore needed to clarify whether this is the case or whether the metabolic abnormalities associated with i-IFG, i-IGT, and IFG/IGT develop simultaneously with the increases in fasting and/or postchallenge plasma glucose levels.
The aim of this study was to describe the natural history of insulin sensitivity and insulin secretion during the progression from normal glucose tolerance (NGT) to the pre-diabetic states of i-IFG, i-IGT, and combined IFG/IGT.
RESEARCH DESIGN AND METHODS
Individuals from the Danish population-based study Inter99 (11) were used as the study population. The Inter99 study is a 5-year nonpharmacological intervention study with the aim of reducing the incidence of ischemic heart disease and type 2 diabetes in the Danish population.
All individuals were invited to a screening program at the Research Centre for Prevention and Health in Glostrup in 1999–2001. At baseline, 6,784 subjects were included in the Inter99 study (52.5% of the invited subjects). After 5 years, all eligible 6,784 individuals were reinvited to a health examination. A total of 4,735 individuals were classified as having NGT at baseline, and 68.2% of these (n = 3,229) attended the 5-year follow-up examination. Individuals with diabetes at the 5-year follow-up (n = 42) or with incomplete measures of fasting plasma glucose (FPG) or 2-h plasma glucose (n = 42) were excluded, leaving 3,145 individuals with baseline and 5-year follow-up data for analysis.
All participants gave written informed consent before taking part in the Inter99 study. The protocol was in accordance with the Helsinki Declaration, approved by the local ethics committee (KA 98 155), and registered as a clinical trial. The Inter99 study is described in detail elsewhere (11,12).
Glucose tolerance status
After an overnight fast, the participants had a standard 75-g oral glucose tolerance test (OGTT). Venous samples for measurement of plasma glucose and serum insulin concentrations were taken before glucose ingestion and after 30 and 120 min. Glucose was analyzed using the hexokinase/G6P-DH technique (Boehringer Mannheim, Mannheim, Germany), and insulin was analyzed with the fluoroimmunoassay technique (AutoDELFIA; Perkin Elmer-Wallac, Turku, Finland). The participants were classified into categories of glucose tolerance according to the World Health Organization 1999 criteria (13).
Data collection
Self-administered general questionnaires were completed before the participants’ first visit to the research center. Family history of diabetes was assessed by asking questions about parents’ and siblings’ histories of diabetes. An estimate of physical activity in minutes per week was obtained by combining answers of commuting and leisure-time physical activity (14). Smoking status was categorized as follows: daily smokers, occasional smokers, previous smokers, and never smokers. A 48-item food-frequency questionnaire was used to assess the participants’ dietary habits. Based on the participants’ intake of vegetables, fruit, fish, and fat, a dietary quality score ranging from 1 (unhealthy dietary habits) to 9 (healthy dietary habits) was developed (15). Measurement of waist circumference was taken to the nearest 0.5 cm halfway between the lowest point of the costal margin and highest point of the iliac crest.
Lifestyle intervention
All participants received individual lifestyle counseling, including a risk assessment based on age, sex, total and HDL cholesterol, systolic blood pressure, smoking, BMI, known diabetes, family predisposition, and previous heart disease (16). Individuals at high risk of developing ischemic heart disease were offered a low- or high-intensity lifestyle intervention at baseline. Individuals in the high-intensity intervention group were offered participation in group meetings with a clinical dietitian. The aim of the meetings was to increase the participants’ knowledge regarding the importance of smoking, diet, and physical activity in the prevention of type 2 diabetes and cardiovascular diseases. Participants in the low-intensity intervention group were only referred to their general practitioner. The randomization and intervention are described in detail elsewhere (11,12). A three-class variable based on the risk and intervention status (low-risk/no intervention, high-risk/low-intensity intervention, and high-risk/high-intensity intervention) was included in the present analyses, together with changes in different lifestyle factors, to adjust for a potential effect of the intervention.
Calculations
Insulin sensitivity was estimated by use of the insulin sensitivity index (ISI), which is based on plasma glucose and insulin measurements during OGTTs as well as information on body weight (17). The ISI correlates relatively well with the M value from a euglycemic-hyperinsulinemic clamp (17). Homeostasis model assessment (HOMA) of insulin sensitivity (HOMA-IS) (1/HOMA of insulin resistance) was also calculated (18). Early-phase insulin release (EPIR) was estimated from fasting insulin, 30-min insulin, and 30-min plasma glucose levels (19). EPIR correlates with first-phase insulin release measured during a hyperglycemic-hyperinsulinemic clamp. An estimate of disposition index (DI) was calculated by multiplying ISI with EPIR. Hence, DI reflects the ability of the β-cell to compensate for insulin resistance. When ISI was plotted against EPIR, the data points approximated a hyperbolic curve, suggesting that DI may be a good surrogate measure of β-cell function.
Statistical methods
The study population was divided into four groups of glucose tolerance based on the classification at the 5-year follow-up: 1) NGT, 2) incident i-IFG, 3) incident i-IGT, and 4) incident IFG/IGT. Estimates of ISI, HOMA-IS, EPIR, and DI were compared among the four groups before the diagnosis (baseline, all had NGT) and at the time of diagnosis (5-year follow-up) by use of the Wald test from multiple linear regression models. Changes in metabolic characteristics during the 5-year follow-up (Δ = 5-year follow-up minus baseline) were also analyzed in multiple linear regression models. Serum insulin levels, HOMA-IS, ISI, and DI were non-normally distributed and therefore were log-transformed before analysis. SAS (version 9.1; SAS Institute, Cary, NC) was used for statistical analysis.
Even though a relatively large number of statistical comparisons were performed in this study, we did not use P corrections, because the majority of the tests were predefined. Thus, some of the borderline and weakly significant findings may be related to noncausal associations and should be interpreted with some caution.
RESULTS
Before the pre-diabetes diagnosis (baseline)
Five years before the development of pre-diabetes, several characteristics differed among the groups (Table 1). The proportion of men and individuals with a family history of diabetes was highest in the groups who later developed i-IFG and IFG/IGT. Physical activity was lower in those who later developed i-IGT, and dietary quality score was lower in all groups progressing to pre-diabetes but only significant in those with subsequent i-IGT. At baseline, plasma glucose and insulin levels were higher, and ISI, HOMA-IS, EPIR, and DI were lower in individuals who progressed to i-IFG, i-IGT, or IFG/IGT than in those who maintained NGT status. Those who later progressed to i-IGT had lower baseline ISI than those who subsequently developed i-IFG. In contrast, baseline EPIR was slightly but significantly lower in those with subsequent i-IFG and IFG/IGT than in those who later developed i-IGT (Table 1).
Table 1.
Characteristics of individuals with NGT before development of i-IFG, i-IGT, or IFG/IGT or maintenance of NGT status
| NGT → NGT | NGT → i-IFG | NGT → i-IGT | NGT → IFG/IGT | P < 0.05* | |
|---|---|---|---|---|---|
| n | 2,842 | 83 | 192 | 28 | |
| Male sex (%) | 46.8 (45.0–48.7) | 77.1 (66.6–85.6) | 53.1 (45.8–60.3) | 67.9 (47.6–84.1) | a, c, d |
| Family history of diabetes (%) | 14.4 (13.2–15.8) | 22.9 (14.4–33.4) | 18.2 (13.0–24.4) | 39.3 (21.5;59.4) | a, c, e, f |
| Age (years) | 45.4 (45.2–45.7) | 48.6 (47.0–50.2) | 47.7 (46.6–48.7) | 49.0 (46.2–51.8) | a, b, c |
| BMI (kg/m2) | 25.2 (25.1–25.3) | 27.2 (26.4–28.1) | 26.4 (25.9–27.0) | 28.2 (26.7–29.6) | a, b, c, f |
| Waist circumference (cm) | 83.4 (82.9–83.8) | 92.3 (89.8–94.8) | 87.6 (85.9–89.2) | 95.4 (91.1–99.7) | a, b, c, e, f |
| Physical activity (min/week) | 305 (299–312) | 295 (260–331) | 275 (251–298) | 336 (276–396) | b |
| Dietary quality score (points) | 4.08 (4.03–4.14) | 3.69 (3.37–4.00) | 3.84 (3.63–4.04) | 3.64 (3.11–4.17) | b |
| Fasting plasma glucose (mmol/l) | 5.3 (5.3–5.3) | 5.7 (5.6–5.8) | 5.4 (5.3–5.4) | 5.8 (5.6–5.9) | a, c, d, f |
| 2-h plasma glucose (mmol/l) | 5.4 (5.4–5.5) | 5.8 (5.6–6.0) | 6.2 (6.0–6.3) | 6.4 (6.0–6.8) | a, b, c, d, e |
| Fasting serum insulin (pmol/l)† | 30 (21–43) | 38 (26–48) | 35 (24–56) | 34 (24–59) | a, b |
| 2-h serum insulin (pmol/l)† | 131 (83–196) | 148 (77–266) | 188 (124–284) | 161 (86–341) | a, b, c, d |
| ISI† | 2.64 (2.16–3.30) | 2.27 (1.85–3.00) | 2.13 (1.85–2.51) | 2.14 (1.63–3.02) | a, b, c, d |
| HOMA-IS† | 0.99 (0.67–1.42) | 0.74 (0.58–1.08) | 0.81 (0.51–1.20) | 0.78 (0.48–1.11) | a, b |
| EPIR† | 755 (559–973) | 637 (449–912) | 689 (494–1,014) | 501 (364–936) | a, c, d, f |
| DI† | 2,037 (1,464–2,728) | 1,507 (1,053–2,175) | 1,586 (1,112–2,182) | 1,209 (699–1,620) | a, b, c |
Data are unadjusted means and proportions (95% CI) unless otherwise indicated. Baseline, n = 3,145. P values are adjusted for age and sex.
P values for BMI were further adjusted for baseline physical activity level. P values for HOMA-IS, ISI, EPIR, and DI were further adjusted for family history of diabetes as well as baseline values of BMI, smoking, physical activity, and dietary quality. Wald test from linear models: a, i-IFG vs. NGT; b, i-IGT vs. NGT; c, IFG/IGT vs. NGT; d, i-IGT vs. i-IFG; e, IFG/IGT vs. i-IFG; f, IFG/IGT vs. i-IGT.
Unadjusted medians (interquartile range).
High-intensity lifestyle intervention was offered to 36.6% of those who maintained NGT status, 50.6% of those who developed i-IFG, 39.1% of those who developed i-IGT, and 46.4% of those who developed IFG/IGT (P < 0.05 for i-IFG versus NGT).
Time of pre-diabetes diagnosis (5-year follow-up)
At the 5-year examination, ISI, HOMA-IS, EPIR, and DI were still low in all three pre-diabetic groups (Table 2). However, ISI was lower in individuals with i-IGT and IFG/IGT than in those with i-IFG, whereas HOMA-IS was lower in individuals with i-IFG and IFG/IGT than in those with i-IGT. EPIR was equally low in individuals with i-IFG and i-IGT, but lower in those with IFG/IGT. DI was lower in individuals with i-IGT and IFG/IGT than in those with i-IFG.
Table 2.
Characteristics of individuals with incident i-IFG, i-IGT, IFG/IGT, or NGT (5-year follow-up)
| NGT → NGT | NGT → i-IFG | NGT → i-IGT | NGT → IFG/IGT | P < 0.05* | |
|---|---|---|---|---|---|
| n | 2,842 | 83 | 192 | 28 | |
| BMI (kg/m2) | 25.6 (25.5–25.8) | 28.2 (27.4–29.1) | 27.4 (26.8–27.9) | 29.1 (27.6–30.6) | a, b, c |
| Waist circumference (cm) | 86.5 (86.1–86.9) | 95.9 (93.4–98.5) | 91.8 (90.1–93.5) | 98.5 (94.1–102.9) | a, b, c, f |
| Physical activity (min/week) | 301 (296–307) | 299 (265–333) | 256 (233–279) | 309 (250–368) | b |
| Dietary quality score (points) | 4.60 (4.54–4.65) | 4.37 (4.06–4.69) | 4.25 (4.04–4.46) | 4.46 (3.89–5.03) | b |
| Fasting plasma glucose (mmol/l) | 5.2 (5.2–5.2) | 6.4 (6.3–6.4) | 5.4 (5.1–5.4) | 6.4 (6.2–6.6) | a, b, c, d, f |
| 2-h plasma glucose (mmol/l) | 5.2 (5.2–5.3) | 5.8 (5.6–6.0) | 8.6 (8.5–8.8) | 8.5 (8.1–8.9) | a, b, c, d, e |
| Fasting serum insulin (pmol/l)† | 27 (20–39) | 48 (34–67) | 37 (25–54) | 56 (37–91) | a, b, c, d, f |
| 2-h serum insulin (pmol/l)† | 139 (85–211) | 202 (103–292) | 365 (231–560) | 361 (246–571) | a, b, c, d, e |
| ISI† | 2.68 (2.19–3.43) | 1.99 (1.69–2.54) | 1.47 (1.27–1.67) | 1.36 (1.20–1.58) | a, b, c, d, e |
| HOMA-IS† | 1.11 (0.75–1.54) | 0.51 (0.36–0.73) | 0.79 (0.54–1.17) | 0.45 (0.33–0.67) | a, b, c, d, f |
| EPIR† | 746 (569–978) | 639 (380–960) | 670 (447–903) | 586 (365–1,001) | a, b, c, f |
| DI† | 2,084 (1,514–2,864) | 1,325 (918–1,949) | 1,047 (656–1,312) | 815 (516–1,185) | a, b, c, d, e |
Data are unadjusted means and proportions (95% CI) unless otherwise indicated. n = 3,145.
P values are adjusted for age, sex, and risk/intervention group. P values for BMI were further adjusted for 5-year physical activity level. P values for ISI, HOMA-IS, EPIR, and DI were further adjusted for family history of diabetes as well as 5-year values of BMI, smoking, physical activity, and dietary quality. Wald test from linear models: a, i-IFG vs. NGT; b, i-IGT vs. NGT; c, IFG/IGT vs. NGT; d, i-IGT vs. i-IFG; e, IFG/IGT vs. i-IFG; f, IFG/IGT vs. i-IGT.
Unadjusted medians (interquartile range).
Cross-sectional data of all individuals with NGT, i-IFG, i-IGT, and IFG/IGT at baseline in the Inter99 study (n = 6,006) showed the same pattern of insulin sensitivity and insulin secretion (supplemental Table A1, available in an online appendix at http://dx.doi.org/10.2337/dc08-1195).
Transition from normal to abnormal glucose regulation (5-year changes)
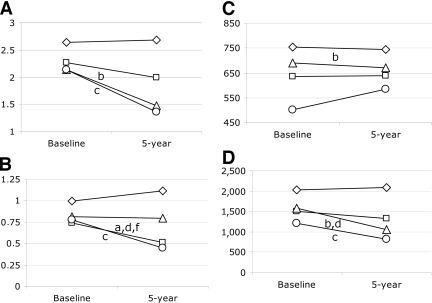
During the 5-year follow-up, different changes in insulin secretion and insulin sensitivity were observed in the groups developing i-IFG, i-IGT, and IFG/IGT compared with those who maintained NGT status (Fig. 1). ISI decreased significantly in individuals progressing to i-IGT and IFG/IGT but not in those who developed i-IFG even though ISI tended to fall (P = 0.227 vs. NGT; Fig. 1A). In contrast, HOMA-IS decreased significantly in individuals with i-IFG and IFG/IGT but not in those with i-IGT (P = 0.851 vs. NGT; Fig. 1B). A minor but significant decline in EPIR was observed for individuals with i-IGT compared with those with NGT (P < 0.001; Fig. 1C). The change in EPIR did not differ among the other pre-diabetes groups and the NGT group (P ≥ 0.154 for all other comparisons), even though EPIR seemed to increase in the IFG/IGT group during the 5-year follow-up (P = 0.393 vs. NGT; Fig. 1C). However, DI decreased significantly in individuals who progressed to i-IGT and IFG/IGT and tended to decrease in those who developed i-IFG (P = 0.066) (Fig. 1D).
Figure 1.
Baseline and 5-year values of ISI (A), HOMA-IS (B), EPIR (C), and DI (D) in 3,145 individuals developing i-IFG (□), i-IGT (▵), or IFG/IGT (○) or maintaining NGT status (⋄). Data are presented as medians. P values for differences in changes (5-year minus baseline) between groups are adjusted for age, sex, family history of diabetes, and risk/intervention group as well as for changes in BMI, smoking, physical activity, and dietary quality during the 5 years of follow-up. Wald test from linear models (P < 0.05): a, i-IFG vs. NGT; b, i-IGT vs. NGT; c, IFG/IGT vs. NGT; d, i-IGT vs. i-IFG, e, IFG/IGT vs. i-IFG; f, IFG/IGT vs. i-IGT.
CONCLUSIONS
This study is the first to report abnormalities in insulin secretion and insulin sensitivity before the development of i-IFG, i-IGT, and IFG/IGT. We found that different disturbances of insulin sensitivity, absolute insulin secretion, and insulin secretion relative to insulin resistance were apparent 5 years before the pre-diabetic states of i-IFG, i-IGT, and IFG/IGT were breached. These findings support the view that i-IFG and i-IGT represent two distinct pathological mechanisms.
Progression from NGT to i-IFG
Absolute and relative insulin secretion (EPIR and DI) were significantly impaired in normoglycemic individuals who subsequently progressed to i-IFG, but during the development of i-IFG, EPIR and DI did not decrease further, indicating that a progressive loss of the ability to secrete sufficient amounts of insulin is not a major feature in the early states of fasting hyperglycemia. Instead, an underlying, more stationary β-cell failure seems to be present in these individuals. Reduced absolute and/or relative insulin secretion has previously been demonstrated in individuals with i-IFG (1,2,5,6,20). However, all of these studies were cross-sectional, and they were therefore not able to detect whether this feature occurred concomitantly with the development of hyperglycemia or whether it was manifest years before overt hyperglycemia was present. In this particular study, family history of diabetes was significantly more prevalent in individuals with i-IFG than in those with NGT. This finding could indicate a role for genetics in the development of i-IFG, which should be examined in future studies.
Prior to the development of i-IFG and at time of diagnosis, ISI was significantly reduced compared with that in individuals with NGT, but ISI did not change significantly during the development of i-IFG. The finding of reduced insulin sensitivity contrasts with recent observations in a smaller subsample of the Inter99 population studied with the euglycemichyperinsulinemic clamp technique (1). In addition, other studies using the euglycemic-hyperinsulinemic clamp technique have reported normal peripheral insulin sensitivity in individuals with i-IFG (1,4,6,7), whereas only a few studies reported low insulin sensitivity (2,3). Accordingly, the low insulin sensitivity we observed in individuals with i-IFG may indicate that estimates of insulin sensitivity derived from OGTTs are not as precise as measures obtained from “gold standards” such as the euglycemic-hyperinsulinemic clamp technique. The clamp technique provides estimates of predominantly peripheral (muscle) insulin action and only to a lesser extent hepatic insulin action. Whether estimates based on glucose and insulin levels during OGTTs also reflect mainly peripheral insulin sensitivity needs to be addressed in future studies. HOMA-IS is based on fasting glucose and insulin levels and therefore is assumed to reflect hepatic insulin sensitivity (5). The pre-diabetic state, i-IFG, therefore appears to be caused by stationary abnormalities in β-cell function in combination with a progressive decline in hepatic insulin sensitivity.
In the present study, the proportion of men was 65% higher in the i-IFG group than in the group with NGT. A higher prevalence of i-IFG in men has previously been reported by others (8,20,21). Because men in general have lower serum insulin levels than women (20), the different sex distributions in the i-IFG and i-IGT groups may have contributed to some of the observed differences in insulin secretion between the groups. Further studies are needed to clarify the impact of sex differences on the pathophysiology of i-IFG and i-IGT.
Progression from NGT to i-IGT
Five years prior to the i-IGT diagnosis, EPIR was not significantly different from that in those who maintained NGT status. However, during the development of i-IGT, small but significant declines in EPIR and DI were observed. This finding indicates that a progressive, and thereby age-dependent, loss of insulin secretion is involved in the development of postchallenge hyperglycemia. Indeed, age-dependent loss of insulin secretion is a well-established feature of overt type 2 diabetes as documented in the UK Prospective Diabetes Study (22). The present data indicate that this feature may be more central to patients who have developed type 2 diabetes via i-IGT compared with via i-IFG, but this suggestion remains to be shown in prospective studies including patients with overt type 2 diabetes studied before and after the diabetes diagnosis.
Numerous cross-sectional studies showed that individuals with i-IGT are insulin resistant (1–7). In this study, we take this finding a step further by documenting that low insulin sensitivity is present already 5 years before the demonstration of i-IGT. The progressive decline of insulin secretion therefore seems to be secondary to the low insulin sensitivity and thus represents an inadequate compensatory insulin secretory response. This primary decline in insulin sensitivity could be caused by an adverse lifestyle, as indicated by the lower levels of physical activity and dietary quality compared with those for individuals with NGT. However, genetic factors or in utero preprogrammed abnormalities could also have contributed.
Progression from NGT to IFG/IGT
Not surprisingly, the IFG/IGT phenotype was characterized by the same abnormalities in insulin sensitivity as seen in those who developed i-IFG (decline in HOMA-IS) and i-IGT (decline in ISI). Interestingly, EPIR tended to increase during the development of IFG/IGT. This finding supports the notion that modest increases in plasma glucose levels may induce β-cell proliferation and survival, whereas prolonged exposure to significant elevations in plasma glucose levels can cause impaired β-cell proliferation and increased β-cell failure and apoptosis (23). Based on our observations, we suggest that some of the mechanisms leading to an early compensatory increase in β-cell function may be initiated by elevated fasting plasma glucose (i-IFG and IFG/IGT) but not by postprandial plasma glucose levels (i-IGT). In that respect, it was of interest that individuals who developed IFG/IGT had more characteristics in common with those who developed i-IFG than with those who developed i-IGT (e.g., sex distribution and family history of diabetes). We therefore suggest that individuals with elevated 2-h plasma glucose levels should be separated into an i-IGT and an IFG/IGT group instead of being classified in one group of IGT individuals as currently suggested by the World Health Organization (13).
Study limitations
Our estimates of insulin secretion and insulin sensitivity were based on OGTTs and therefore may correlate with the classification of the pre-diabetic groups. In addition, it should be noted that disposition indexes based on estimates of insulin secretion and insulin sensitivity derived from the same test (e.g., an OGTT) may not be as solid and reliable as disposition indexes based on independent tests. Ideally, estimates of insulin secretion and action should be based on “gold standard” tests such as the glucose clamp technique. However, this is not feasible in large-scale epidemiological studies, and we believe that proxy measures are reliable with large datasets such as that in this study. However, caution should be taken when one is comparing estimates of insulin secretion based on intravenous versus oral glucose tolerance tests. In particular, the gut incretin hormones glucagon-like-peptide-1 and glucose-dependent insulinotropic polypeptide may influence the results, because the secretion of these hormones seems to differ significantly among individuals with different types of pre-diabetes (1).
The classification of glucose tolerance status was based on single OGTTs. Accordingly, it is likely that some individuals may have been misclassified because of normal day-to-day variations in plasma glucose. In addition, the intraindividual variation in serum insulin levels is large (24), affecting the estimates of insulin secretion and action. Small changes in the estimates would therefore be expected if the same measurements were repeated on a separate day.
In general, the changes in insulin secretion and insulin sensitivity during the 5-year follow-up were relatively small. However, because even small changes in plasma glucose and insulin levels may have consequences for glucose homeostasis, we believe our findings are biologically relevant. Nevertheless, the biological significance of such small disturbances needs to be clarified in other studies.
Finally, it is possible that individuals who participated in group lifestyle counseling (high-intensity intervention) may have changed their physical activity level, smoking status, diet, and body composition more than those who were not included in the high-intensity intervention during the 5-year follow-up. However, by adjusting for intervention status as well as for changes in body composition and lifestyle factors, we believe that our results are reliable and can be generalized to other white Caucasian populations.
In summary, this study showed that impairments in glucose metabolism occur many years before it is possible to classify individuals as abnormally hyperglycemic either in the fasting or postchallenge state. Hyperglycemia in the fasting state (i-IFG) seems primarily to be caused by an inherent insulin secretory dysfunction followed by a decline in hepatic insulin sensitivity. In contrast, the development of postchallenge hyperglycemia (i-IGT) mainly seems to be caused by low whole-body insulin sensitivity followed by a progressive decline in β-cell function, indicating a loss of β-cell compensation. This study supports the notion that the pre-diabetic states of i-IFG and i-IGT may have different etiological and pathophysiological origins, which in turn may have implications for future prevention and treatment of overt type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported by the Danish Ministry of Science, Technology and Innovation, the Danish Diabetes Association, the Novo Nordisk Foundation, the Foundation of Gerda and Aage Haensch, and an EXGENESIS grant (005272) from the European Union. The Inter99 study was initiated by T. Jørgensen (principal investigator [PI]), K. Borch-Johnsen (co-PI), H. Ibsen, and T. Thomsen. The Inter99 steering committee comprises T. Jørgensen, K. Borch-Johnsen, and C. Pisinger.
K.B.-J. is employed as director and professor of the Steno Diabetes Center, a hospital providing health service for the public health care system but owned by Novo Nordisk A/S, Bagsvaerd, Denmark; holds stock shares in Novo Nordisk; and has received honoraria for invited lectures by Novo-Nordisk, Bristol-Myers Squibb, Novartis, Pfizer, Hermedico, and AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
We thank the staff of Inter99 and all the participants as well as statistician, Dorte Vistisen, PhD.
Published ahead of print at http://care.diabetesjournals.org on 10 December 2008.
Clinical trial reg. no. NCT00289237, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Færch K, Vaag A, Holst J, Glümer C, Pedersen O, Borch-Johnsen K: Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic α and β cell function but differential roles of incretin hormones and insulin action. Diabetologia 51:853–861, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48:2197–2203, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Laakso M, Zilinskaite J, Hansen T, Boesgaard T, Vänttinen M, Stancáková A, Jansson PA, Pellmé F, Holst J, Kuulasmaa T, Hribal M, Sesti G, Stefan N, Fritsche A, Häring H, Pedersen O, Smith U, EUGENE: Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 51:502–511, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bock G, Man CD, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R: Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 55:3536–3549, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55:1430–1435, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Meyer C, Szoke E, Pimenta W, Mitrakou A, Woerle HJ, Gerich J, Van-Haeften T: Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29:1909–1914, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Wasada T, Kuroki H, Katsumori K, Arii H, Sato A, Aoki K, Jimba S, Hanai G: Who are more insulin resistant, people with IFG or people with IGT? Diabetologia 47:759–760, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T: Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care 26:868–874, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen T, Borch-Johnsen K, Thomsen TF, Ibsen H, Glümer C, Charlotta P: A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehab 10:377–386, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Research Centre for Prevention and Health, Glostrup Hospital, Denmark: The Inter99 Study [Internet], 2008. Available from http://www.Inter99.dk. Accessed 14 October 2008
- 13.World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999
- 14.von Huth Smith L, Borch-Johnsen K, Jørgensen T: Commuting physical activity is favourably associated with biological risk factors for cardiovascular disease. Eur J Epidemiol 22:771–779, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Toft U, Kristoffersen LH, Lau C, Borch-Johnsen K, Jørgensen T: The Dietary Quality Score: validation and association with cardiovascular risk factors: the Inter99 study. Eur J Clin Nutr 61:270–278, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Thomsen TF, Davidsen M, Ibsen H, Jørgensen T, Jensen G, Borch-Johnsen K: A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD and the Copenhagen Risk Score. J Cardiovasc Risk 8:291–297, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB: Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Res Clin Pract 47:177–184, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, Renn W, Gerich J: Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23:295–301, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Williams JW, Zimmet PZ, Shaw JE, De-Court, Cameron AJ, Chitson P, Tuomilehto J, Alberti KGMM: Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius: does sex matter? Diabet Med 20:915–920, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE: Differences in height explain gender differences in the response to the oral glucose tolerance test—the AusDiab study. Diabet Med 25:296–302, 2008 [DOI] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 44:1249–1258, 1995 [PubMed] [Google Scholar]
- 23.Chang-Chen KJ, Mullur R, Bernal-Mizrachi E: β-Cell failure as a complication of diabetes. Rev Endocr Metab Disord 9:329–343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, Heine RJ: Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia 39:298–305, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.