Abstract
OBJECTIVE—Recent evidence suggests that, in children, traditional markers of metabolic disturbance are related only weakly to physical activity. We therefore sought to establish the corresponding relationships with newer metabolic markers.
RESEARCH DESIGN AND METHODS—This was a nonintervention longitudinal study of 213 healthy children recruited from 54 schools in Plymouth, U.K. MTI accelerometers were used to make objective 7-day recordings of physical activity at ages 5 ± 0.3 (mean ± SD), 6, 7, and 8 years. Overall physical activity was taken as the average of the four annual time points. The metabolic markers at 8 years were adiponectin, leptin, high-sensitivity C-reactive protein (hsCRP), and insulin resistance (homeostasis model assessment). Potential confounders included percent body fat measured by dual-energy X-ray absorptiometry and diet measured by food frequency questionnaire.
RESULTS—Whereas physical activity did not correlate with insulin resistance (r = −0.01), leptin (r = +0.04), or hsCRP (r = +0.01) independently of percent body fat, it did correlate with adiponectin, but inversely (r = −0.18, P = 0.02). This unexpected inverse relationship was strongest among the less active children (physical activity < median: r = −0.30, P = 0.01) but negligible in the more active children (physical activity > median: r = +0.04, P = 0.76). Adiponectin was significantly higher (0.52 SD, P < 0.01) in the least active tertile compared with the other two tertiles. Insulin resistance, however, did not differ across the physical activity tertiles (P = 0.62).
CONCLUSIONS—Adiponectin levels in children are highest among those who are least active, but their insulin resistance is no different. Adiponectin has a known insulin-sensitizing effect, and our findings are consistent with a selective effect at low levels of physical activity.
Childhood obesity is increasing throughout the industrialized world, and both inactivity and overnutrition are believed to contribute (1). Obesity is of concern because it drives the insulin resistance that is believed to underlie diabetes, cardiovascular disease, and metabolic syndrome (2).
Two reviews looking at the relationship between physical activity and the traditional metabolic markers in children showed only weak associations. Wareham et al. (3) reviewed 12 such studies relating habitual physical activity to insulin resistance and found five that reported a weak-to-moderate correlation. Froberg and Andersen (4) reported similar findings with studies measuring physical activity and lipids, in which five reported no association and the remaining six a weak association. The same authors also reported six studies in which the relationship between physical activity and blood pressure was investigated: three showed an association and three did not.
Markers such as leptin, adiponectin, and C-reactive peptide (CRP) have received less attention with respect to physical activity, although all three show weak-to-moderate associations with the traditional markers of metabolic health in children. Leptin appears to be positively correlated with insulin resistance (5) and adiponectin inversely correlated (6), although Ong et al. (7) did suggest a weak positive association for adiponectin in boys. A study of adolescents reported significantly higher CRP levels in young male subjects with three or more traditional risk factors compared with those who had fewer risk factors, although no such association was found in young female subjects (8).
Thus, whereas there are (weak) associations in children between physical activity and the more traditional metabolic markers, the newer markers do not appear simply to be surrogates for them. Given that the mechanisms whereby physical activity improves metabolic health are incompletely understood and that metabolic disease is believed to be an inflammatory state (9), it seemed appropriate to consider whether the impact of physical activity on metabolic health might be mediated by or reflected in adipokines (e.g., adiponectin and leptin) and/or inflammatory reactants (e.g., high-sensitivity CRP [hsCRP]).
Only five studies of children/adolescents have investigated the relationship between physical activity and one or more of these novel metabolic markers (10–14). Three studies of three reported no significant association between physical activity and adiponectin (10,12,13), and only one of three reported an association with CRP (14). Two of two reported that serum leptin was inversely related to physical activity, independently of body composition (10,11). However, none of these five studies measured physical activity objectively. Three were exercise training intervention studies (11,13,14), and the other two were cross-sectional studies that measured physical activity using self-report questionnaires (10,12).
In this study we investigated the relationships between physical activity and three of the more recent markers of metabolic disturbance using an objective measure of physical activity whose reliability was optimized by a longitudinal design.
RESEARCH DESIGN AND METHODS
EarlyBird is a nonintervention prospective cohort study of 307 healthy children (170 boys and 137 girls). The children were recruited at age 4.9 ± 0.3 years (school entry: January 2000–January 2001) from 54 Plymouth schools. Schools were stratified into quartiles according to proportion of pupils entitled to free school meals. A random selection was made from each, ensuring a wide socioeconomic mix representative of the Plymouth area (Index of Multiple Deprivation 2004 score: cohort 26.1, Plymouth 26.3, and England 21.7). Most children (98%, n = 302) were white Caucasian and five children (2%) were of mixed race, reflecting the racial mix of the area. Local research ethics committee approval was obtained in 1999, and the study's rationale, recruitment procedures, and protocol have been reported in detail elsewhere (15).
Measurements
Physical activity.
Actigraphs (formerly CSA accelerometers; Manufacturing Technology, Fort Walton Beach, FL) are small (50 × 41 × 15 mm), lightweight (43 g), robust, tamperproof, and of good technical reproducibility (between-monitor coefficient of variation [CV] 5% and within-monitor CV <2%), (16) and correlate well with criterion measures of free-living activity-related energy expenditure by indirect calorimetry (r = 0.92 with body weight) (17). They record changes in vertical acceleration 600 times per minute, which for the present study data were integrated into 1-minute epochs. The actigraphs were worn on an adjustable elastic belt around the child's waist and were set to run continuously for 7 days at each of the four annual time points, 5, 6, 7, and 8 years (mean interval between follow-ups was 1.0 years, SD 1 month). The children were asked to wear the actigraph every day from when they got up in the morning to when they went to bed at night, taking it off only for water-based activities (e.g., swimming, bathing, or showering). The actigraphs were worn during the week that followed the measurement of metabolic health to eliminate any acute effect that physical activity may have on such factors. Parent-reported periods of noncompliance and periods with zero accelerometer counts for ≥17 consecutive minutes (assumed to be unreported noncompliance) were replaced with the mean of counts recorded at the same clock time on the other days. The sensitivity of each accelerometer was measured under controlled conditions by a motorized turntable (16). Seasonality was measured on a continuous scale by the number of relevant daylight hours (from 8 a.m.–9 p.m.) for the week the accelerometer was worn.
Two components of physical activity were calculated from the actigraph data: total physical activity volume and minutes spent in moderate- and vigorous-intensity physical activity (MVPA). MVPA here is equivalent to ≥3 METs (18) (resting energy expenditure = 1 MET) and equates to ≥2,500 actigraph counts/min. Others have equated 3 METs to a walking speed of ∼4 km/h (18), and our own calibration trials (unpublished data) indicate that prepubertal children walking at 4 km/h average ∼2,500 actigraph counts/min. These data are similar to calibration data reported by Schmitz et al. (8- to 12-year-old children walking at 4 km/h = 3.2 METs = 2,359 counts/min) (17). We reported previously that the average of two annual time points of physical activity recordings is required to provide >80% reliability in ranking/positioning individual children (19). For this reason, only children who wore the actigraph on at least two of the four annual time points (each for a minimum of 9 h/day for ≥5 days) were deemed to have a sufficiently reliable measure of physical activity.
Diet.
A validated food frequency questionnaire, completed by the parents, was used to measure the quality of the child's diet (20) at each of the four annual time points. The frequency of foods consumed per week that were either high in fat or high in sugar were derived from 11 and 6 questions, respectively.
Body fat.
Percent body fat at age 8 years was obtained from a whole-body dual-energy X-ray absorptiometry scan performed with the Lunar DPX-L pencil beam densitometer. BMI was calculated from measures of weight and height and age-adjusted standard deviation scores (SDSs) for BMI were derived from 1990 U.K. reference data.
Metabolic markers.
A venous blood sample was taken at ∼9 a.m. after an overnight fast. Serum insulin was measured using an Immulite analyzer (Diagnostic Products). The cross-reactivity with proinsulin was <1%, the interassay CV was ∼9%, and the detection limit of the assay was 2.0 mU/l. Glucose was measured using a Cobas Integra 700 analyzer (Roche Diagnostics, Welwyn Garden City, Herfordshire, U.K.), with an interassay CV of ∼2%. The values for insulin and glucose were used to derive a measure of insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR) program. HOMA-IR has been validated against the euglycemic clamp (r = 0.91) in healthy children (21). Adiponectin, leptin, and hsCRP were measured on thawed serum (stored at −85°C for between 6 and 18 months) at the Department of Vascular Biochemistry, University of Glasgow. Adiponectin was measured by ELISA (R&D Systems, Abingdon, U.K.) with an interassay CV of 7%. Leptin was measured by an in-house radioimmunoassay validated against the commercially available Linco assay. The interassay CV was <10% over the sample concentration range and the detection limit of the assay was 0.5 ng/ml. CRP was measured by an automated particle-enhanced immunoturbidimetric assay (Roche Diagnostics). The method has a lower limit of sensitivity of 0.1 mg/l and interassay CV of <3%.
Statistical analysis
Each 7-day sample of physical activity was adjusted for seasonality and for the sensitivity of each actigraph device before the annual recordings were averaged. Diet quality was also averaged over the four annual time points. The distribution of BMI, percent body fat, and all four metabolic markers was positively skewed, and therefore all analyses were carried out on log-transformed data. Analysis of covariance was performed, controlling for age, to establish sex differences for physical activity, BMI, percent body fat, and the four metabolic markers. Means (95% CI) of logged data were back-transformed. Hierarchical linear regression analysis was performed to quantify the association between physical activity (total physical activity and MVPA) and the metabolic markers with 1) age and 2) age, diet, and percent body fat already entered into the model as potential confounders. This regression analysis was carried out on boys and girls separately and together (controlling for sex). ANCOVA was also used to compare the distribution of insulin resistance and adiponectin (both internally derived SDSs) across tertiles of physical activity independent of sex, age, diet, and percent body fat. All data analysis was performed using SPSS version 14.0.
RESULTS
Attrition/compliance
During the 3-year period (5–8 years of age), 21 boys and 16 girls of the 307 recruited left the study. There was no difference in the baseline BMI SDSs between those who left by the 8-year visit and those who remained (difference 0.06 SDS, P = 0.76). Of the 270 children who remained in the study at 8 years, one child was entering puberty (established by a detectable level of the luteinizing hormone), 30 children did not have a sufficiently reliable measure of physical activity, and a further 18 children did not have a measure of body fat from dual-energy X-ray absorptiometry. Of the remaining 221 children a further 8–19 children did not have a measure for one or more of the metabolic markers. The final analyses were therefore based on 202–213 children (55% boys), depending on the risk factor being analyzed. There was no difference in BMI SDS at 8 years between those included and those excluded from the analysis (difference 0.03 SDS, P = 0.85). Sample sizes of n ≥ 200 can deem partial correlations of r ≥ 0.20 statistically significant (P < 0.05) with 80% power.
Subject characteristics
A summary of the subject characteristics by sex is presented in Table 1. The girls were less active than the boys, spending on average 11 min/day less in MVPA. The frequency with which children consumed foods that were high in fat or high in sugar did not differ by sex. BMI (measured as weight in kilograms divided by the square of height in meters and age/sex-specific SDSs) and percent body fat were significantly higher in the girls than in the boys. Levels of HOMA-IR, leptin, and hsCRP were significantly less favorable (higher) in the girls, yet their levels of adiponectin were slightly, although not significantly, more favorable (higher) than those in the boys.
Table 1.
Subject characteristics
| Boys | Girls | P | |
|---|---|---|---|
| n | 117 | 96 | |
| Age (years) | 7.83 (7.76–7.90) | 7.83 (7.77–7.89) | 0.92 |
| Physical activity | |||
| Total volume (units × 105) | 38.1 (36.5–39.7) | 34.9 (33.7–36.1) | <0.001 |
| Moderate and vigorous (min/day) | 56.0 (52.0–60.0) | 45.1 (42.0–48.1) | <0.001 |
| Diet | |||
| Foods high in fat (frequency/week) | 19.0 (18.1–19.9) | 18.8 (17.8–19.8) | 0.81 |
| Foods high in sugar (frequency/week) | 21.2 (20.2–22.2) | 22.0 (20.7–23.2) | 0.38 |
| Body composition | |||
| BMI* (kg/m2) | 16.5 (15.8–17.2) | 17.5 (17.0–18.0) | <0.01 |
| BMI (SDS) | 0.27 (0.02–0.51) | 0.56 (0.33–0.78) | 0.06 |
| Body fat (%)* | 16.4 (14.1–18.7) | 23.8 (22.0–25.5) | <0.001 |
| Metabolic markers | |||
| Insulin resistance (HOMA-IR)* | 0.37 (0.32–0.43) | 0.46 (0.41–0.51) | <0.01 |
| Adiponectin (μg/ml)*† | 11.6 (10.5–12.8) | 12.6 (11.6–13.6) | 0.11 |
| Leptin (ng/ml)*‡ | 3.22 (2.63–3.95) | 5.05 (4.33–5.87) | <0.001 |
| hsCRP (mg/l)*§ | 0.43 (0.30–0.58) | 0.60 (0.46–0.77) | 0.05 |
Data are means (95% CI).
Values are back-transformed means and 95%CI of log data.
Boys n = 116, girls n = 94.
Boys n = 115, girls n = 93.
Boys n = 111, girls n = 91.
Associations
Table 2 reports the associations between physical activity (total physical activity and MVPA) and the metabolic markers. There were no clinically or statistically significant associations in either sex between physical activity and insulin resistance, leptin, or hsCRP, before or after controlling for diet and percent body fat. In girls, there were moderate inverse correlations between physical activity and adiponectin, which strengthened slightly after adjustment for diet and percent body fat. The association was strongest for MVPA, explaining nearly 11% (r = −0.33, P < 0.01) of the variation in adiponectin. The corresponding associations in the boys were also inverse although weaker and not statistically significant (r = −0.13, P = 0.21). When all children were combined (controlling for sex), the overall association between physical activity and adiponectin was significant (r = −0.20, P = 0.01). Further analysis revealed that this inverse linear relationship was largely attributable to the children whose physical activity lay below the median (r = −0.31, P < 0.01) rather than to those above the median (r = +0.05, P = 0.68) where the gradient of association leveled off (interaction term P = 0.03). A model containing a logarithmic function of physical activity confirmed this curvilinear association over the entire range of physical activity, improving the r value slightly from −0.20 to −0.23.
Table 2.
Associations between objectively-measured physical activity (5–8 years) and metabolic markers at 8 years: partial correlation (p)
| Metabolic risk factors | Model | All*
|
Boys
|
Girls
|
|||
|---|---|---|---|---|---|---|---|
| Total PA | MVPA (min) | Total PA | MVPA (min) | Total PA | MVPA (min) | ||
| Insulin resistance | Model 1 | −0.06 (0.46) | −0.07 (0.33) | −0.06 (0.56) | −0.03 (0.80) | −0.06 (0.59) | −0.13 (0.25) |
| Model 2 | −0.01 (0.88) | 0.00 (0.98) | −0.02 (0.85) | +0.05 (0.62) | −0.03 (0.80) | −0.12 (0.24) | |
| Adiponectin | Model 1 | −0.17 (0.02) | −0.17 (0.02) | −0.14 (0.16) | −0.14 (0.14) | −0.20 (0.08) | −0.22 (0.05) |
| Model 2 | −0.20 (0.01) | −0.20 (0.01) | −0.14 (0.16) | −0.13 (0.21) | −0.30 (<0.01) | −0.33 (<0.01) | |
| Leptin | Model 1 | −0.06 (0.38) | −0.04 (0.56) | −0.01 (0.96) | −0.01 (0.92) | −0.14 (0.20) | −0.11 (0.30) |
| Model 2 | 0.00 (0.96) | +0.02 (0.64) | +0.02 (0.77) | −0.01 (0.90) | −0.03 (0.66) | +0.05 (0.50) | |
| hsCRP | Model 1 | −0.03 (0.71) | −0.02 (0.80) | −0.11 (0.30) | −0.09 (0.35) | +0.13 (0.26) | +0.12 (0.26) |
| Model 2 | −0.01 (0.93) | +0.01 (0.91) | −0.12 (0.24) | −0.10 (0.29) | +0.14 (0.18) | +0.15 (0.17) | |
Data are partial r (P). Model 1 controlled for age, seasonality, and between-actigraph variation. Model 2 as model 1 with further adjustment for diet and percent body fat.
Controlled for sex. PA, physical activity.
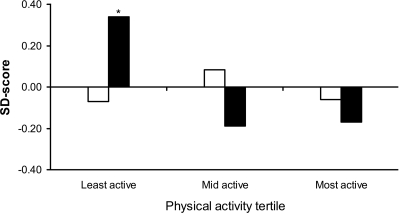
Figure 1 shows mean insulin resistance and adiponectin (both internally derived SDSs) by tertile of physical activity. Whereas levels of insulin resistance did not differ across the tertiles (P = 0.47), adiponectin was significantly higher (effect size 0.52 SD, P < 0.01) in the least active group compared with the middle and most active groups who had adiponectin levels similar to each other (effect size 0.02 SD, P = 0.96).
Figure 1.
Insulin resistance and adiponectin by tertiles of physical activity. *P < 0.01 for least active versus mid active and least active versus most active. □, insulin resistance; ▪, adiponectin.
CONCLUSIONS
This study in children reports findings similar to those of others for insulin resistance, leptin, and hsCRP but reveals an association with adiponectin that has not been reported previously. Insulin resistance, leptin, and hsCRP measured at age 8 years were unrelated to physical activity. Adiponectin, on the other hand, was inversely related to physical activity and remained so after adjustment for body fat and diet quality. This finding seems at first glance paradoxical, given that lower metabolic risk in children is reportedly associated with both higher physical activity (3,4) and higher adiponectin (6).
Of the three previous studies to have investigated the link between physical activity and adiponectin in children (10,12,13), two showed trends consistent in direction with the findings of the present study, but neither reached statistical significance. Platat at el. (10) reported that adiponectin levels were 9% higher (effect size ∼0.2 SD, P = 0.09) in the least physically active tertile compared with the most active tertile. Nassis at al. (13) found that the mean adiponectin level was 5% higher (effect size ∼0.2 SD, P > 0.05) before than after a 12-week exercise training program. The smaller effect sizes in these studies compared with those we observed (∼0.5 SD) may be due to methodological differences. Platat et al. used questionnaires to categorize the children according to participation in “organized leisure-time physical activity” rather than total physical activity, which may underestimate differences between the most and least physically active groups. The exercise training program delivered by Nassis et al. may not have increased “total” physical activity by as much as intended perhaps because of poor compliance and/or the possibility that children offset session-related increases with less activity than usual during the rest of the week. In addition, Nassis et al. measured adiponectin before and after 12 weeks of “increased” activity, whereas in the present study we compared adiponectin levels between groups of children whose average activity levels have been very different for at least 3 years (5–8 years of age).
Yatagai et al. (22) studied the relationship between adiponectin and physical activity in adults and, in line with the findings of Nassis et al. (13) in children, showed that serum adiponectin fell significantly in response to a 6-week exercise training program in men (n = 12). Despite a strong inverse relationship between insulin resistance and adiponectin before the intervention (r = −0.63), the preintervention to postintervention decrease in adiponectin was accompanied by a decrease in insulin resistance (measured by intravenous glucose tolerance tests), although fasting insulin did not change. The authors suggested that “increased insulin action induced by the exercise” may suppress the expression and/or secretion of adiponectin. This interpretation is supported by the in vitro study by Fasshauer et al. (23), who demonstrated a dose-dependent reduction in the expression of adiponectin mRNA in 3T3-L1 adipocytes in response to insulin. Our data are consistent with this finding insofar as adiponectin levels were higher in the least active tertile of children, whereas insulin resistance remained constant across the physical activity range. We interpret the observation to mean that when levels of physical activity are insufficient to maintain insulin sensitivity, adiponectin may be secreted to compensate. In support of this hypothesis, we found that the association between adiponectin and insulin resistance differed by activity group. Although the interaction fell just short of statistical significance (P = 0.08), the correlation between adiponectin and insulin resistance appeared to be stronger when physical activity levels were lower (r = −0.21 physical activity < median versus r = +0.08 for physical activity > median). A review of the adult literature was also consistent with this hypothesis: 11 of 14 studies showed that exercise decreased insulin resistance (or surrogate of it) without changing adiponectin (24). These findings suggest that the compensatory secretion of adiponectin may not be required when physical activity levels are sufficient to improve insulin sensitivity.
The weak independent association between physical activity and insulin resistance shown in the present study (r = −0.07 to 0.00) is consistent with the reports reviewed by Wareham et al. (3). Although half of the studies did report a significant inverse association, none of these measured physical activity objectively. Since that review, the European Youth Heart Study reported an inverse association (r = −0.17) between objectively measured physical activity and insulin resistance in older children (aged 9–15 years), although not independently of body fat (25). Others have found an inverse relationship between leptin and physical activity (10,11), which in the present study we did not. CRP was not associated with physical activity in this study nor in two of the three other studies that have reported it (10,13), suggesting that this marker of low-grade inflammation is not influenced by the level of habitual physical activity in children. The independent associations of physical activity with insulin resistance, leptin, and hsCRP were never greater than r = 0.02, and, therefore, it would seem unlikely that this study has failed to detect any meaningful underlying relationships.
This study has strengths and limitations. As the “true” underlying associations between physical activity and metabolic markers are likely to be subtle, both the exposure and outcome variables need to be measured reliably to reveal them. Fewer studies are now using questionnaires to measure physical activity, and more are using objective assessment methods such as accelerometers, which offer a greater degree of reliability. However, although physical activity is relatively consistent from year to year (r = ∼0.5), reliability is lost by sampling just 1 week per year. By taking the average across two, three, or four annual time points (mean 3.5 in this report), we were able to improve the reliability of the physical activity measure from 71% (one time point) to 88%. The present study measured total adiponectin rather than the high-molecular-weight adiponectin (deemed the biologically active form). However, owing to the less precise measure of biologically active adiponectin, it is unlikely that the associations reported here overestimate the true underlying association between adiponectin and physical activity. Potential confounders such as age, diet quality, and body fat did not account, even partly, for the inverse relationship observed between physical activity and adiponectin but in fact appeared to strengthen it. Finally, this study is based on a single population of Caucasian children living in southwest England. The homogeneity of age and race may have been important to revealing the associations we have reported, but the findings may not be generalizable to other racial groups or to older age-groups (adolescents or adults).
To our knowledge, this is the first study to investigate the relationship of adiponectin, leptin, and hsCRP to objectively measured free-living physical activity in children. It may also be the first of its kind to examine the interaction between insulin resistance, adiponectin, and physical activity in prepubertal children. Although a novel inference, it seems possible that adiponectin is secreted to modulate insulin sensitivity when activity levels are insufficient to do so. Such a compensatory mechanism, if present, would support the concept that adiponectin is a selectively controlled modulator of insulin sensitivity. A randomized controlled trial would be needed to establish the cause and effect of this relationship.
Acknowledgments
The EarlyBird study has been supported by Diabetes U.K., Bright Futures Trust, Smith's Charity, Child Growth Foundation, Diabetes Foundation, Beatrice Laing Trust, Abbott, Astra-Zeneca, GlaxoSmithKline, Ipsen, and Roche.
No potential conflicts of interest relevant to this article were reported.
The authors thank Dr. Patrick English for helpful discussion, Rosemary Snaith and Jenny Perkins for their help with data collection, and Dr. Lynne Cherry, Pauline Watt and Anne Kelly for their technical assistance.
Parts of this study were presented in abstract form at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007.
Published ahead of print at http://care.diabetesjournals.org on 25 November 2008.
None of the sources funding this study had any involvement in its design, analysis, interpretation, or writing.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Goran MI, Treuth MS: Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am 48:931–953, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Weiss R, Caprio S: The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab 19:405–419, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Wareham NJ, Brage S, Franks PW, Abbott RA: Relationship between physical activity and insulin resistance. In insulin resistance: insulin action and its disturbances in disease. Kumar S, O'Rahilly S, Eds. Chichester, Wiley, 2004, p. 317–400
- 4.Froberg K, Andersen LB: Mini review: Physical activity and fitness and its relations to cardiovascular disease risk factors in children. Int J Obes (Lond) 29(Suppl. 2):S34–S39, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Slinger JD, van BE, Keizer H, Rump P, Hornstra G, Kuipers H: Insulin resistance, physical fitness, body composition and leptin concentration in 7–8 year-old children. J Sci Med Sport 11:132–138, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Bunt JC, Salbe AD, Funahashi T, Matsuzawa Y, Tataranni PA: Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J Clin Endocrinol Metab 87:4652–4656, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Ong KK, Frystyk J, Flyvbjerg A, Petry CJ, Ness A, Dunger DB: Sex-discordant associations with adiponectin levels and lipid profiles in children. Diabetes 55:1337–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ferreira I, Boreham CA, Twisk JW, Gallagher AM, Young IS, Murray LJ, Stehouwer CD: Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts Project. J Hypertens 25:1009–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B: Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12, 2006 [PubMed] [Google Scholar]
- 10.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C: Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia 49:2078–2085, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gutin B, Ramsey L, Barbeau P, Cannady W, Ferguson M, Litaker M, Owens S: Plasma leptin concentrations in obese children: changes during 4-mo periods with and without physical training. Am J Clin Nutr 69:388–394, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Punthakee Z, Delvin EE, O'Loughlin J, Paradis G, Levy E, Platt RW, Lambert M: Adiponectin, adiposity, and insulin resistance in children and adolescents. J Clin Endocrinol Metab 91:2119–2125, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS: Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism 54:1472–1479, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W: Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol 48:1865–1870, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Voss LD, Kirkby J, Metcalf BS, Jeffery AN, O'Riordan C, Murphy MJ, Wilkin TJ: Preventable factors in childhood that lead to insulin resistance, diabetes mellitus and the metabolic syndrome: the EarlyBird diabetes study 1. J Pediatr Endocrinol Metab 16:1211–1224, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Metcalf BS, Curnow JS, Evans C, Voss LD, Wilkin TJ: Technical reliability of the CSA activity monitor: the EarlyBird study. Med Sci Sports Exerc 34:1533–1537, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Treuth M, Hannan P, McMurray R, Ring KB, Catellier D, Pate R: Predicting energy expenditure from accelerometry counts in adolescent girls. Med Sci Sports Exerc 37:155–161, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recommendations for active living throughout the lifecourse. In At Least Five a Week: Evidence on the Impact of Physical Activity and Its Relationship to Health. A report of the Chief Medical Officer, 2004, Chapter 3. Available from http://www.dh.gov.uk/assetRoot/04/08/09/81/04080981.pdf. Accessed 15 April 2007
- 19.Metcalf BS, Voss LD, Hosking J, Jeffery AN, Wilkin TJ: Physical activity at the government-recommended level and obesity-related health outcomes: a longitudinal study (EarlyBird 37). Arch Dis Child 93:772–777, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hammond J, Nelson M, Chinn S, Rona RJ: Validation of a food frequency questionnaire for assessing dietary intake in a study of coronary heart disease risk factors in children. Eur J Clin Nutr 47:242–250, 1993 [PubMed] [Google Scholar]
- 21.Gungor N, Saad R, Janosky J, Arslanian S: Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 144:47–55, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Yatagai T, Nishida Y, Nagasaka S, Nakamura T, Tokuyama K, Shindo M, Tanaka H, Ishibashi S: Relationship between exercise training-induced increase in insulin sensitivity and adiponectinemia in healthy men. Endocr J 50:233–238, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R: Hormonal regulation of adiponectin gene expression in 3T3–L1 adipocytes. Biochem Biophys Res Commun 290:1084–1089, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Simpson KA, Singh MA: Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring )16:241–256, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, Anderssen SA: Physical activity and clustered cardiovascular risk in children: a cross-sectional study (the European Youth Heart Study). Lancet 368:299–304, 2006 [DOI] [PubMed] [Google Scholar]