Abstract
OBJECTIVE—The aim of this study was to investigate the role of inflammatory markers as potential predictors of cardiovascular events in subjects with and without the metabolic syndrome.
RESEARCH DESIGN AND METHODS—This was a post hoc analysis from the Diet and Omega-3 Intervention Trial (DOIT), comprising 563 elderly men with (n = 221) and without (n = 342) metabolic syndrome. Circulating inflammatory markers were measured.
RESULTS—During 3 years, 68 cardiovascular events were recorded. In the total population, C-reactive protein (CRP) (P < 0.001), interleukin-18 (IL-18) (P = 0.008), and IL-6 (P = 0.003) were elevated in subjects with events. In subjects with metabolic syndrome, IL-18 was the strongest predictor (adjusted odds ratio 2.9 [95% CI 1.1–7.8]). In subjects without metabolic syndrome, only CRP seemed to be an independent predictor (3.3 [1.5–7.3]). There was a significant interaction between fasting glucose and IL-18 (P = 0.008) and IL-6 (P = 0.024) but not CRP. Elevated fasting glucose (>6.2 mmol/l) markedly increased the predictive power of inflammatory markers (IL-18: 5.5 [1.4–21.1], IL-6: 3.5 [1.0–11.8], and CRP: 3.5 [1.0–11.9]). For IL-18, there was a stepwise increase in event rate by quartiles of fasting glucose.
CONCLUSIONS—IL-18 was an independent predictor of cardiovascular events in subjects with metabolic syndrome and even more so in the presence of elevated fasting glucose. Our findings suggest a mutually potentiating effect of hyperglycemia and inflammation in cardiovascular risk prediction.
The metabolic syndrome is a cluster of risk factors for cardiovascular disease (CVD), including insulin resistance, obesity, hypertension, elevated triglycerides, and low levels of HDL cholesterol (1,2). There is increasing evidence that the metabolic syndrome is associated with a proinflammatory state, and whether a measure of inflammation should be included in the definition of the syndrome is currently being discussed (1,3). However, to be included, such a marker should preferably enhance the prediction of CVD, and debate on whether this is the case is ongoing.
Cross-sectional studies have shown added prognostic information by risk stratification with C-reactive protein (CRP) in populations with diabetes and metabolic syndrome (4). One prospective study reported that combining metabolic syndrome and CRP enhanced the prognostic information for both future CVD and new-onset diabetes (5), whereas another prospective study showed that although CRP and metabolic syndrome were independent predictors of CVD, the combination of the two did not increase the predictive value (6).
Interleukin-18 (IL-18) is a potent proinflammatory cytokine that has been reported to be associated with multiple components of metabolic syndrome and to predict the development of type 2 diabetes (7,8). Furthermore, IL-18 has been shown to be associated with the formation, progression, and vulnerability of atherosclerotic plaque (9–11). However, data regarding IL-18 in predicting coronary events have been conflicting (10,11), and IL-18 as a potential predictor of cardiovascular events in populations with metabolic syndrome remains to be investigated. The aim of the present study was to investigate inflammatory markers as potential predictors of cardiovascular events in a population of elderly men with or without metabolic syndrome.
RESEARCH DESIGN AND METHODS
The basis for recruitment into the present study was a follow-up of the participants from the Oslo Diet and Anti-smoking Study carried out in 1972–1977, comprising 1,232 men with a high risk of CVD (12). Twenty-five years later the survivors of this population were invited to participate in the Diet and Omega-3 Intervention Trial on Atherosclerosis (DOIT), a 3-year intervention trial with the aim of investigating the effect of n-3 polyunsaturated fatty acid (PUFA) supplementation and/or dietary intervention on markers of atherosclerosis. The study had a 2 × 2 factorial design and was placebo controlled for the n-3 PUFA capsules (13). Altogether, a total of 563 subjects, aged 64–76 years, were included in the DOIT study. The study was carried out in compliance with the Declaration of Helsinki and was approved by the regional ethics committee. All subjects gave their written informed consent to participate.
Definition of the metabolic syndrome
To be classified as having the metabolic syndrome, participants needed to have three or more of the following criteria, as defined by the revised Adult Treatment Panel III (ATP-III) (1): 1) abdominal obesity (defined as waist circumference >102 cm); 2) serum triglyceride level ≥1.7 mmol/l; 3) serum HDL cholesterol level <1.0 mmol/l; 4) blood pressure ≥130/85 mmHg or drug treatment for elevated blood pressure; and 5) fasting plasma glucose level ≥5.6 mmol/l or drug treatment for elevated blood glucose.
Cardiovascular events
The cardiovascular events were a composite of fatal and nonfatal CVD, defined as myocardial infarction, revascularization procedures, aortic aneurism, peripheral arterial occlusive disease, and cerebrovascular events, according to medical records and the official death certificates held by Statistics Norway.
Laboratory methods
Fasting blood samples, without intake of any medications, were collected between 8 and 10 a.m. by standard venipuncture. Serum was prepared by centrifugation within 1 h at 2,500g for 10 min for determinations of CRP, tumor necrosis factor-α (TNF-α), IL-6, IL-8, IL-10, IL-18, and adiponectin. Monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor type-1 (PAI-1) activities were determined in citrated plasma (0.129 mmol/l in dilution 1:10) and CD40 ligand (CD40L) in EDTA plasma, all stored on ice and separated within 30 min by centrifugation at 4°C and 3,000g for 20 min to obtain platelet-poor plasma. All samples were stored at −80°C until analysis.
CRP was determined by an enzyme-linked immunosorbent assay (DRG Instruments, Marburg/Lahn, Germany) (detection limit 0.1 mg/l). The enzyme-linked immunosorbent assay method from Medical Biological Laboratories (Naku-ku, Nagoya, Japan) was used for analysis of IL-18. PAI-1 activity was determined by Spectrolyse PL (Biopool, Umeå, Sweden). TNF-α, IL-6, IL-8, IL-10, MCP-1, CD40L, and adiponectin were all measured by enzyme immunoassays from R&D Systems Europe (Abingdon, Oxon, U.K.) according to the manufacturer's instructions. For IL-10, 21 samples were below the detection limit (0.5 pg/ml). In our laboratory the interassay coefficients of variation were as follows: CRP <5%, TNF-α 8.5%, IL-6 10.5%, IL-8 10.5%, IL-10 6.2%, IL-18 6.5%, MCP-1 9.0%, CD40L 9.5%, adiponectin 9.5%, and PAI-1 activity 6.8%.
Serum lipids, insulin, and glucose were determined by conventional enzymatic methods. Insulin resistance was estimated according to the homeostasis model assessment score, calculated with the following formula: (fasting insulin [picomoles per liter]/7.2)/(22.5/fasting glucose [millimoles per liter]) (14).
Statistics
Because the distribution of several biochemical markers was skewed, data are presented as medians (25th, 75th percentiles), and nonparametric statistics were used. For comparison of baseline characteristics, the Mann-Whitney U test was used for continuous data and the Pearson χ2 test was used for categorical data. Analyses of trends through quartiles of inflammatory markers were performed with the χ2 linear-by-linear association. Odds ratios (ORs) were calculated for prediction of events by inflammatory markers and established cardiovascular risk factors. Cutoffs for continuous variables were defined as greater than the 50 percentile, except for glucose, which was divided at the 75 percentile (6.2 mmol/l), as there was a marked increase in events above this level (supplemental Fig. A1 in an online appendix, available at http://dx.doi.org/10.2337/dc08-1710). Statistically significant ORs were adjusted for traditional risk factors, use of medication, inflammatory markers, and intervention principle. Variables were entered in the multivariate model if P < 0.25 in the univariate model. Diabetes was excluded from the model because of a high correlation with fasting glucose (r = 0.63). Potential interactions were tested in a multiple regression model. A significance level of 0.05 was used. The statistical analyses were performed with SPSS (version 15.0; SPSS, Chicago, IL).
RESULTS
Baseline characteristics
Baseline characteristics according to metabolic syndrome status are given in Table 1. As defined by the ATP-III criteria, 221 (39%) of the participants had metabolic syndrome at baseline assessment. Serum levels of several proinflammatory markers were elevated among subjects with metabolic syndrome.
Table 1.
Baseline characteristics in subjects with and without the metabolic syndrome
| With metabolic syndrome | Without metabolic syndrome | P value* | |
|---|---|---|---|
| n | 221 | 342 | |
| Age (years) | 69.5 (67.2, 72.5) | 70.4 (67.7, 72.7) | 0.150 |
| Previous CVD (%) | 28 | 28 | 0.883 |
| Current smokers (%) | 28 | 38 | 0.022 |
| Use of medication (%) | |||
| Statins | 26 | 28 | 0.697 |
| ACE inhibitors | 22 | 10 | <0.001 |
| Diabetes (%) | 29 | 6 | <0.001 |
| Impaired fasting glucose (%) | 56 | 32 | <0.001 |
| Treated hypertension (%) | 40 | 24 | <0.001 |
| BMI (kg/m2) | 28.4 (26.6, 30.6) | 25.3 (23.5, 27.0) | <0.001 |
| Waist circumference (cm) | 104 (99, 109) | 95 (90, 99) | <0.001 |
| Triglycerides (mmol/l) | 2.0 (1.8, 2.5) | 1.3 (1.0, 1.6) | <0.001 |
| HDL cholesterol (mmol/l) | 1.2 (1.0, 1.4) | 1.5 (1.3, 1.7) | <0.001 |
| Cholesterol (mmol/l) | 6.3 (5.5, 7.0) | 6.4 (5.8, 7.0) | 0.107 |
| Systolic blood pressure (mmHg) | 152 (141, 164) | 146 (133, 159) | <0.001 |
| Diastolic blood pressure (mmHg) | 85 (80, 92) | 82 (74, 90) | <0.001 |
| Fasting glucose (mmol/l) | 6.1 (5.7, 6.8) | 5.4 (5.1, 5.8) | <0.001 |
| Homeostasis model assessment score | 5.4 (3.9, 7.3) | 3.7 (2.9, 4.6) | <0.001 |
| CRP (mg/l) | 3.64 (2.16, 6.53) | 2.88 (1.50, 5.82) | 0.013 |
| IL-18 (pg/ml) | 292 (231, 377) | 265 (202, 341) | 0.002 |
| IL-6 (pg/ml) | 1.61 (1.08, 2.62) | 1.45 (0.92, 2.41) | 0.047 |
| IL-8 (pg/ml) | 18.8 (15.0, 24.7) | 17.4 (13.6, 23.0) | 0.106 |
| IL-10 (pg/ml) | 1.39 (0.70, 3.64) | 1.74 (0.77, 3.98) | 0.194 |
| CD40L (pg/ml) | 57 (46, 74) | 52 (43, 65) | 0.003 |
| TNF-α (pg/ml) | 1.21 (0.82, 2.05) | 1.05 (0.77, 1.86) | 0.025 |
| MCP-1 (pg/ml) | 441 (393, 563) | 426 (355, 499) | 0.001 |
| Adiponectin (ng/ml) | 6,703 (4,371, 10,870) | 9,459 (6,167, 13,875) | <0.001 |
| PAI-1 activity (units/ml) | 18.8 (12.1, 27.6) | 11.4 (7.6, 18.2) | <0.001 |
Data are median values (25th, 75th percentiles).
P values refer to differences between groups.
Predictors of cardiovascular events in the total population
A total of 68 cardiovascular events (12.1%) were recorded during 3 years of follow-up. Among inflammatory markers, CRP (P < 0.001), IL-18 (P = 0.008), and IL-6 (P = 0.003) were elevated in subjects with cardiovascular events. The other inflammatory markers did not differ between subjects with or without cardiovascular events (supplemental Table A1 of the online appendix). Unadjusted and adjusted ORs for the total population are given in Table 2. In multivariate analyses, CRP, systolic blood pressure, fasting glucose, and smoking habits remained independent predictors. Metabolic syndrome was not independently predictive of cardiovascular events in the present population.
Table 2.
Univariate and multivariate analyses of predictors of cardiovascular events (n = 68)
| Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value | |
|---|---|---|---|---|
| CRP >3.29 mg/l | 3.7 (2.1–6.7) | <0.001 | 3.0 (1.6–5.5) | 0.001 |
| Systolic blood pressure >148 mmHg | 2.4 (1.4–4.2) | 0.001 | 2.6 (1.4–4.5) | 0.001 |
| IL-6 >1.53 pg/ml | 2.1 (1.2–3.6) | 0.006 | 1.4 (0.81–2.6) | 0.213 |
| Fasting glucose >6.2 mmol/l | 2.0 (1.2–3.4) | 0.012 | 1.8 (1.0–3.3) | 0.041 |
| IL-18 >277 pg/ml | 1.9 (1.1–3.3) | 0.015 | 1.6 (0.90–2.8) | 0.109 |
| Smoking (yes/no) | 1.9 (1.1–3.1) | 0.018 | 2.4 (1.4–4.2) | 0.003 |
| Previous CVD | 1.5 (0.88–2.6) | 0.138 | ||
| Cholesterol >6.3 mmol/l | 1.4 (0.82–2.3) | 0.238 | ||
| Diet intervention (yes/no) | 0.72 (0.43–1.2) | 0.203 |
Total population (n = 563). Age, metabolic syndrome, triglycerides, BMI, statin use, ACE inhibitors, and n-3 PUFA intervention were excluded from the model (P > 0.25). Cutoffs are as described in Statistics.
Predictors of cardiovascular events in subjects with or without metabolic syndrome
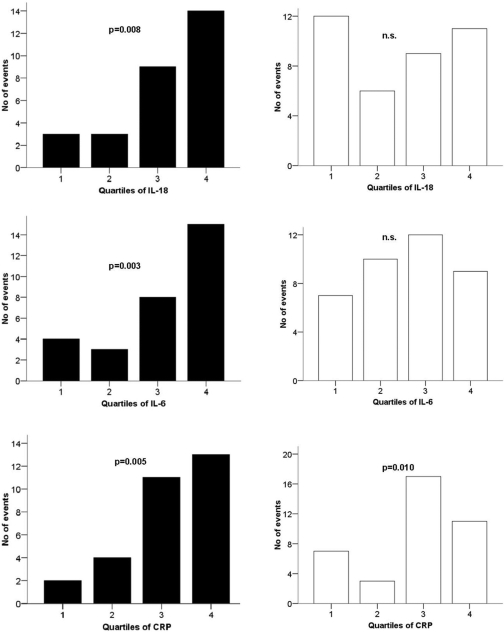
In subjects with metabolic syndrome, 29 cardiovascular events (13.1%) occurred during the study period. Analysis for trend through quartiles showed a statistically significant association with cardiovascular events for IL-18 (P = 0.008), IL-6 (P = 0.003), and CRP (P = 0.005) (Fig. 1). Furthermore, in univariate analyses, IL-18, IL-6, and CRP but not conventional risk factors were significantly predictive of cardiovascular events. After adjustment for relevant covariates, only IL-18 remained a statistically significant predictor (Table 3).
Figure 1.
Number of cardiovascular events by quartiles of IL-18, IL-6, and CRP in subjects with (▪, n = 221) and without (□, n = 342) the metabolic syndrome. P values refer to trend analysis. MetS+, with metabolic syndrome; MetS−, without metabolic syndrome; n.s., not significant.
Table 3.
Univariate and multivariate analyses of predictors of cardiovascular events in subjects with and without metabolic syndrome
| With metabolic syndrome (29 events)
|
Without metabolic syndrome (39 events)
|
|||
|---|---|---|---|---|
| Univariate OR (95% CI) | Multivariate OR (95% CI) | Univariate OR (95% CI) | Multivariate OR (95% CI) | |
| n | 221 | 342 | ||
| IL-18 >277 pg/ml | 3.6 (1.4–9.3)* | 2.9 (1.1–7.8)† | 1.3 (0.66–2.6) | |
| IL-6 >1.53 pg/ml | 3.5 (1.4–8.6)* | 2.5 (0.97–6.6) | 1.5 (0.76–3.0) | |
| CRP >3.29 mg/l | 3.6 (1.4–9.1)* | 2.5 (0.89–6.9) | 3.8 (1.8–8.1)* | 3.3 (1.5–7.3)* |
| Systolic blood pressure >148 mmHg | 2.0 (0.85–4.5) | 2.8 (1.4–5.6)* | 3.1 (1.4–6.5)* | |
| Smoking (yes/no) | 1.8 (0.83–4.1) | 2.0 (1.0–3.9)† | 2.5 (1.1–5.3)† | |
| Fasting glucose >6.2 mmol/l | 2.1 (0.97–4.7) | 1.8 (0.75–4.5) | ||
| Previous CVD | 1.3 (0.59–3.0) | 1.6 (0.81–3.3) | ||
| Diet intervention (yes/no) | 0.95 (0.44–2.0) | 0.57 (0.28–1.1) | ||
Cholesterol, age, triglycerides, BMI, statin use, ACE inhibitors, and n-3 PUFA intervention were excluded from the model (P > 0.25 in univariate analysis). Cutoffs are as described in Statistics.
P < 0.01;
P < 0.05.
In subjects without metabolic syndrome, 39 cardiovascular events (11.4%) were recorded. Analysis for trend through quartiles showed a statistically significant association with cardiovascular events for CRP (P = 0.010) but not for IL-18 or IL-6 (Fig. 1). CRP but not IL-18 or IL-6 appeared to be independently predictive of cardiovascular events (Table 3).
To further explore the observed difference in cardiovascular prediction in the presence and absence of metabolic syndrome, we tested for interactions and found significant interactions between elevated fasting glucose and IL-18 (P = 0.008) and IL-6 (P = 0.024) but not CRP (P = 0.240). There were no significant interactions between the inflammatory markers and other covariates.
Figure 2 illustrates number of cardiovascular events by quartiles of IL-18, IL-6, and CRP in subjects with (n = 139) and without (n = 424) elevated fasting glucose (upper quartile >6.2 mmol/l). The event rate by levels of IL-18 increased stepwise for each quartile of glucose in the total population (supplemental Table A2 and supplemental Fig. A2, available in the online appendix). For all inflammatory markers, ORs were higher in subjects with elevated fasting glucose (IL-18: adjusted OR 5.5 [95% CI 1.4–21.1], IL-6: 3.5 [1.0–11.8], and CRP: 3.5 [1.0–11.9]). Using the definition of impaired fasting glucose (5.6–6.9 mmol/l) (1), the same pattern of risk stratification was observed (data not shown).
Figure 2.
Number of cardiovascular events by quartiles of IL-18, IL-6, and CRP in subjects with (▪, n = 139) and without (□, n = 424) elevated fasting glucose (>6.2 mmol/l). P values refer to trend analysis. n.s., not significant.
CONCLUSIONS
The main finding in this study is that IL-18 strongly predicted cardiovascular events in subjects with metabolic syndrome, and the prediction was even more pronounced in the sole presence of elevated fasting glucose. Indeed, there was a significant interaction between elevated glucose and IL-18, and a stepwise increase in the predictive power of IL-18 by quartiles of glucose, consistent with a synergistic effect of IL-18 and hyperglycemia in cardiovascular risk prediction.
Previous studies on IL-18 in predicting coronary events have been conflicting (10,11). In the Dallas Heart Study, IL-18 was associated with multiple metabolic syndrome components but not with subclinical atherosclerosis after adjustment for traditional risk factors (15). The authors suggested that the associations between IL-18 and atherosclerosis depend on the presence of other risk factors such as obesity and diabetes and that IL-18 may mediate some of the proatherogenic effects of these factors (15).
A very recent publication reported that inflammatory markers including CRP, IL-6, and IL-18 added prognostic information regarding cardiovascular mortality in subjects with known coronary artery disease and metabolic syndrome (16). In line with our results, only IL-18 could be identified as an independent predictor in multivariate analyses (16). We found IL-18 to be even more predictive in the presence of elevated glucose, and as most subjects with metabolic syndrome had impaired fasting glucose or diabetes, it is likely that the predictive value of IL-18 depends on hyperglycemia in the present study.
IL-18 has been shown to predict the development of type 2 diabetes (8). On the other hand, experimental hyperglycemia has been shown to increase concentrations of IL-18 (17). It has also been reported that in the setting of acute myocardial infarction, IL-18 and CRP were elevated in parallel with troponin I in patients with elevated glucose, suggesting that inflammation might explain some of the excess myocardial damage in hyperglycemic patients (18).
Our results expand these findings by suggesting that hyperglycemia is not only associated with increased levels of IL-18 but also fuels the potentially harmful effects of a given cytokine level. Our findings introduce the possibility of a mutually potentiating effect of IL-18, and elevated fasting glucose. IL-18 can stimulate both type 1 helper T (Th1) and Th2 responses, depending on its cytokine milieu (19), and acts synergistically with IL-12 to stimulate a Th1 response with production of interferon-γ, a central feature in the atherosclerotic lesion (20). Recently, expression of IL-12 in macrophages has been shown to be increased by experimental hyperglycemia (21). Thus, we speculate that our findings could in part be explained by a Th1 response mediated by IL-18 acting in synergy with a hyperglycemic proinflammatory milieu.
Another main finding in this study is that CRP independently predicted cardiovascular events in elderly high-risk men, in contrast to a recent prospective study reporting limited value of CRP in risk stratification of elderly men and women (22). The discrepancy might be due to the fact that our study cohort consisted of only men and that a large proportion had impaired fasting glucose. Elevated glucose was an independent predictor of events, and for all inflammatory markers, the event rates were higher in subjects with elevated glucose. Furthermore, we found a significant interaction between elevated fasting glucose and IL-6 in the cardiovascular risk prediction. In total, our results point to a more generalized concept with increased susceptibility to inflammation in the presence of hyperglycemia.
A few experimental studies may point to the potential mechanisms involved. In an in vitro study, it was shown that the proatherogenic effects of CRP were potentiated by hyperglycemia, by increased expression of adhesion molecules and MCP-1 in endothelial cells (23). From other in vitro studies, it has been reported that preexposure with hyperglycemia increases lipopolysaccharide-induced secretion of proinflammatory cytokines (24) and induction of Toll-like receptor expression in human monocytes (25).
Our study has several limitations. First, we acknowledge that our results represent a post hoc analysis from an intervention trial and should be interpreted with caution, although we have tested for interactions and adjusted for intervention principles as appropriate. Second, the limited number of end points may increase the risk of type II errors, especially in subgroup analyses. Thus, although multiple tests were performed, the strong association between IL-18 and end points is likely to be reliable. Furthermore, lack of power only allows analyses of the combined end point, although different mechanisms might exist. Third, the study subjects were quite heterogeneous, with a broad spectrum of morbidity and medication. Fourth, the subjects consisted of long-time survivors from a high-risk population, raising the possibility of survivor bias. Finally, this study comprised a selected group of elderly Caucasian men. Different results may be obtained from other demographic groups, and younger cohorts could provide valuable information regarding long-term outcomes.
Our study also has several strengths, one of which is that metabolic syndrome is well characterized according to the ATP-III criteria, whereas other studies have used BMI as a surrogate marker of waist circumference (5,16). Furthermore, our study is one of few prospective studies to investigate the combined effect of metabolic syndrome and inflammatory markers in cardiovascular risk prediction.
In summary, IL-18 was an independent predictor of cardiovascular events in subjects with metabolic syndrome and even more in the presence of elevated fasting glucose. Moreover, our findings suggest the possibility of a mutually potentiating effect of inflammation and hyperglycemia in cardiovascular risk prediction. Further studies are needed to confirm and elucidate our findings.
Supplementary Material
Acknowledgments
This work was supported by Helse Sør-Øst, Norway, and by the Norwegian Retail Company RIMI. No other potential conflicts of interest relevant to this article were reported.
We gratefully thank the laboratory staff at the Center for Clinical Heart Research, especially medical technologist Sissel Åkra for performing the biochemical analyses.
Published ahead of print at http://care.diabetesjournals.org on 17 December 2008.
Clinical trial reg. no. NCT00764010, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112:2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome [article online], 2006. Available from http://www.idf.org/webdata/docs/MetS_def_update2006.pdf. Accessed 5 March 2008
- 3.Haffner SM: The metabolic syndrome: inflammation, diabetes mellitus and cardiovascular disease. Am J Cardiol 97:3A–11A, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Malik S, Wong ND, Franklin S, Pio J, Fairchild C, Chen R: Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care 28:690–693, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J: Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland coronary prevention study. Circulation 108:414–419, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Wilson PW: C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham offspring study. Circulation 110:380–385, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP: Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol 25:1268–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C, Ilig T, Martin S, Herder C: Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg study 1984–2002. Diabetes 54:2932–2938, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Mallat Z, Corbaz A, Scoazec A, Besnard S, Lesèche G, Chvatchko Y, Tedgui A: Expression of IL-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 104:1598–1603, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Blankenberg S, Luc G, Ducimetiere P, Arveiler D, Ferrieres J, Amouyel P, Evans A, Cambien F, Tiret L, PRIME Study Group: IL-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 108:2453–2459, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Koenig W, Khuseyinova N, Baumert J, Thorand B, Loewel H, Chambless L, Meisinger C, Schneider A, Martin S, Kolb H, Herder C: Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol 26:2745–2751, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Hjermann I, Velve Byre K, Holme I, Leren P: Effect of diet and smoking intervention on the incidence of coronary heart disease: report from the Oslo Study Group of a randomised trial in healthy men. Lancet 2:1303–1310, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, Aase O, Klemsdal TO, Hjermann I, Arnesen H: Effect of diet and/or very long chain n-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima media thickness and by pulse wave propagation in elderly men with hypercholesterolemia. Eur J Cardiovasc Prev Rehab 13:325–333, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA: Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 27:2043–2049, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Genth-Zotz S, Post F, Munzel T, Blankenberg S: Impact of inflammatory markers on cardiovascular mortality in patients with metabolic syndrome. Eur J Cardiovasc Prev Rehab 15:278–284, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D: Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Marfella R, Siniscalchi M, Esposito K, Sellito A, de Fanis U, Romano C, Portoghese M, Siciliano S, Nappo F, Sasso FC, Mininni N, Cacciapuoti F, Luivero G, Giunta R, Verza M, Giugliano D: Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Diabetes Care 26:3129–3135, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H: Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev 12:53–72, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hansson GK: Inflammation, atherosclerosis, and coronary disease. N Engl J Med 352:1685–1695, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL: Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147:2518–2525, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Sattar N, Murray HM, McConnachie A, Blauw GJ, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Murphy MB, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, Shepherd J: C-reactive protein and prediction of coronary heart disease and global vascular events in the prospective study of elderly at risk (PROSPER). Circulation 115:981–989, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Wang CH, Weisel RD, Badiwala MV, Li SH, Fedak PW, Li RK, Mickle DA: Hyperglycemia potentiates the proatherogenic effects of C-reactive protein: reversal with rosiglitazone. J Mol Cell Cardiol 35:417–419, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Nareika A, Maldonado A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y: High glucose-boosted inflammatory response to lipopolysaccharide are suppressed by statin. J Periodont Res 42:31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Dasu MR, Devaraj S, Ling Z, Hwang DH, Jialal I: High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57:3090–3098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.