The prevalence of obesity appears to have reached a plateau in the U.S. between 2003 and 2007 (1), but the obesity epidemic is still rampant in many other countries—especially in the developing world (2)—in adults as well as children (http://www.who.int/topics/obesity/en/). A recent analysis of a large prospective cohort of individuals 50–71 years old (3) has generated a precise dose-response gradient for the positive association of BMI and relative risk of death independent of other risk factors (especially smoking and preexisting disease, which cause weight loss). Yet, long-term observational studies have generally found that weight loss, whether spontaneous or intentional, is associated with increased, rather than decreased, overall mortality (rev. in 4).
Lifestyle intervention (diet and exercise), behavioral management, and drug therapy for obesity deliver a degree of weight loss that is usually modest (and therefore unattractive to patients) and short-lived (6 months to 1 year at best) and carry considerable side effects. Moreover, despite the attenuation of risk factors such as diabetes and dyslipidemia, trial evidence for an effect of these weight-control approaches on reducing cardiovascular disease or mortality is still lacking (rev. in 5). On the other hand, and perhaps as a consequence, surgery for the treatment of severe obesity is gaining increasing favor. The annual U.S. frequency of hospital discharges that included bariatric surgery increased sevenfold (from 3.5 to 24.0 per 100,000) between 1996 and 2002 (6). According to a review of 85,048 morbidly obese patients (7), early (≤30 days) and late (30 days to 2 years) mortality rates for bariatric surgery trend downward (0.28 and 0.35%, respectively). Surgical treatment of massive obesity is being extended to adolescents, seemingly with similar success and risk rates as in adults (8–10). Recently, the 10.9-year follow-up of the Swedish Obese Subjects Study reported a 30% risk reduction for overall mortality in 2,010 obese patients who had undergone bariatric surgery (11). Likewise, in a retrospective cohort of 7,925 surgical patients (12), mortality from any cause was 40% lower than in 7,925 nonsurgical obese patients.
These findings are striking, particularly when considering the high rate of failure of other treatment modalities, such as caloric restriction (13) and antiobesity drugs (14), and may foster a change in the indications for bariatric surgery (15). They also raise a number of questions. The question we address here is the impact of bariatric surgery on type 2 diabetes, its size, and mechanisms.
Bariatric surgery and type 2 diabetes
A systematic review and meta-analysis of the English literature including >22,000 patients (73% women, mean BMI 47 kg/m2) reported complete resolution of type 2 diabetes (defined as discontinuation of all diabetes-related medications and blood glucose levels within the normal range) in 77% of cases. This percentage increased to 85% when counting patients reporting improvement of glycemic control, and diabetes resolution occurred in concomitance with an average weight loss of 41 kg (∼65% of the excess weight) (16). In the analysis by Adams et al. (12), deaths attributed to diabetes were reduced by a phenomenal 92%. Thus, there can be little doubt that in very obese patients with type 2 diabetes bariatric surgery in general is a highly effective means of curing type 2 diabetes. However, the most frequent kind of type 2 diabetes, i.e., the hyperglycemia surfacing after the fourth decade of life in moderately obese subjects, is a progressive disease (17) that rarely undergoes resolution, whether spontaneously or with treatment. One possibility is that the hyperglycemia of morbid obesity (BMI between 35 and 70 kg/m2) has a pathogenesis different from that of the hyperglycemia of moderately obese (BMI between 27 and 34 kg/m2) or lean type 2 diabetes. Another possibility is that bariatric surgery per se interferes with glucose metabolism in ways that none of the other antidiabetes treatments do. Because circulating glucose levels quantitatively result from the insulin sensitivity of glucose uptake (in peripheral tissues) or release (by the liver) and the dynamics (amount and time course) of insulin made available by β-cells, we shall first review the evidence linking surgery-induced weight loss with changes in insulin sensitivity and β-cell function. Next, other potential interactions will be discussed.
Bariatric operations
A number of surgical approaches to induce weight loss have been developed, and several are in current use (cf. ref. 18 for a detailed description). In general, they can be grouped into purely restrictive, mostly restrictive, and mostly malabsorptive procedures. In the first group, the most common procedure is laparoscopic adjustable gastric banding (LAGB), which consists of placing a band around the upper part of the stomach, thereby creating a small pouch that empties into the lower stomach without bypassing the foregut (see Figure A1 in the online appendix available at http://dx.doi.org/10.2337/dc08-1762). In the cited meta-analysis (16), LAGB was associated with the loss of 32–70% of excess weight. Vertical banded gastroplasty is a variant of LAGB, typically leading to the loss of 48–93% of excess weight. With either of these techniques, the mechanism essentially hinges upon generating effective satiety signals for small amounts of ingested food.
Probably the most common weight-loss surgery is the Roux-en-Y gastric bypass (RYGB), in which the stomach is reduced to a small pouch (<30 ml) that is connected via a tight outlet to the jejunum just past the duodenum while the jejunal stump is anastomosed to the lower jejunum in a Y conformation (Fig. 1). Here, a degree of gastric restriction comparable with that of LAGB (but not adjustable) is coupled with the bypass of the duodenum and upper jejunum, making RYGB a mostly restrictive procedure. Between 33 and 77% of excess weight can be lost following RYGB (16).
Figure 1.
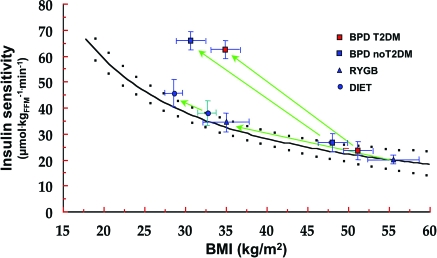
The results (symbols) are means ± SEM in patients undergoing weight loss by diet (ref. 69), RYGB, or BPD (ref. 68). Green arrows connect pretreatment to posttreatment values. The black line and the black dots are the fitting function and 95% CIs of the data in ref. 68 (same as in online appendix Fig. A2, bottom graph). FFM, fat-free mass; T2DM, type 2 diabetes.
With the jejunoileal bypass, the gastric content is emptied directly into the terminal ileum, thereby inducing major malabsorption with no restriction of food intake. Now abandoned, this procedure led to the development of current malabsorptive approaches, the prototype of which is biliopancreatic diversion (BPD). Here, a 60% distal gastric resection with stapled closure of the duodenal stump results in a residual stomach volume of ∼300 ml. The small bowel is transected 2.5 m from the ileocecal valve, and its distal end is anastomosed to the remaining stomach. The proximal end of the ileum, comprising the remaining small bowel carrying the biliopancreatic juice and excluded from food transit, is anastomosed to the bowel 50 cm proximal to the ileocecal valve. Consequently, the total length of absorbing bowel is 250 cm, the final 50 cm of which represents the site where ingested food and biliopancreatic juices mix (Fig. 1). This mostly malabsorptive approach is associated with the highest (62–75%) (16) and most durable (19) degree of excess weight loss.
Weight loss and insulin sensitivity: preliminary considerations
Adiposity is one of the physiological determinants of insulin sensitivity (20). Weight loss has been shown to enhance insulin sensitivity under all circumstances (rev. in 21) except wasting, major stress, and HIV infection (22–24). Surgically induced weight loss, in general, appears to take no exception (see below). However, selective surgical removal of fat tissue from subcutaneous depots does not improve insulin resistance. In a well-controlled study of 15 obese women (7 of whom had diabetes), removal of 9–10 kg of subcutaneous abdominal fat by liposuction did not change insulin-mediated glucose disposal (on a euglycemic-hyperinsulinemic clamp) despite the expected drop in circulating leptin levels (25). Although other studies using liposuction have reported some improvement of insulin sensitivity in the longer term (26), this observation clearly implies that factors other than the sheer mass of subcutaneous adipose tissue have an impact on insulin action. Thus, large adipocytes, such as those that are deposited in subcutaneous depots during weight gain, are less sensitive to insulin than small adipocytes (27); their degree of insulin unresponsiveness in vitro correlates with in vivo insulin insensitivity (28). Ectopic fat, accumulated in abdominal visceral depots, skeletal muscle, and liver, has been specifically linked with the presence of insulin resistance independently of total adiposity. In support of these findings, surgical removal of visceral, but not subcutaneous, fat from aging or diabetic rats restores insulin sensitivity and glucose tolerance (29). In obese humans, the metabolic changes induced by weight loss, i.e., insulin sensitization of both glucose metabolism and lipolysis, have been related to the depletion of ectopic fat stores (30–35). Importantly, whereas fasting for 3 days depresses insulin action in obese subjects (36), caloric restriction enhances it (37), accounting for as much increase in insulin sensitivity as weight loss itself (38). Finally, several peptides expressed and released by adipose tissue are metabolically active. For example, adiponectin upregulates insulin action in liver and skeletal muscle, whereas high circulating levels of some adipocytokines (e.g., tumor necrosis factor-α and retinol binding protein 4) correlate with in vivo insulin resistance (39).
Collectively, these observations suggest the following considerations when interpreting the evidence linking surgically induced weight loss (or, for that matter, any weight change) and insulin sensitivity:
quality and site are as important as amount of fat gained or lost;
energy balance matters to insulin action independently of weight changes;
the endocrine activity of adipose tissue itself may interfere with the relationship between changes in adiposity and insulin sensitivity;
coordinate changes in body weight and insulin sensitivity may differ between diabetic and nondiabetic subjects;
metabolic abnormalities may emerge at different BMI thresholds in individuals of diverse ethnic background (40);
specifically for bariatric surgery, an understanding of how gastrointestinal transit is altered by the operation may be key to interpreting metabolic effects for any given amount of weight loss.
Bariatric surgery and insulin sensitivity: results
Online appendix Table A1 lists the studies in which data on insulin sensitivity have been reported for obese type 2 diabetic patients undergoing bariatric surgery. With the proviso that our literature search has likely missed diabetic patients included in series that did not provide results separately for diabetic and nondiabetic subjects, the studies in online appendix Table A1 include ∼450 type 2 diabetic patients reported over a period of ∼25 years. As can be appreciated, type of surgery, duration of follow-up, and methodology vary enough to preclude precise quantitation of the impact of weight loss on insulin sensitivity. Nevertheless, some information can be derived from these data (with no pretense of bona fide meta-analysis). Thus, the weighted mean of preoperative BMI in all 423 patients was 46.4 kg/m2, which reflects the current indications for bariatric surgery (BMI ≥40 kg/m2 or ≥35 kg/m2 in complicated obesity). In the 204 patients in whom homeostasis model assessment (HOMA) (HOMA of insulin resistance, in this case) was used to assess changes in insulin sensitivity ≥4 months after surgery (weighted mean 12 months), insulin sensitivity improved by 51% for a mean BMI drop of 32% (∼40 kg). The percent changes in BMI and HOMA across the different studies were loosely related to one another. In the 79 patients in whom HOMA was measured <4 months after surgery (weighted mean 1.5 months), insulin sensitivity improved by 49% for a BMI decrease of 10% (with no correlation between the respective decrements). The few studies in online appendix Table A1 using different methods of estimating insulin sensitivity (insulin sensitivity test, insulin tolerance test, frequently sampled intravenous glucose tolerance test, and clamp [60–65]) do not in general run contrary to this result. Therefore, with all the approximations of this sort of analysis, the notions emerge that 1) bariatric surgery is capable of improving the insulin resistance of type 2 diabetes by ∼50% while at the same time causing a ∼30% decrease in BMI and 2) this improvement of insulin sensitivity may be seen already ∼6 weeks following surgery, at which time BMI may be decreased by only 11%. The latter apparent paradox may result from the use of surrogate measures of insulin sensitivity (HOMA). It can also be at least partly explained by the fact that early after surgery, caloric restriction per se (whether achieved by lower intake as per restrictive procedures or reduced absorption as with malabsorptive operations) plays a major role in improving insulin action. Months to years after surgery, weight loss has generally leveled off; a quantitative relationship between the changes in BMI and insulin sensitivity is now usually evident. The weakness of the correlation between the loss of weight and the gain in insulin sensitivity has been repeatedly noted. For example, in a nonrandomized study comparing LAGB and RYGB in a large number of nondiabetic patients (66), HOMA at 30 months postsurgery was similar but RYGB induced a significantly larger weight loss than LAGB (−33 vs. −22%). It should be considered that, in addition to the confounding factors mentioned in the previous section, an important determinant of weight loss is the initial body weight. For example, in a prospective 2-year follow-up of 107 men and women undergoing BPD at one center, there was a strong correlation (r = 0.68) between initial weight and achieved weight loss, which was entirely driven by initial fat mass (r = 0.64) and not fat-free mass (FFM) (67). This phenomenon is reminiscent of the common clinical observation that with any treatment the response is proportional to the initial height of the abnormality (e.g., A1C or arterial blood pressure). As with these other variables, the biology underlying this apparent rule is unclear.
The questions now arise 1) whether insulin sensitivity is fully restored despite the fact that postoperative BMI typically remains in the obese range, 2) whether the heightened insulin sensitivity is alone responsible for the resolution/improvement of hyperglycemia, and 3) whether the different surgical approaches differ from one another in their insulin-sensitizing power.
Some relevant information can be derived from the few studies that have employed the clamp technique to directly measure insulin sensitivity. In two different databases using the same exogenous insulin infusion rate, the dependency of whole-body insulin sensitivity on BMI is best described by curvilinear fits (online appendix Fig. A2). Both in the European Group for the Study of Insulin Resistance (EGIR) cohort (20) and in the study by Muscelli et al. (68), a 30% reduction in BMI (from 46 kg/m2) predicts a 50% increase in insulin sensitivity. However, both interpolating functions also predict that insulin sensitivity would not be fully restored by a 30% weight decrement (to the level of 42 μmol · kgFFM−1 · min−1 associated with a BMI of 25 kg/m2).
We then used these cross-sectional relationships to scale the results of insulin clamp studies in subjects losing weight by different approaches. In nondiabetic subjects on a calorie-restricted diet (69) or undergoing RYGB (68), the data fall well within the 95% CIs of the fit (Fig. 1)—in other words, when weight-stabilized subjects had gained insulin sensitivity in exact proportion to the weight change. Strikingly similar results are obtained when plotting data from two other clamp studies, using caloric restriction (38) or RYGB (70). In contrast, in 107 patients, 35 of whom with type 2 diabetes, undergoing BPD and restudied 2 years later, the increase in insulin sensitivity definitely exceeded the prediction; i.e., insulin resistance was normal or supernormal at BMI values still in the obese range (67). Furthermore, in one study (71) where insulin sensitivity was estimated at variable (but not sequential) time intervals following BPD, completely normal rates of insulin-mediated glucose clearance were found as early as 10 days after surgery; at this time, marked insulin resistance was present in a control group of morbidly obese patients undergoing major nonbariatric abdominal surgery.
With regard to hepatic insulin resistance, we could find no study that measured endogenous glucose production in obese diabetic patients before and after surgery. However, given that hepatic and peripheral insulin resistance correlate with each other, the pattern of results outlined for insulin-mediated glucose uptake can probably be extrapolated to the effects of insulin on endogenous (hepatic) insulin sensitivity. As for fat distribution, in a large cohort of subjects (including type 2 diabetic patients) undergoing LAGB, the ratio of visceral to subcutaneous abdominal fat (measured by ultrasound) was significantly reduced 1 year postoperatively in concomitance with a weight loss of 8 BMI units (43). This finding is consistent with the notion that fat is more rapidly lost from visceral than subcutaneous depots. Whereas this fat redistribution may contribute to the improvement of other metabolic abnormalities, it is doubtful that selective fat removal in very obese subjects who lose substantial portions of excess weight affects insulin action over and above what is engendered by weight loss itself.
Patients with diabetes appear to lose significantly less weight than equally obese nondiabetic subjects following gastric bypass (72). Whether the gain in insulin sensitivity in obese type 2 diabetic patients is any different from that of the nondiabetic obese individuals for the same weight reduction has not been examined systematically. In one series (71), insulin sensitivity was fully restored in subjects with normal glucose tolerance, impaired glucose tolerance, or type 2 diabetes following BPD; because type 2 diabetic patients had lower presurgery levels of insulin sensitivity, their gain was the highest.
The picture emerging from this analysis is as follows: Surgical procedures that greatly restrain food intake (RYGB) or absorption (BPD) may induce some recovery of insulin sensitivity before any large weight loss has occurred. However, when tested under conditions of stable energy balance, insulin resistance is increased by surgically induced weight loss quantitatively. Therefore, long-term–postsurgery morbidly obese patients (nondiabetic and diabetic alike) are likely to retain a degree of insulin resistance if their BMI is still in the overweight/obese range. Malabsorptive procedures take exception in that they may improve insulin sensitivity beyond the effect of weight loss. This fundamental difference between mainly restrictive and malabsorptive procedures needs to be proven by prospective, randomized studies.
Bariatric surgery and β-cell function
Assessing β-cell function is problematic because currently no clinical test has been agreed upon as being the gold standard in the way that the clamp has for the measurement of insulin sensitivity. Furthermore, insulin secretion is intrinsically complex because β-cells must adapt to chronic stimuli (e.g., weight changes) as well as respond to acute challenges (i.e., the succession of fasting and feeding). For the sake of the following discussion, it is important to distinguish between secretory indexes that reflect long-term adaptation from those that result from the dynamic behavior of the β-cell. Basal insulin secretion and total insulin output in response to a standard stimulus are set points of secretory capacity, whereas β-cell glucose sensitivity (or the slope of the insulin secretion/plasma glucose dose-response relationship) is the ability to control glycemia by promptly releasing sufficient hormone. This key function is reflected in empirical indexes such as the acute insulin response (AIR) to intravenous glucose or the insulinogenic index on the oral glucose tolerance test or a mixed meal.
Data on changes in β-cell function with bariatric surgery are few but relatively consistent. Fasting insulin concentrations (44,51) or secretion rates (54) and total insulin output in response to intravenous (47) or oral glucose (71) or mixed meals (54) all have been found to decrease after any bariatric procedure. This is the expected consequence of the reduced adipose mass and improved insulin sensitivity (73). In contrast, in type 2 diabetic patients, HOMA of β-cell function after LAGB (45), AIR and the insulinogenic index after RYGB (49,52), and AIR after BPD (47) all increased to a variable extent. Model-derived β-cell glucose sensitivity was fully normalized in 10 type 2 diabetic patients 2 years post-BPD in parallel with the normalization of daylong plasma glucose concentrations (54).
Thus, surgical weight loss generally lowers the set point but heightens the dynamic responsivity of the β-cell. Whether β-cell function is fully restored appears to depend principally on the severity of diabetes (relative to duration, degree of metabolic control, and intensity of antidiabetes treatment) (45,55). With purely or mostly restrictive procedures, β-cell function shows progressive improvement over time, paralleling ongoing weight loss. With BPD, almost complete recovery of AIR has been reported in a small group of type 2 diabetic patients as early as 1 month postsurgery (59).
All in all, diabetes in the very obese does not appear to differ in pathophysiology from the more common variety of moderately obese diabetes. Clearly, insulin resistance is more severe because of its quantitative relation to BMI, and drastic increments in insulin action with major weight loss underlie the spectacular remission rates. β-Cell function recovers in large part or in full probably depending on its initial, genetically determined quality.
Mechanisms
Food intake, transit, and absorption are regulated by a complex network including the gastrointestinal system, the liver, and the brain (rev. in 74). Surgical restriction of the stomach may change circulating concentrations of ghrelin, a hormone secreted by endocrine cells in the fundus. When infused into healthy volunteers, ghrelin induces acute insulin resistance (75). However, changes in ghrelin levels after bariatric surgery have been inconsistent and unrelated to the ensuing changes in insulin sensitivity (76). Changes in other gastrointestinal hormones have been analyzed for those bariatric procedures that alter food transit. Glucagon-like peptide-1 (GLP-1) potentiates insulin release in a glucose-dependent manner (74). GLP-1 responses (to glucose or mixed meals) are impaired in association with both type 2 diabetes and obesity (77), and GLP-1 levels increase early after both RYGB (58,78) and BPD (53) but apparently not after diet (58). Thus, one possibility is that heightened GLP-1 levels contribute to the improvement in β-cell function detectable early after surgery. It is not clear what the precise mechanism is by which GLP-1 release is revved up by anatomical rearrangements that either bypass the duodenum and upper jejunum (RYGB) or exclude the larger part of the entire gastrointestinal tract from food transit (BPD). However, in one study, GLP-1 at 6 weeks postsurgery was increased in normotolerant and impaired glucose-tolerant subjects but not in type 2 diabetic patients despite similar improvements in insulin resistance and β-cell dysfunction (49). Also, infusion of GLP-1 to pharmacological levels fails to stimulate insulin-mediated glucose disposal in healthy volunteers or under experimental conditions where its effects on endogenous insulin release are prevented (as in type 1 diabetic patients) (rev. in 79). Coupled with the current notion that GLP-1 receptors have not been demonstrated in liver, skeletal muscle, or adipose tissue, one can conclude that GLP-1 is an unlikely candidate to explain the remission of insulin resistance in bariatric patients. The involvement of glucose-dependent insulinotropic polypeptide (GIP) is even less clear. GIP resistance has been repeatedly described in type 2 diabetes (e.g., ref. 77), and GIP knockout in mice improves insulin action (80). However, following RYGB, increased (58) or unchanged (81) GIP levels have been reported in type 2 diabetic patients. On the other hand, GLP-1 has been implicated in the dumping syndrome and the reactive hypoglycemia that may follow gastric surgery (82). Its trophic actions on β-cells have been called upon to explain six cases of histologically proven nesidioblastosis after RYGB (83).
Further involvement of the gastrointestinal tract has been inferred from the outcome of novel surgical approaches. In Goto-Kakizaki rats, Rubino et al. (84) found that excluding a short segment of proximal intestine from food passage (via a duodenal-jejunal bypass or a gastrojejunostomy) improved glucose tolerance, whereas restoring duodenal transit reestablished glucose intolerance. This observation has led to a foregut hypothesis, which holds that contact of nutrients with the duodenal mucosa generates signals (hormonal and/or neural) that interfere with glucose metabolism and insulin action; bypassing duodenal passage (as in RYGB and BPD) would remove this inhibition.
In the rat, Strader et al. (85) showed that effects similar to those of duodenal exclusion could be produced by transposing an ileal segment into the upper intestine. In studies in type 2 diabetic patients with a BMI <35 kg/m2, DePaula et al. (86) reported that ileal interposition with a sleeve gastrectomy (with or without diversion) resulted in weight loss and a drastic fall in A1C, fasting glucose, and HOMA-IR 7 months later. A “hindgut hypothesis” thus proposes that contact with undigested nutrients triggers an ileal brake, i.e., a combination of effects influencing eating behavior and digestion.
Finally, in rats, diversion of the bile flow from the duodenum into the second jejunal loop leads to improved tolerance to oral as well as intravenous glucose compared with sham-operated animals (87). BPD is the only bariatric approach that causes major lipid malabsorption at the same time as it depletes intramyocellular lipids and returns insulin sensitivity to normal or supranormal levels in the longer term (88). Fat starvation in rats reproduces the metabolic picture of BPD with surprising precision (89). Therefore, lipids and lipid signal molecules—and their control by the bile—must be among key messengers released by a subverted gastrointestinal tract.
CONCLUSIONS
Bariatric surgery is a highly effective means of inducing diabetes remission in very obese patients with type 2 diabetes. Rank order of increasing efficacy of the most common surgical procedures progresses from the purely restrictive to the mostly restrictive to the mostly malabsorptive; several new approaches, however, are under very active investigation. Diabetes remission results from the joint improvement of insulin resistance and β-cell dysfunction. Better insulin action on glucose metabolism relieves secretory pressure on the β-cell, resulting in reduced insulin output; dynamic β-cell responses, however, are restored. Rates of complete diabetes remission or glycemic improvement depend essentially on the severity of diabetes (as indexed by duration, A1C levels, presence of complications, and intensity of treatment). Factors such as family history, interaction with previous antidiabetes therapies, or evidence of autoimmunity have not been analyzed specifically.
Caloric restriction and weight loss are the dominant mechanisms of improved glucose metabolism. The former appears to account for the early postsurgical recovery of insulin sensitivity and secretory dynamics; the latter is the final determinant of the outcome once weight and caloric balance have stabilized. When food transit is surgically altered, changes in the pattern of gastrointestinal hormone release may support the early adaptation of β-cell function but are unlikely to make a major contribution to insulin action.
Whether bariatric operations exert an intrinsic antidiabetes action beyond weight loss remains unproven. The best of available evidence indicates that malabsorptive operations presently offer the highest chances of revealing weight-independent mechanisms of diabetes resolution, but smart manipulations of food passage may open entirely new avenues. Experimenting in less obese or nonobese diabetes or minimizing weight loss may provide further evidence. Difficult as they may be, randomized controlled clinical studies using state-of-the-art methodology are required to prove the worth of metabolic surgery.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM: Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El NM: Obesity and diabetes in the developing world: a growing challenge. N Engl J Med 356:213–215, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF: Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355:763–778, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Nilsson PM: Is weight loss beneficial for reduction of morbidity and mortality? Diabetes Care 31(Suppl. 2):S278–S283, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bessesen DH: Update on obesity. J Clin Endocrinol Metab 93:2027–2034, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Davis MM, Slish K, Chao C, Cabana MD: National trends in bariatric surgery, 1996–2002. Arch Surg 141:71–74, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I: Trends in mortality in bariatric surgery: a systematic review and metaanalysis. Surgery 142:621–632, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG: Bariatric surgery for severely obese adolescents. J Gastrointest Surg 7:102–107, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Barnett SJ, Stanley C, Hanlon M, Acton R, Saltzman DA, Ikramuddin S, Buchwald H: Long-term follow-up and the role of surgery in adolescents with morbid obesity. Surg Obes Relat Dis 1:394–398, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Nadler EP, Youn HA, Ren CJ, Fielding GA: An update on 73 US obese pediatric patients treated with laparoscopic adjustable gastric banding: comorbidity resolution and compliance data. J Pediatr Surg 43:141–146, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgeson J, Agren G, Carlsson LM, Swedish Obese Subjects Study: Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC: Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Hu FB: Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med 4:34–41, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Maglione M, Tu W, Mojica W, Arteburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC: Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 142:532–546, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ahima RS: Should eligibility for bariatric surgery be expanded? Gastroenterology 134:15, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K: Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Turner RC, Cull CA, Frighi V, Holman RR: Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281:2005–2012, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Crookes PF: Surgical treatment of morbid obesity. Annu Rev Med 57:243–264, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF: Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care 28:2406–2411, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G: Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 100:1166–1173, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAuley K, Mann J: Thematic review series: patient-oriented research. Nutritional determinants of insulin resistance. J Lipid Res 47:1668–1676, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Pidcoke HF, Wade CE, Wolf SE: Insulin and the burned patient. Crit Care Med 35(Suppl. 9):S524–S530, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Brandi LS, Santoro D, Natali A, Altomonte F, Baldi S, Frascerra S, Ferrannini E: Insulin resistance of stress: sites and mechanisms. Clin Sci 85:525–535, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Grinspoon S: Mechanisms and strategies for insulin resistance in acquired imuune deficiency syndrome. Clin Infect Dis 37(Suppl. 2):S85–S90, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Klein S, Fontana L, Young L, Coggan AR, Kilo C, Patterson BW, Mohammed BS: Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350:2549–2557, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Rizzo MR, Paolisso G, Grella R, Barbieri M, Grella E, Ragno E, Grella R, Nicoletti G, D'Andrea F: Is dermolipectomy effective in improving insulin action and lowering inflammatory markers in obese women? Clin Endocrinol (Oxf )63:253–258, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Foley JE, Laursen AL, Sonne O, Gliemann J: Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia 19:234–241, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW: Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia 50:625–633, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N: Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 51:2951–2958, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Knittle JL, Ginsberg-Fellner F: Effect of weight reduction on in vitro adipose tissue lipolysis and cellularity in obese adolescents and adults. Diabetes 21:754–761, 1972 [DOI] [PubMed] [Google Scholar]
- 31.Purnell JQ, Kahn SE, Albers JJ, Nevin DN, Brunzell JD, Schwartz RS: Effect of weight loss with reduction of intra-abdominal fat on lipid metabolism in older men. J Clin Endocrinol Metab 85:977–982, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Goodpaster BH, Theriault R, Watkins SC, Kelley DE: Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49:467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Kotronen A, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H: Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia 51:130–138, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Klein S, Luu K, Gasic S, Green A: Effect of weight loss on whole body and cellular lipid metabolism in severely obese humans. Am J Physiol 270:E739–E745, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Pontiroli AE, Frigé F, Paganelli M, Folli F: In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg 2008 July 16 [Epub ahead of print] [DOI] [PubMed]
- 36.DeFronzo RA, Soman V, Sherwin RS, Hendler R, Felig P: Insulin binding to monocytes and insulin action in human obesity, starvation, and refeeding. J Clin Invest 62:204–213, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Henry RR, Scheaffer L, Olefsky JM: Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 61:917–925, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M: Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 77:1287–1293, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Badman MK, Flier JS: The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132:2103–2115, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Razak F, Anand S, Vuksan V, Davis B, Jacobs R, Teo KK, Yusuf S: Ethnic differences in the relationships between obesity and glucose-metabolic abnormalities: a cross-sectional, population-based study. Int J Obes (Lond) 29:656–667, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G: Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes. Am J Med 77:7–17, 1984 [DOI] [PubMed] [Google Scholar]
- 42.Gelonese B, Tambascia MA, Pareja JC, Repetto EM, Magna LA: The insulin tolerance test in morbidly obese patients undergoing bariatric surgery. Obes Res 9:763–769, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Pontiroli AE, Pizzocri P, Librenti MC, Vedani P, Marchi M, Cucchi E, Orena C, Paganelli M, Giacomelli M, Ferla G, Folli F: Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab 87:3555–3561, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Dixon JB, O'Brien PE: Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care 25:358–363, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Dixon JB, Dixon AF, O'Brien PE: Improvements in insulin sensitivity and ß-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med 20:127–34, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, Minar E, Roka R, Schernthaner G: Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol 23:1042–7, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK: Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 52:1098–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Veronelli A, Laneri M, Ranieri R, Koprivec D, Vardaro D, Paganelli M, Folli F, Pontiroli AE: White blood cells in obesity and diabetes: effects of weight loss and normalization of glucose metabolism. Diabetes Care 27:2501–2502, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J: GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 16:1594–1601, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Coppini LZ, Bertevello PL, Gama-Rodrigues J, Waitzberg DL: Changes in insulin sensitivity in morbidly obese patients with or without metabolic syndrome after gastric bypass. Obes Surg 16:1520–1525, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Ballantyne GH, Farkas D, Laker S, Wasiliewski A: Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg 16:1189–1197, 2006 [DOI] [PubMed] [Google Scholar]
- 52.García-Fuentes E, García-Almeida JM, García-Arnéz J, Rivas-Marín J, Gallego-Perales JL, González-Jiménez B, Cardona I, García-Serrano S, Garriga MJ, Gonzalo M, Ruiz de Adana MS, Soriguer F: Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg 16:1179–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G: Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 55:2025–2031, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Camastra S, Manco M, Mari A, Greco AV, Frascerra S, Mingrone G, Ferrannini E: ß-cell function in severely obese type 2 diabetic patients. Long-term effects of bariatric surgery. Diabetes Care 30:1002–1004, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Vidal J, Ibarzabal A, Nicolau J, Vidov M, Delgado S, Martinez G, Balust J, Morinigo R, Lacy A: Short-term effects of sleeve gastrectomy on type 2 diabetes mellitus in severely obese subjects. Obes Surg 17:1069–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Gannagé-Yared MH, Yaghi C, Habre B, Khalife S, Noun R, Germanos-Haddad M, Trak-Smayra V: Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol 158:353–359, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M: Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B: Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Briatore L, Salani B, Andraghetti G, Danovaro C, Sferrazzo E, Scopinaro N, Adami GF, Maggi D, Cordera R: Restoration of acute insulin response in T2DM subjects 1 month after biliopancreatic diversion. Obesity 16:77–81, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Shen SW, Reaven GM, Farquhar JW: Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest 49:2151–2160, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M: Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab 68:374–378, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ: Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677, 1979 [DOI] [PubMed] [Google Scholar]
- 65.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 66.Lee W-J, Lee Y-C, Ser K-H, Chen J-C, Chen SC: Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg 18:1119–1125, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Valera-Mora ME, Simeoni B, Gagliardi L, Scarfone A, Nanni G, Castagneto M, Manco M, Mingrone G, Ferrannini E: Predictors of weight loss and reversal of comorbidities in malabsorptive bariatric surgery. Am J Clin Nutr 81:1292–1297, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrannini E: Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med 118:51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Muscelli E, Emdin M, Natali A, Pratali L, Camastra S, Gastaldelli A, Baldi S, Carpeggiani C, Ferrannini E: Autonomic and hemodynamic responses to insulin in lean and obese humans. J Clin Endocrinol Metab 83:2084–2090, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Bobbioni-Harsch E, Bongard O, Habicht F, Weimer D, Bounameaux H, Huber O, Chassot G, Morel P, Assimacopoulos-Jeannet F, Golay A: Relationship between sympathetic reactivity and body weight loss in morbidly obese subjects. Int J Obes Relat Metab Disord 28:906–911, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, Ferrannini E: Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia 49:2136–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Carbonell AM, Wolfe LG, Meador JG, Sugerman NJ, Kellum JM, Maher JW: Does diabetes affect weight loss after gastric bypass? Surg Obes Relat Dis 4:441–444, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Ferrannini E, Camastra S, Gastaldelli A, Sironi AM, Natali A, Muscelli E, Mingrone G, Mari A: ß-cell function in obesity: effects of weight loss. Diabetes 53(Suppl. 3):S26–S33, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Cummings DE, Overduin J: Gastrointestinal regulation of food intake. J Clin Invest 117:13–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO: Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 57:3205–3210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanusch-Enserer U, Cauza E, Brabant G, Dunky A, Rosen H, Pacini G, Tüchler H, Prager R, Roden M: Plasma ghrelin in obesity before and after weight loss after laparoscopical adjustable gastric banding. J Clin Endocrinol Metab 89:3352–3358, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E: Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57:1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E: The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240:236–242, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holst JJ: The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Flatt PR: Effective surgical treatment of obesity may be mediated by ablation of the lipogenic gut hormone gastric inhibitory polypeptide (GIP): evidence and clinical opportunity for development of new obesity-diabetes drugs? Diab Vasc Dis Res 4:151–153, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Whitson BA, Leslie DB, Kellog TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S: Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res 141:31–39, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto H, Mori T, Tsuchihashi H, Akabori H, Naito H, Tani T: A possible role of GLP-1 in the pathophysiology of early dumping syndrome. Dig Dis Sci 50:2263–2267, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV: Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353:249–254, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J: The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244:741–749, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ: Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:E447–E453, 2005 [DOI] [PubMed] [Google Scholar]
- 86.DePaula AL, Macedo ALV, Rassi N, Machado CA, Schraibman V, Silva LQ, and Halpern, H: Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc 22:706–716, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Manfredini G, Ermini M, Scopsi L, Bonaguidi F, Ferrannini E: Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol 249:G519–G527, 1985 [DOI] [PubMed] [Google Scholar]
- 88.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E: Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51:144–151, 2002 [DOI] [PubMed] [Google Scholar]
- 89.McGarry JD: Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18, 2002 [DOI] [PubMed] [Google Scholar]