Autoimmune pancreatitis (AIP) is a relatively new clinical entity whose acceptance as a distinct systemic disease has grown in the last decade with the emergence of improved diagnostic criteria and management options. AIP has characteristic clinical, radiological, serological, and histological features (1). A subset of patients with AIP develops fulminant type 1 diabetes (2,3). The pathogenesis remains unresolved, but immune-mediated mechanisms that compromise endocrine and exocrine pancreatic function appear to be involved.
Characteristic imaging criteria include pancreatic enlargement with an irregular, narrow pancreatic duct. The presence of diffuse swelling and “halo” features on computed tomography appear to be predictors of a favorable response to treatment with corticosteroids, whereas the presence of ductal strictures and focal swelling may predict suboptimal response to corticosteroids, the current first-line treatment (4). Cases of AIP with focal enlargement, distinct mass, normal pancreas, and focal acute pancreatitis have been observed as well as involvement of other organs, such as intrahepatic biliary strictures. Serum IgG or IgG4 levels are elevated in a majority of cases. Histologic features include the presence of lymphoplasmacytic sclerosing pancreatitis and infiltration of tissues with IgG4-positive cells (5) (Fig. 1). It is important to rule out pancreatobiliary cancer because of overlap in imaging criteria and other clinical features. The identification of a sensitive and specific biomarker for autoimmune pancreatitis detection of autoantibody responses directed to amylase α-2A represents a significant advance for the diagnosis and management of this condition.
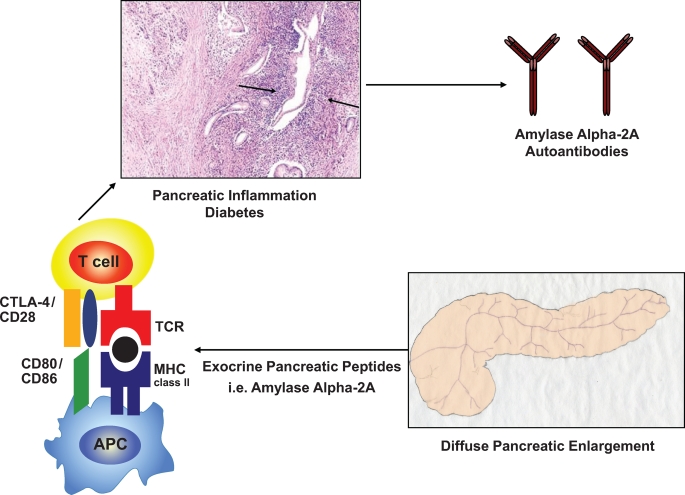
FIG. 1.
Hypothetical mechanisms causing autoimmune pancreatitis. Although the pathogenesis of AIP and fulminant type 1 diabetes remains to be elucidated, AIP, similar to other autoimmune diseases, is associated with polymorphisms within the HLA complex. In the Japanese population, the highest risk for autoimmune pancreatitis is conferred by the HLA DRB1*0405-DQB1*0401 haplotype (ref. 11). Each allele is simply given a number that represents a unique amino acid sequence, and each sequence binds only certain peptides, which could be amylase α-2A peptides. Antigen-presenting cells (APC) present these peptides to lymphocytes and provide signals that stimulate the proliferation and differentiation of lymphocytes, generally T-lymphocytes. Interestingly, regulatory T-cell–deficient NOD CD28KO mice develop spontaneous AIP, which in this model is not a consequence of preexisting β-cell autoimmunity (ref. 13). This mechanism could also play a role in the pathogenesis of AIP in humans. The histologic pattern of AIP in humans includes a periductal collar of lymphoplasmacytic inflammation (arrows, hematoxylin and eosin image reprinted with permission from Finkelbert et al. [ref. 16]). MHC, major histocompatibility complex; TCR, T-cell receptor. (Please see http://dx.doi.org/10.2337/db08-1655 for a high-quality digital representation of this figure.)
While the pathogenesis of AIP and fulminant type 1 diabetes remains to be elucidated, both entities appear to share pancreatic autoimmune manifestations. A biphasic mechanism has been proposed recently consisting of “induction” and “progression” stages (6). An initial induction response to self-antigens and molecular mimicry is mediated by decreased naïve regulatory T-cells, and T-helper cells release proinflammatory cytokines. In the chronic phase, progression is mediated by increased memory regulatory T- and T–helper cell immune responses. The classic complement pathway may be activated by the IgG1 immune complex. A number of important details of this pathway remain to be elucidated.
In Japanese patients, type 1 diabetes is associated with the HLADRB1, DQA1, DQB1 *0405, *0303, *0401 or *0901, *0302, *0303 haplotype (7–9). Interestingly, in this issue of Diabetes, Endo et al. (10) report that 5 out of 15 patients with AIP were heterozygous for the HLA DRB1* 0405-DQB1*0401 haplotype, which confirms previously published results in a Japanese population (11,12). It is noteworthy that a recent study provided convincing evidence that CD4+ T-cells were found to be both necessary and sufficient for the development of AIP in regulatory T-cell–deficient, CD28-deficient NOD mice (13). Autoantibodies and autoreactive T-cells from these mice recognized pancreatic amylase, and the administration of tolerogenic amylase-coupled fixed spleen cells significantly improved disease severity, suggesting that this protein is a significant autoantigen in this model of AIP.
A subset of patients with AIP develops fulminant type 1 diabetes. Identification of a sensitive and specific biomarker for this population would represent a significant advance for the diagnosis and management of these individuals. Endo et al. report that autoantibodies directed against amylase α-2A appear to be a specific marker for patients with AIP and fulminant type 1 diabetes. The authors screened a λTriplEx2 human pancreas cDNA library with serum from a patient with AIP and obtained positive clones. Subsequently, the authors developed an enzyme-linked immunosorbent assay (ELISA) to detect autoantibodies against human amylase α-2A using a recombinant COOH-terminal amylase α-2A protein. Sera from patients with AIP, chronic alcoholic pancreatitis, and pancreas tumor were screened, and only the AIP patients demonstrated autoantibodies that recognized the recombinant protein. Of interest, autoantibody against amylase α-2A was detected in 88% of patients with fulminant type 1 diabetes, 21% of patients with acute-onset type 1 diabetes, and 6% of patients with type 2 diabetes. The authors conclude that autoantibody against amylase α-2A represents a novel diagnostic marker for both AIP and fulminant type 1 diabetes. It will be important to determine the sensitivity and specificity of the radiobinding assay based on in vitro transcribed/translated [35S]methionine-labeled recombinant amylase α-2A, which has been described by Endo et al. This assay format could be used for large-scale screening purposes. It is widely known that the overall performance of ELISA assays detecting autoantibodies directed to type 1 diabetes–related autoantigens, i.e., insulin or GAD65 autoantibodies, is poor (14).
The clinical and morphological changes that are characteristically observed in patients with AIP and fulminant type 1 diabetes are nonspecific. Therefore, the availability of a specific serologic biomarker for this disorder would be very useful, particularly in helping to guide the clinician to intervene with an immunosuppressant, such as a corticosteroid, relatively early to help preserve pancreatic exocrine and endocrine function. Based on the data presented by the authors, the presence of elevated titers of autoantibodies directed against amylase α-2A in the appropriate clinical setting may represent a specific biomarker to identify patients with AIP and fulminant type 1 diabetes. The issue of specificity has been partially addressed by the observation that sera from patients with either chronic alcoholic pancreatitis or pancreas tumor did not recognize the recombinant protein in a fluid-phase assay format. However, sera from 21% of patients with acute-onset type 1 diabetes and 6% of patients with type 2 diabetes did demonstrate elevated titers of autoantibody directed against amylase α-2A. Therefore, the specificity of amylase α-2A autoantibodies as a unique biomarker for AIP and fulminant type 1 diabetes requires further evaluation and testing in other racial groups. For example, a recent report supports the presence of distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes and nonalcoholic chronic pancreatitis (15). Autoantibodies against carbonic anhydrase II (CAIIAb) and lactoferrin (LACAb) (both of which are exocrine pancreatic antigens) were screened with ELISA. A significantly higher prevalence of CAIIAb and/or LACAb was found for patients with type 1 diabetes (29.2%) and nonalcoholic chronic pancreatitis (22.9%) than in control subjects (0%). There was a significant association between CAIIAb and LACAb titers both for patients with type 1 diabetes (P = 0.042) and for patients with nonalcoholic chronic pancreatitis (P < 0.001). Therefore, a subpopulation of European Caucasian patients with type 1 diabetes and nonalcoholic chronic pancreatitis have autoantibodies against the exocrine pancreatic antigens CAIIAb and LACAb. Autoantibodies directed against amylase α-2A were not screened in this report. It is noteworthy that Endo et al. observed that 100% of the sera from patients with AIP and 88% of the sera from patients with fulminant type 1 diabetes demonstrated elevated titers of the autoantibody against the recombinant human amylase α-2A protein.
It is unresolved whether the titer of autoantibodies directed against amylase α-2A is causally linked to severity of the pathophysiology in patients with AIP and fulminant type 1 diabetes or merely correlates with severity. The favorable response to intervention with corticosteroids and the observed decrease in titers of autoantibody suggest that there is an association between the titers of antibody and severity but do not establish a potential contributing role for the amylase α-2A autoantibodies to the pathophysiology of this disorder, beyond being a biomarker for AIP and fulminant type 1 diabetes.
The presence of elevated titers of autoantibodies directed against amylase α-2A may represent a novel specific biomarker to help identify patients at risk for autoimmune pancreatitis and fulminant type 1 diabetes. This population appears to benefit from early intervention with corticosteroids, which helps preserve pancreatic exocrine and endocrine function. This interesting and potentially clinically relevant observation requires confirmation by other laboratories and further characterization regarding its specificity and sensitivity. Mechanistic studies in humans will be necessary to assess whether there is a pathogenic role for T-cells and/or autoantibodies reactive with α-2A and whether a combination of specific T-effector–inactivating therapies along with immunomodulatory agents can alter disease course in patients affected by autoimmune pancreatitis.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
See accompanying original article, p. 732.
REFERENCES
- 1.Kahara T, Takamura T, Ando H, Sakurai M, Ota T, Misaki T, Oba S, Iguchi M, Komori K, Kobayashi K: Fulminating onset type 1 diabetes with positivity for anti-GAD antibody and elevated pancreatic exocrine enzyme concentrations. Intern Med 42: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Imagawa A, Hanafusa T, Miyagawa J-I, Matzuzawa Y, for the Osaka IDDM Study Group: A novel subtype of type 1 diabetes mellitus characterized by rapid onset and absence if diabetes-related antibodies. N Engl J Med 342: 301–307, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Imagawa A, Hanafusa T: Fulminant type 1 diabetes mellitus. Endocr J 53: 577–584, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sahani DV, Sainani NI, Deshpande V, Shaikh MS, Frinkelberg DL, Fernandez-Del Castillo C: Autoimmune pancreatitis: disease evolution, staging, response assessment, and CT features that predict response to corticosteroid therapy. Radiology. In press [DOI] [PubMed]
- 5.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB: Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 4: 1010–1016, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Okazaki K, Uchida K, Fukui T: Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol 43: 409–418, 2008 [DOI] [PubMed] [Google Scholar]
- 7.She J-X: Susceptibility to type I diabetes: HLA-DQ and DR revisited. Immunol Today 17: 323–329, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Pietropaolo M, Trucco M: Genetics of type 1 diabetes mellitus. In Contemporary Endocrinology: Type 1 Diabetes: Etiology and Treatment. Sperling MA, Ed. Totowa, NJ, Humana Press Inc., 2003, p. 23–54
- 9.Morran MP, Omenn GS, Pietropaolo M: Immunology and genetics of type 1 diabetes. Mt Sinai J Med 75: 314–327, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, Kobayashi T: Amylase α-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 58: 732–737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, Hasebe O, Kiyosawa K: HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology 122: 1264–1269, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S, Kobayashi T, Nakanishi K, Koyama R, Okubo M, Murase T, Odawara M, Inoko H: Association of HLA-DQ genotype in autoantibody-negative and rapid-onset type 1 diabetes. Diabetes Care 25: 2302–2307, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Meagher C, Tang Q, Fife BT, Bour-Jordan H, Wu J, Pardoux C, Bi M, Melli K, Bluestone JA: Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol 180: 7793–7803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H: Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 74: 1040–1044, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Hardt PD, Ewald N, Brockling K, Tanaka S, Endo T, Kloer HU, Bretzel RG, Jaeger C, Shimura H, Kobayashi T: Distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP 9: 683–689, 2008 [PubMed] [Google Scholar]
- 16.Finkelberg DL, Sahani D, Deshpande V, Brugge WR: Autoimmune pancreatitis. N Engl J Med 355: 2670–2676, 2006 [DOI] [PubMed] [Google Scholar]