Abstract
OBJECTIVE—Leptin is an adipocyte hormone that plays a major role in energy balance. Leptin receptors in the hypothalamus are known to signal via distinct mechanisms, including signal transducer and activator of transcription-3 (STAT3) and phosphoinositol-3 kinase (PI 3-kinase). Here, we tested the hypothesis that extracellular signal–regulated kinase (ERK) is mediating leptin action in the hypothalamus.
RESEARCH DESIGN AND METHODS—Biochemical, pharmacological, and physiological approaches were combined to characterize leptin activation of ERK in the hypothalamus in rats.
RESULTS—Leptin activates ERK1/2 in a receptor-mediated manner that involves JAK2. Leptin-induced ERK1/2 activation was restricted to the hypothalamic arcuate nucleus. Pharmacological blockade of hypothalamic ERK1/2 reverses the anorectic and weight-reducing effects of leptin. The pharmacological antagonists of ERK1/2 did not attenuate leptin-induced activation of STAT3 or PI 3-kinase. Blockade of ERK1/2 abolishes leptin-induced increases in sympathetic nerve traffic to thermogenic brown adipose tissue (BAT) but does not alter the stimulatory effects of leptin on sympathetic nerve activity to kidney, hindlimb, or adrenal gland. In contrast, blockade of PI 3-kinase prevents leptin-induced sympathetic activation to kidney but not to BAT, hindlimb, or adrenal gland.
CONCLUSIONS—Our findings indicate that hypothalamic ERK plays a key role in the control of food intake, body weight, and thermogenic sympathetic outflow by leptin but does not participate in the cardiovascular and renal sympathetic actions of leptin.
Leptin is a largely adipocyte-derived hormone that can act in the central nervous system to decrease appetite and increase energy expenditure, thereby leading to decreased body weight (1). Central actions of leptin play an important role in the regulation of several other physiological functions, including reproductive function (2), bone formation (3), and regional sympathetic nerve activity (SNA) subserving thermogenic metabolism and cardiovascular function (4).
Leptin exerts its effects via interaction with specific receptors located in distinct classes of neurons. While several isoforms of the leptin receptor have been identified, the Ob-Rb form that includes the long intracellular domain that has signaling capacity appears to mediate most of the biological effects of leptin (5,6). The signal transducer and activator of transcription-3 (STAT3) pathway was the first signaling mechanism associated with the leptin receptor (7). Neural-specific inactivation of STAT3 leads to hyperphagia and obesity in mice (8). In addition, disrupting the ability of the leptin receptor to activate the STAT3 pathway in mice leads to severe obesity and several other neuroendocrine abnormalities (9–11). More recently, other intracellular signaling mechanisms, including phosphoinositol-3 kinase (PI 3-kinase) (12), AMP-activated protein kinase (13), and mammalian target of rapamycin (14), have been shown to play an important role in the action of leptin on food intake.
Extracellular signal–regulated kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family, is an additional downstream pathway of the leptin receptor (15). Leptin was shown to activate ERK1/2 in a time- and dose-dependent manner in cultured cells (16–18). Activation of ERK1/2 by leptin seems to be mediated through Src homology–containing tyrosine phosphatase 2 (Shp2) emanating from the tyrosine 985 (Tyr985) of the leptin receptor (19,20). Stimulation of ERK by leptin can also be achieved by direct interaction with Jak2 (15,19,20). In turn, in cell lines, ERK appears to mediate the activation of c-fos (20) and ribosomal S6 kinase and S6 (21) by the leptin receptor.
This ERK pathway has been reported to mediate leptin effects in several tissues, including cardiomyocytes (22,23), the immune system (24,25), and kidney (26). However, the physiological significance of this pathway for the hypothalamic-mediated effects of leptin remains poorly characterized. This study depicts the effect of leptin on hypothalamic ERK and investigates the potential role of this ERK pathway in mediating the effect of leptin on food intake, body weight, and regional sympathetic outflow.
RESEARCH DESIGN AND METHODS
Male Sprague-Dawley rats and lean and obese Zucker (fa) rats were obtained from Harlan Sprague-Dawley. Rats were housed at 23°C with a 12-h light/dark cycle (light on at 6:00 a.m.) and allowed free access to standard rat chow and water. Rats receiving injections in the third cerebral ventricle were equipped with intracerebroventricular cannulas at least 1 week before the experimentation as described previously (27). Ethical approval of all of the studies was granted by the University of Iowa Animal Research Committee.
Biochemical studies.
Rats were fasted overnight before intracerebroventricular administration of murine leptin (R&D Systems). Rats were killed at the indicated time points by CO2 asphyxiation. The mediobasal hypothalamus was quickly removed from each rat, and the total proteins were extracted and stored at −80°C. Protein samples of homogenized tissues or immunoprecipitates [to assess the effect of leptin on PI 3-kinase, immunoprecipitates were obtained by incubating protein samples with anti–IRS-1 antibody (E-12; Santa Cruz Biotechnology) in the presence of protein A–sepharose] were resolved with 10% SDS-PAGE, and PVDF membranes were incubated with specific antibodies for STAT3 (C-20; Santa Cruz Biotechnology), phospho-STAT3 (Tyr705; Cell Signaling), ERK1/2 (Cell Signaling), phospho-ERK1/2 (Thr202/Tyr204; Cell Signaling), p38 MAPK (H-147; Santa Cruz Biotechnology), phospho-p38 MAPK (Thr180/Tyr182; Cell Signaling), or PI 3-kinase p85 (Cell Signaling). Blots were detected with enhanced chemiluminescence, and their intensity was measured for quantitative analysis. To examine the effect of JAK2 blockade on the increase in ERK1/2 phosphorylation induced by leptin, different doses of AG490 were administered intracerebroventricularly 1 h before leptin (10 μg). To assess the effect of leptin on p38 MAPK in the skeletal muscle, rats were injected intraperitoneally with leptin (1 μg/g body wt) and killed 10 min after the treatment. Skeletal muscle from the hindlimb was removed, and the extracted proteins were assayed for phospho-p38 MAPK as described above.
Immunohistochemistry.
Rats were fasted overnight and treated with vehicle or leptin either intracerebroventricularly (10 μg) or intraperitoneally (1 μg/g body wt). Five to 20 min after the treatment, rats were killed by CO2 asphyxiation and then perfused transcardially with PBS followed by 4% paraformaldehyde in PBS. The brains were removed and postfixed in 4% paraformaldehyde at 4°C overnight. Fixed brains were washed three times with PBS and incubated in 30% sucrose in PBS. Coronal sections (30 μm) were cut with a freezing Microm cryostat. Free-floating sections were washed with PBS and permeabilized with 0.1% Triton X-100 in PBS. Sections were then incubated overnight at 4°C with a mouse phospho-ERK antibody (1:100; sc-7383; Santa Cruz Biotechnology) in 0.2% goat serum followed by 1-h incubation at room temperature with a secondary antibody, rhodamine (TRITC)-conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch Laboratories).
For double labeling, brains sections were processed for the localization of phospho-ERK as above. Rabbit antibodies recognizing proopiomelanocortin (POMC; 1:50; Phoenix Pharmaceuticals) or neuropeptide Y (NPY; 1:50; Chemicon International) were used to identify the neurons in which leptin activates ERK. Biotin-SP–conjugated donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch Laboratories) was used as secondary antibody. Primary antibodies were tested separately before performing the double immunostaining. Additional control experiments consisted in the omission of primary or secondary antibodies in each case.
Further processing for immunodetection was performed using kits (Vector Laboratories) following the manufacturer's instruction. Slices were mounted on slides, coverslipped, and visualized using a Nikon eclipse E600 fluorescence microscope equipped with a SPOT RT digital camera.
Food intake and body weight studies.
Food was removed from the individually caged rats the day before the study. Rats were given a single intracerebroventricular injection of PD98059 (5 μg), U0126 (7 μg), vehicle (DMSO; 2 μl), or artificial cerebrospinal fluid (2 μl) followed 15 min later by an intraperitoneal administration of leptin (1 μg/g body wt) or vehicle (saline) or an intracerebroventricular injection of 5 μg Melatonan II (MTII) or corticotrophin-releasing factor. The doses of the various drugs were based on our previous studies (27,28). Food was returned 1 h after the last injection corresponding to the onset of the dark cycle. Food intake and body weight were then recorded after 4 and 24 h.
Study of the sympathetic nervous system.
Anesthetized rats were instrumented for direct multifiber recording of regional SNA as described previously (27,28). Brown adipose tissue (BAT) SNA was recorded simultaneously with SNA to kidney, hindlimb, or adrenal gland. After baseline recordings of SNA were obtained, each animal received two intracerebroventricular injections. Rats received first PD98059 (5 μg), U0126 (7 μg), LY294002 (5 μg), or vehicle (DMSO; 2 μl) followed 15 min later by leptin (10 μg) or saline. After intracerebroventricular administration of experimental agents, SNA measurements were made every 15 min for 6 h. The data for SNA are expressed as percentage change from baseline.
Statistical analysis.
All results are expressed as means ± SE and analyzed using Student's t test, one- or two-way ANOVA. When ANOVA reached significance, a post hoc comparison was made using Bonferroni or Newman-Keuls test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
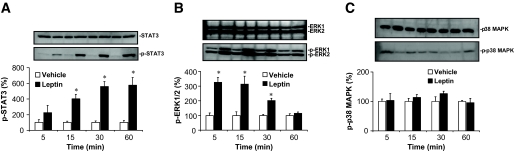
To investigate the hypothesis that the ERK pathway is important for leptin action in the central nervous system, we first assessed whether leptin activates this enzyme in the hypothalamus, in vivo. The effect of intracerebroventricular administration of leptin on the activity of STAT3 and ERK1/2 in the hypothalamus was examined in Sprague-Dawley rats. As previously reported (7,12,13), leptin caused a robust activation of the STAT3 in mediobasal hypothalamic extracts (Fig. 1A). Leptin also caused a rapid activation of hypothalamic ERK1/2 with a maximum effect at 5–15 min (Fig. 1B), consistent with previous findings (29,30). This response was dose dependent (5 and 10 μg leptin increased ERK1/2 activity by 3.4- and 4.3-fold, respectively). In peripheral tissues, such as the skeletal muscle, leptin has been shown to activate different isoforms of MAPK (31). Therefore, we examined whether leptin alters the activity of another hypothalamic isoform of MAPK. Leptin did not affect the activity of p38 MAPK in the hypothalamus (Fig. 1C). Intraperitoneal (IP) administration of leptin (1 μg/g body wt) did, however, cause a 3.1-fold increase (P < 0.01) in p38 MAPK in skeletal muscle (data not shown).
FIG. 1.
Leptin activation of STAT3 and ERK in the hypothalamus. Time course of activation of STAT3 (A) and ERK1/2 (B) in mediobasal hypothalamic explants after intracerebroventricular administration of murine leptin (10 μg) in rat. C: Lack of effect of intracerebroventricular leptin on the phosphorylation of hypothalamic p38 MAPK. Data represent means ± SE; n = 5 rats per group. *P < 0.001 vs. vehicle; †P < 0.001 vs. leptin alone.
Leptin activation of ERK1/2 appears to be mediated by the leptin receptor because it activates hypothalamic ERK1/2 in the Zucker lean rat, but not in the leptin receptor–deficient obese Zucker rat (Fig. 2A). Blockade of JAK2 with AG490 inhibited, in a dose-dependent manner, the activation of ERK1/2 by leptin (Fig. 2B), demonstrating that the leptin receptor modulates the activity of ERK1/2 via JAK2. These data demonstrate that in the hypothalamus, the effect of leptin on ERK is receptor mediated, involves JAK2, and is specific to ERK1/2 isoforms.
FIG. 2.
Leptin activation of ERK is receptor mediated and involves JAK2. A: Intracerebroventricular leptin caused a significant increase in the phosphorylation of hypothalamic ERK1/2 in the Zucker lean (ZL) but not in the leptin receptor–deficient Zucker obese (ZO) rats. B: AG490, an inhibitor of JAK2, administered intracerebroventricularly caused a dose-dependent blockade of intracerebroventricular leptin-induced hypothalamic ERK1/2 activation. Data represent means ± SE; n = 5 rats per group. *P < 0.001 vs. vehicle; †P < 0.001 vs. leptin alone.
Within the hypothalamus, the leptin receptor has been detected in several nuclei, including the arcuate, ventromedial, paraventricular, and dorsomedial nuclei (32,33). We used an immunohistochemical approach to identify the hypothalamic nuclei in which ERK1/2 is activated by leptin. Both systemic and central administration of leptin caused a marked increase in the immunoreactive ERK1/2 in the arcuate nucleus (Fig. 3A). In contrast, no increase in ERK activity was observed in hypothalamic nuclei other than the arcuate nucleus (in Fig. 3B, the paraventricular nucleus is shown as an example) or in extrahypothalamic nuclei, including the nucleus tractus solitarii in the brainstem (data not shown). These data suggest that leptin activation of hypothalamic ERK1/2 is selectively localized in the arcuate nucleus.
FIG. 3.
Immunohistochemical analysis of leptin activation of ERK in the hypothalamus. Intracerebroventricular and intraperitoneal (IP) leptin activate ERK1/2 in the arcuate nucleus (ARC; A) but not in the paraventricular nucleus (PVN; B). Five rats per treatment were examined. (Please see http://dx.doi.org/10.2337/db08-0822 for a high-quality digital representation of this figure.)
Two classes of neurons account for leptin sensitivity within the arcuate nucleus (32–34): first, a catabolic pathway represented mainly by POMC neurons that is activated by leptin; and second, an anabolic pathway represented principally by the NPY neurons that is inhibited by leptin. We therefore used double staining to determine whether activation of ERK1/2 by leptin occurs in one specific neuronal population. Interestingly, all the neurons in which the ERK1/2 immunoreactivity was increased by leptin were POMC positive (supplemental Fig. 1A, available in an online appendix at http://dx.doi.org/10.2337/db08-0822). No activation of ERK1/2 was observed in NPY neurons (supplemental Fig. 1B). These data seem to suggest that ERK mediates leptin action through an effect on POMC neurons.
To test the hypothesis that ERK is crucial for the physiological action of leptin, we assessed the effect of ERK inhibition (PD98059 and U0126) (35–37) on the feeding and body weight responses to leptin. We first verified that baseline food intake and body weight were not altered by the administration of the vehicle (DMSO) or the ERK inhibitors in the third cerebral ventricle (Fig. 4A–C). Treatment with leptin at the onset of the dark phase significantly reduced food intake at both 4 h (Fig. 4A) and 24 h (Fig. 4B). This effect was accompanied by decreased body weight at 24 h after treatment with leptin (Fig. 4C). Pretreatment with the ERK inhibitors (PD98059 or U0126) reversed the decrease in food intake induced by leptin at 4 h (Fig. 4A) and 24 h (Fig. 4B). The ability of leptin to decrease body weight was also blocked by PD98059 and U0126 (Fig. 4C). To exclude the possibility that blockade of the anorectic and weight-reducing actions of leptin by PD98059 and U0126 may be due to inhibition of other mediators of leptin action, such as STAT3 and PI 3-kinase (which are known to play an important role in leptin effects on food intake) (9,12), we tested the effect of leptin on the activity of ERK1/2, STAT3, and PI 3-kinase in the presence of PD98059 and U0126. As expected, leptin activation of hypothalamic ERK1/2 was prevented in the presence of PD98059 or U0126 (Fig. 5). In contrast, stimulation of hypothalamic PI 3-kinase and STAT3 by leptin was not affected by the presence of these inhibitors (Fig. 5). These data demonstrate that blockade of the effect of leptin on food intake and body weight by PD98059 and U0126 is due to inhibition of ERK1/2 and not to blockade of leptin-induced STAT3 or PI 3-kinase activation.
FIG. 4.
Role of ERK in mediating the effect of leptin on food intake and body weight. Intracerebroventricular (ICV) administration of ERK inhibitors PD98059 (PD; 5 μg) or U0126 (U; 7 μg) 15 min before intraperitoneal (IP) leptin prevents the 4 h (A) and 24 h (B) decrease in food intake and the weight loss (C) induced by leptin. ERK inhibitors do not prevent the 4 h (D) and 24 h (E) decrease in food intake and the weight loss (F) induced by intracerebroventricular MTII. Data represent means ± SE; n = 8 rats per group. *P < 0.001 vs. vehicle. Lep, leptin; Veh, vehicle.
FIG. 5.
Presence of ERK inhibitors blocks specifically leptin stimulation of hypothalamic ERK. Intracerebroventricular (ICV1) administration of ERK inhibitors PD98059 (PD; 5 μg) or U0126 (U; 7 μg) 15–20 min before intracerebroventricular (ICV2) leptin prevents the increase in hypothalamic phospho-ERK1/2, but not activation of PI 3-kinase or STAT3, induced by leptin. Each blot is representative of three to four experiments.
Because ERK is a key enzyme for many intracellular signaling processes, we addressed the specificity of blockade of leptin-induced anorexia and weight loss by testing the feeding- and weight-reducing actions of other stimuli, i.e., an agonist of the melanocortin receptors MTII and corticotrophin-releasing hormone. Intracerebroventricular MTII caused a significant decrease in food intake and body weight at 4 and 24 h (Fig. 4D–F; data not shown). Intracerebroventricular pretreatment with PD98059 or U0126 did not alter the effect of intracerebroventricular MTII on food intake and body weight (Fig. 4D–F). In addition, the anorectic and weight-reducing actions of intracerebroventricular administration of corticotrophin-releasing hormone were not affected by the ERK inhibitors (data not shown). Together, these findings show that the pharmacological inhibitors of ERK produce a selective blockade of the effects of leptin and do not attenuate the responses to other agonists, such as MTII and corticotrophin-releasing hormone.
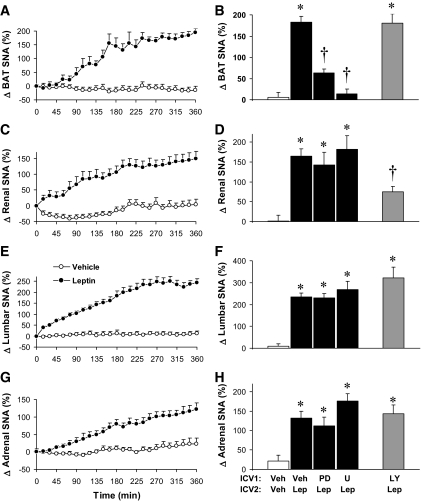
Leptin stimulation of the sympathetic nervous system regulates diverse physiological processes, including energy expenditure and cardiovascular function. The possible role of hypothalamic ERK in the control of the sympathetic nerve traffic by leptin was investigated. Intracerebroventricular leptin caused regional sympathetic activation, including increases in sympathetic nerve outflow to BAT (Fig. 6A), kidney (Fig. 6C), hindlimb (Fig. 6E), and adrenal gland (Fig. 6G). Selective inhibition of ERK prevented leptin-induced sympathetic activation to thermogenic BAT (Fig. 6B). This effect of ERK inhibition on BAT sympathetic activation to leptin was dose dependent (BAT sympathetic activation to leptin was 183 ± 13, 114 ± 19, and 14 ± 11% in the presence of vehicle, 3 μg U0126, and 7 μg U0126, respectively, P < 0.001). In contrast, PD98059 or U0126 did not alter renal (Fig. 6D), lumbar (Fig. 6F), and adrenal (Fig. 6G) sympathetic nerve responses to leptin. To examine whether the control of sympathetic outflow to thermogenic BAT by leptin exclusively involves ERK, we assessed the effect of inhibition of another pathway that has been shown to play a major role in the feeding and sympathetic responses to leptin, PI 3-kinase (12,38). Inhibition of PI 3-kinase with LY294002 significantly attenuated renal sympathetic activation to leptin (Fig. 6D), which is consistent with our previous report (38). In contrast, PI 3-kinase inhibition with LY294002 failed to alter BAT (Fig. 6A), lumbar (Fig. 6F), or adrenal (Fig. 6G) sympathetic nerve responses to leptin. Taken together, these results demonstrate that leptin regulates different regional sympathetic nerve activities through distinct and contrasting intracellular signaling pathways with ERK contributing to the thermogenic BAT sympathetic response but not to renal, lumbar, or adrenal sympathetic nerve responses to leptin.
FIG. 6.
Role of ERK in mediating the sympathetic effects of leptin is specific for the thermogenic BAT. Left panels (A, C, E, and G) show the time course of SNA responses to intracerebroventricular leptin. Right panels (B, D, F, and H) depict the effects of ERK and PI 3-kinase inhibitors on regional SNA response to leptin. B: Intracerebroventricular (ICV1) administration of ERK inhibitors PD98059 (PD; 5 μg) or U0126 (U; 7 μg), but not of PI 3-kinase inhibitor LY294002 (LY; 5 μg), 15 min before intracerebroventricular (ICV2) leptin prevents the increase in BAT SNA induced by leptin. Inhibition of ERK does not prevent the SNA increase induced by leptin to kidney (D), hindlimb (F), and adrenal gland (H). Data represent means ± SE; n = 8–20 rats per group. *P < 0.001 vs. vehicle; †P < 0.001 vs. leptin alone.
DISCUSSION
We have characterized a new hypothalamic signaling mechanism of the leptin receptor. Our results show that leptin activates ERK1/2 in the arcuate nucleus and that this pathway contributes to leptin control of food intake, body weight, and thermogenic sympathetic outflow. Hypothalamic ERK appears to mediate selective leptin actions because its inhibition prevented some but not all actions of leptin. This suggests that modulation of the hypothalamic ERK pathway could affect the metabolic actions of leptin (appetite and thermogenic sympathetic metabolism) without altering its sympathetic cardiovascular and renal actions.
Previous studies have implicated hypothalamic ERK in the regulation of energy homeostasis. Fasting was shown to activate ERK in the hypothalamic arcuate and paraventricular nuclei in mice (39,40). Fasting-induced activation of hypothalamic ERK was reversed by re-feeding, suggesting that activation of ERK in the hypothalamus is relevant for energy balance (40). Our current findings that hypothalamic ERK contributes to the actions of leptin extend the role of this ERK pathway in the control of energy homeostasis. Our results are in line with the observation that the development of obesity in mice with neuronal-specific ablation of Shp2 is related to the inability of leptin to stimulate ERK (41). To study the role of neuronal Shp2, Zhang et al. (41) used the cre-loxP system to create a conditional Shp2 mutant allele in mice. This allowed selective deletion of Shp2 in postmitotic forebrain neurons. Surprisingly, the predominant phenotype exhibited by this mouse model was the development of early-onset obesity. In subsequent studies, Zhang et al. found that leptin-induced phosphorylation of ERK1/2 in the arcuate nucleus of hypothalamus was dramatically reduced in mice with neuronal-specific ablation of Shp2 compared with the controls (41). In contrast, the ability of leptin to induce phosphorylation of arcuate STAT3 was preserved in this mouse model. Although the signaling cascade leading to the activation of ERK by the leptin receptor remains unclear (15), the inability of leptin to stimulate ERK in the absence of Shp2 suggests that this protein mediates leptin activation of ERK. However, recent findings challenge the importance of Shp2 in mediating leptin effects (42). This is based on the observation that in mice, mutation of the Tyr985 of the leptin receptor that blocks Shp2 recruitment did not recapitulate the obesity phenotype observed in mice in which Shp2 was deleted in the forebrain neurons (42), suggesting that, in vivo, Jak2-dependent mechanism may be the predominant pathway for the stimulation of ERK by the leptin receptor.
The downstream hypothalamic pathways controlled by the leptin receptor–ERK axis remain to be elucidated. Importantly, leptin activation of ERK seems to occur in the POMC neurons in the arcuate nucleus of the hypothalamus, which narrows the search for the mechanisms that are controlled by the leptin receptor–ERK axis. However, additional studies are needed to analyze in more detail the role of ERK in POMC vs. NPY neurons in mediating leptin action.
A key downstream target of the POMC neurons are neurons expressing melanocortin 4 receptors (MC4Rs) that are activated by α-melanocyte stimulating hormone (product of POMC) (33,34). Pharmacological blockade of MC4Rs reverses the effect of leptin on body weight and food intake (43) and deletion of the MC4Rs leads to severe obesity in mice (44). We have previously shown that blockade of the brain MC4R inhibits the renal, but not the BAT, sympathetic nerve response to leptin (27). We also showed that leptin-induced renal sympathetic activation is absent in the homozygous MC4R knockout mice, demonstrating the importance of this receptor in the control of renal sympathetic outflow by leptin (45). In addition, blockade of PI 3-kinase also inhibits renal, but not BAT, SNA. In contrast to the role of MC4R and PI 3-kinase, blockade of ERK in this study inhibited SNA to BAT but not kidney. This supports the concept that differential intracellular mechanisms are involved in leptin-induced sympathetic activation to kidney and thermogenic BAT. Sympathetic activity to BAT is ERK dependent, whereas sympathetic activity to kidney is PI 3-kinase and MC4R dependent.
These findings are in line with the notion that leptin controls various physiological processes through a variety of signaling mechanisms. For instance, the STAT3 pathway appears to be involved in mediating the effects of leptin on food intake and energy homeostasis but not on reproductive function, growth, or glucose homeostasis (9). However, the relative role of each of the downstream pathways associated with the leptin receptor in the control of food intake by leptin remains perplexing, because disrupting any of these pathways seems to have a profound effect on leptin-induced food intake. Disruption of the leptin receptor–STAT3 pathway causes hyperphagia in mice (9,10), which demonstrates that STAT3 is important for the control of food intake by leptin. In addition, the anorectic response to leptin can be reversed by blockade of PI 3-kinase (12,38) and ERK (present study).
Some limitations of the present study need to be addressed. First, our conclusions regarding the role of ERK in mediating leptin effects were based on acute studies. Whether chronic inhibition of these signaling pathways will result in a similar effect as in the acute studies remains to be determined, but deletion of Shp2 is accompanied by increased food intake and obesity, presumably through disruption of ERK signaling. Second, the inhibitors that we used in our studies to block ERK might have other nonspecific actions. However, cell-based assays and in vitro studies have shown that both PD98059 and U0126 appear to specifically suppress ERK signaling (35–37). These inhibitors have been widely used to suppress activation of ERK and to examine the physiological roles of these enzymes. In addition, we have shown that pretreatment with ERK inhibitors did not alter leptin-induced activation of STAT3 or PI 3-kinase and did not affect the appetite- and weight-reducing actions of MTII and corticotrophin-releasing hormone. Third, our studies lack the neuroanatomical specificity regarding the brain nuclei where the ERK signaling pathway mediates the effects of leptin on food intake, body weight, and BAT sympathetic outflow, because the inhibitors were administered intracerebroventricularly. Nonetheless, using an immunohistochemical approach, we have shown that leptin activation of ERK occurs in the arcuate nucleus.
In conclusion, our experiments provide evidence that hypothalamic ERK is a significant downstream target for the effects of leptin to regulate food intake, body weight, and thermogenic sympathetic outflow to BAT. However, ERK does not appear to be involved in leptin activation of the sympathetic nervous system to other tissues, such as kidney, hindlimb, and adrenal gland. These findings provide new insights into the intracellular mechanisms engaged by the leptin receptor to control various physiological functions.
Supplementary Material
Acknowledgments
K.R. has received Scientist Development Grant 0530274N from the American Heart Association National Center. These studies were supported by National Heart, Lung, and Blood Institute Grant HL084207.
No potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://diabetes.diabetesjournals.org on 9 December 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 513.
REFERENCES
- 1.Friedman JM, Halaas JL: Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Chehab FF, Lim ME, Lu R: Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G: Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Rahmouni K, Correia ML, Haynes WG, Mark AL: Obesity-associated hypertension: new insights into mechanisms. Hypertension 45: 9–14, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP: Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia LA: The leptin receptor. J Biol Chem 272: 6093–6096, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM: Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY: Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A 101: 4661–4666, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG Jr: STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Filer E, Myers MG: LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53: 3067–3073, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Piper ML, Unger EK, Myers MG, Xu AW: Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 22: 751–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW: Intracellular signalling: key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB: AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ: Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Myers MG: Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59: 287–304, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K: Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem 272: 12897–12900, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K: Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun 246: 752–759, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Bjorbaek C, Uotani S, da Silva B, Flier JS: Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272: 32686–32695, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG Jr, Flier JS: Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276: 4747–4755, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Banks AS, Davis SM, Bates SH, Myers MG: Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Muenzberg H, Myers MG: The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem 282: 31019–31027, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M: The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res 93: 277–279, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Tajmir P, Ceddia RB, Li RK, Coe IR, Sweeney G: Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology 145: 1550–1555, 2004 [DOI] [PubMed] [Google Scholar]
- 24.van den Brink GR, O'Toole T, Hardwick JC, van den Boogaardt DE, Versteeg HH, van Deventer SJ, Peppelenbosch MP: Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol Cell Biol Res Commun 4: 144–150, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Najib S, Sanchez-Margalet V: Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol 220: 143–149, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Wojcicka G, Jamroz-Wisniewska A, Widomska S, Ksiazek M, Beltowski J: Role of extracellular signal-regulated kinases (ERK) in leptin-induced hypertension. Life Sci 82: 402–412, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL: Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33: 542–547, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG: Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ: Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res 13: 48–57, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Benomar Y, Wetzler S, Larue-Achagiotis C, Djiane J, Tome D, Taouis M: In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol Cell Endocrinol 242: 59–66, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Maroni P, Bendinelli P, Piccoletti R: Early intracellular events induced by in vivo leptin treatment in mouse skeletal muscle. Mol Cell Endocrinol 201: 109–121, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Elmquist JK, Elias CF, Saper CB: From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221–232, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG: Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Flier JS: Obesity wars: molecular progress confronts an expanding epidemic. Cell 116: 337–350, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM: Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR: PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Davies SP, Reddy H, Caivano M, Cohen P: Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmouni K, Haynes WG, Morgan DA, Mark AL: Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 41: 763–767, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Morikawa Y, Ueyama E, Senba E: Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J Neuroendocrinol 16: 105–112, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ueyama E, Morikawa Y, Yasuda T, Senba E: Attenuation of fasting-induced phosphorylation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus in response to refeeding. Neurosci Lett 371: 40–44, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Zhang EE, Chapeau E, Hagihara K, Feng GS: Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A 101: 16064–16069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG: Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117: 1354–1360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW: Melanocortin receptors in leptin effects. Nature 390: 349, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F: Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Rahmouni K, Haynes WG, Morgan DA, Mark AL: Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.